Introduction
Lung cancer is the second most prevalent type of
malignancy and the primary cause of cancer-associated mortality
worldwide (1). Numerous advances have
been made in diagnostic, surgical and staging techniques for cancer
over the past decade, as well as in the development of novel
chemotherapy and radiotherapy treatments (2). Following initial tumorigenesis, cancers
may progress via the following three distinct pathways: Local
invasion into adjacent structures; lymphatic spread to regional
lymph nodes; and the hematogenous spreading of distant metastases
(2).
The chemokine family consists of 48 chemotactic
cytokines, which interact with chemokine receptors under
physiological and pathological conditions in order to regulate
immune cell trafficking (3). The
presence of a limited number of chemokines and chemokine receptors
has been reported in cancer tissues (4). In tumor cells, the chemokine system is
utilized and manipulated in order to promote local tumor growth and
distant dissemination. The promotion of tumor cell growth, survival
and neo-angiogenesis occurs through autocrine and paracrine
interactions between chemokines and chemokine receptor loops in the
tumor microenvironment (5). At
distant sites, endogenous tissue-produced chemokines attract and
navigate tumor cells expressing chemokine receptors in order for
metastasis to occur (3–5).
Chemokine (C-X-C motif) receptor (CXCR)4 is the most
prevalent type of chemokine receptor expressed by malignant tumors,
the only ligand for which is chemokine (C-X-C motif) ligand
(CXCL)12/stromal cell-derived factor (SDF)-1 (3). Previous studies regarding CXCL12-CXCR4
focus on its pathologic role and the potential therapeutic
implications of targeting this axis for the treatment of lung
cancer (2,3,6,7). In addition, CXCL8/interleukin-8 (IL-8),
a potent angiogenic and autocrine growth factor, was reported to be
associated with metastasis of lung cancer (8). The IL-8 receptors, CXCR1 and CXCR2, have
been identified in numerous tumor cells; however, the presence of
these receptors has not yet been reported in lung cancer. Chemokine
(C-C motif) receptor (CCR)7, which is the receptor for two major
chemokines, chemokine (C-C motif) ligand (CCL)19 and CCL21, was
also demonstrated to have an important role in tumor metastasis and
was associated with poor prognosis (9,10). The aim
of the present study was to investigate the role of three chemokine
ligand-receptor pairs, CXCL12-CXCR4, CXCL8-CXCR1/CXCR2,
CCL19/CCL21-CCR7, in the malignant progression and metastasis of
lung carcinoma.
Materials and methods
Human tissue collection
Lung carcinoma and non-malignant lung tissues were
obtained from patients who underwent pulmonary lobe resection or
pneumonectomy at Tianjin Chest Hospital (Tianjin, China) between
2007 and 2009. Of the 120 cancer patients studied, 79 were men and
41 were female, with an average age at the time of surgery of
59.7±6.53 years (range, 31–79 years). Clinicopathological
information was recorded, including patient characteristics,
histological subtype, tumor grade and tumor-node-metastasis (TNM)
staging results. The identification of tumor types was performed by
two professional pathologists. Of these patients, 30 cases were
adenocarcinomas (Adc), 54 cases were squamous cell carcinomas
(Scc), 24 cases were adenosquamous carcinomas (Asc) and 12 cases
were combined small-cell carcinoma (Csc). The stages of tumors were
estimated according to the 7th edition of the new TNM staging
system suggested by the International Association for the Study of
Lung Cancer in 2009 (11). Written
informed consent was obtained from the patients. All procedures
involving participants in the study were approved by Peking
University Institutional Review Board (approval no.
IRB00001052-10004; the approval no. is the same as that of a
previous study by the same authors).
Immunohistochemistry
All tumor and normal control tissue samples were
formalin fixed and paraffin embedded. Sections of 4-µm-thick were
then cut onto glass slides and dewaxed in xylene, then rehydrated
through graded alcohols (100, 95, 85 and 70%). Antigen retrieval
was performed using citrate buffer (10 mM, pH 6.0) in a pressure
cooker (model EPC450; Elecpro Electric Appliance Holding Co., Ltd,
Guangdong, China; heated to 117.5°C for 3 min and then cooled to
room temperature). Following antigen retrieval, 0.3%
H2O2 in phosphate buffer solution was
employed to block endogenous peroxidase activity in the sections.
The aforementioned chemical reagents were purchased from Sinopharm
Chemical Reagent Beijing Co., Ltd. (Beijing, China). Following
treatment with protein-blocking serum to block non-specific
binding, the sections were incubated overnight at 4°C with the
following monoclonal mouse anti-human antibodies (dilution, 25
µg/ml; R&D Systems Europe, Abingdon, United Kingdom): CXCR1
(clone 79018), CXCR2 (clone 48311), CXCL8 (clone 6217), CXCR4
(clone 44716), CXCL12/SDF-1 (clone 51505), CCL19 (clone 54909),
CCL21 (clone 59106) and CCR7 (clone 150503). A streptavidin/biotin
detection reagent kit with 3, 3′-diaminobenzidine
tetrahydrochloride was used for signal detection and Harris
hematoxylin was used to counter-stain slides. The reagents for
immunohistochemical analysis, including the buffer, serum,
detection kit, DAB and hematoxylin, were obtained from Maixin
Biotech Co., Ltd. (Fuzhou, China). For each staining, negative
control slides were processed without the primary antibody.
The mean percentage of positive tumor cells was
determined in at least five fields of vision (magnification, x200)
using Leica DM1000 microscope (Leica Microsystems, Solms, Germany).
All slides were evaluated by experienced pathologists who reviewed
the slides together and reached a consensus. The staining of
chemokines and chemokine receptors was primarily located in the
cytoplasm or cytomembrane. Tumors were assigned scores based on the
percentage of positively-stained cells and the intensity of
immunostaining. The scoring for the percentage of
positively-stained tumor cells was as follows: 0, <5%; 1, 5–25%;
2, 25–50%; 3, 50–75%; and 4, >75%. Immunostaining intensity was
scored as follows: 0, none; 1+, weak; 2+, moderate; and 3+,
intense. The two scores were multiplied together to achieve a
weighted score for each case. Cases with weighted scores of 0 or 1
were defined as negative; cases with scores of ≥2 were defined as
positive.
RNA extraction and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
analysis
Within 10 min after surgery, tissues without
necrosis and hemorrhage were dissected from the tumor, followed by
flash freezing in liquid nitrogen and storing at −70°C. Total
cellular RNA was extracted from frozen samples using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions. A Nanodrop 2000 (Thermo Scientific
Inc., Wilmington, DE, USA) was used to determine RNA quality. RNA
(2 µg) from each sample was used for complementary (c)DNA
production using Moloney murine leukemia virus reverse
transcriptase and random hexamers (Promega Corp., Madison, WI,
USA). Amplification of control GAPDH was performed using diluted
cDNA (1:10) in order to determine RT efficiency as well as RNA
integrity. RT-qPCR was performed in a total volume of 50 µl
containing 1X GoTaq flexi buffer, 5 mM MgCl2, 200 µM
each deoxynucleotide, 2.5 Units GoTaq DNA polymerase (Promega
Corp.), 500 nM primers (as shown in Table
I), SYBR-Green I (1:20,000; Invitrogen Biotechnology Co., Ltd.,
Shanghai, China) and 2.5 µl diluted cDNA. The PCR cycling was
performed using an ABI StepOne Real-Time Cycler (Applied
Biosystems, Framingham, MA, USA) as follows: 3 min initial
denaturation at 95°C and 40 cycles of amplification at 94°C for 30
sec, 55°C for 45 sec and 72°C for 45 sec. Fluorescence readings
were collected at 72°C. GAPDH amplification was used as an internal
control. The Ct value of each target gene was normalized to the
Ct-value of GAPDH by subtracting the GAPDH Ct-value from the target
Ct value. The relative expression levels for each target PCR was
calculated using the equation (12):
Relative expression=2−[Ct(target)-Ct(GAPDH)] ×
10,000.
 | Table I.Gene-specific primers used in reverse
transcription quantitative polymerase chain reaction for
chemokine-chemokine receptors. |
Table I.
Gene-specific primers used in reverse
transcription quantitative polymerase chain reaction for
chemokine-chemokine receptors.
| Target gene | Primer pairs | Products (bp) | Accession no. |
|---|
| CCL19 | F:
5′TCCCCAGCCCCAACTC 3′ | 247 | NM_006274 |
|
| R:
5′TGCGGCGCTTCATCTT 3′ |
|
|
| CCL21 | F:
5′AGGACCCAAGGCAGTGAT 3′ | 248 | NM_002989 |
|
| R:
5′CCCTGGGCTGGTTTCTG 3′ |
|
|
| CCR7 | F:
5′CAGAGAGCGTCATGGACC 3′ | 248 | NM_001838 |
|
| R:
5′GACCAGCCCATTGCCC 3′ |
|
|
| CXCL12/SDF-1 | F:
5′TCTGCCTCAGCGACGG 3′ | 237 | NM_199168 |
|
| R:
5′TTGGCTGTTGTGCTTACTTG 3′ |
|
|
| CXCR4 | F:
5′CCAGTAGCCACCGCATCT 3′ | 259 | NM_003467 |
|
| R:
5′TGTCCGTCATGCTTCTCAG 3′ |
|
|
| CXCL8/IL8 | F:
5′CAGCCTTCCTGATTTCTGC 3′ | 245 | NM_000584 |
|
| R:
5′AAACTTCTCCACAACCCTCTG 3′ |
|
|
| CXCR1/IL8R A | F:
5′CCCTCTAGCTGTTAAGTCACTCT 3′ | 255 | NM_000634 |
|
| R:
5′AACACTAGGGCATAGGCGAT 3′ |
|
|
| CXCR2/IL8R B | F:
5′ACCTCATTGTTCCTCTGTGG 3′ | 241 | NM_001557 |
|
| R:
5′TCCTGACTGGGTCGCTG 3′ |
|
|
| GAPDH | F:
5′GTGAAGGTCGGAGTCAACGG 3′ | 225 | NM_002046 |
|
| R:
5′CTGGAAGATGGTGATGGGAT 3′ |
|
|
Statistical analysis
SPSS 13.0 software for Windows (SPSS, Inc., Chicago,
IL, USA) was used for all statistical analyses. The association
between chemokine or chemokine receptor expression and
clinicopathologic parameters was analyzed using the χ2
test. Correlations among the levels of chemokine or chemokine
receptor messenger (m)RNA expression in tumor tissues were
determined using Pearson's correlation coefficient analysis.
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
Expression of CXCL12/SDF-1 and CXCR4
in lung carcinoma
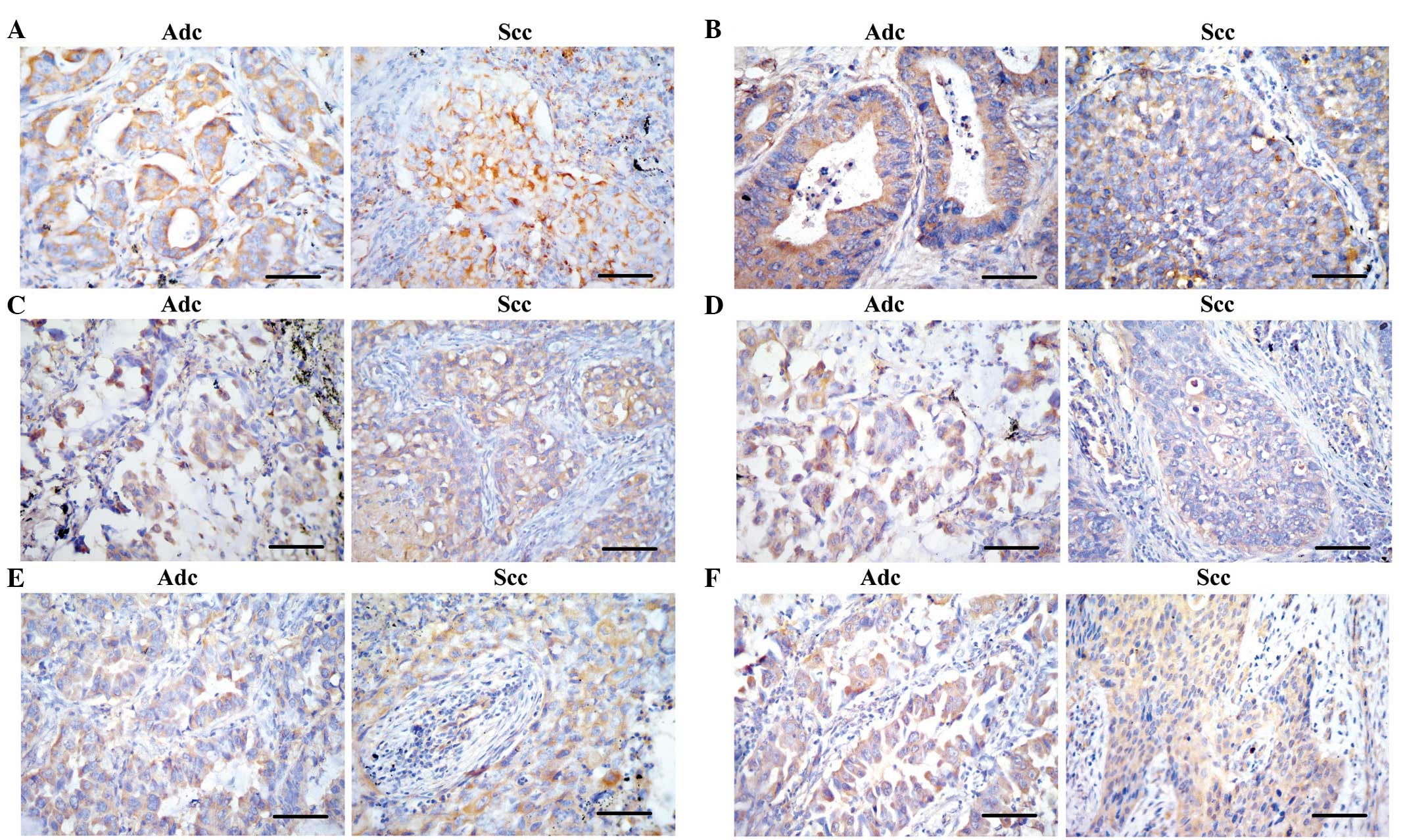
The immunostaining of CXCR4 and CXCL12/SDF-1 was
present in tumor cells and stromal inflammatory cells with no
expression evident on alveolar epithelial cells or vascular
endothelial cells, whereas staining for CXCL12/SDF-1 appeared
weakly in the bronchial epithelial cells. Representative images of
immunohistochemical staining of CXCR4 and CXCL12/SDF-1 are shown in
Fig. 1A and B, respectively. As shown
in Table II, expression of
CXCL12/SDF-1 and CXCR4 was detected in 61.7% (74/120) and 28.3%
(34/120) of lung carcinomas, respectively. There were no
significant differences for the positive percentage of CXCL12/SDF-1
or CXCR4 among different histological subtypes. However, CXCR4 and
CXCL12/SDF-1 expression were found to be positively correlated with
lymph node metastasis (χ2=5.36 and 8.48, respectively;
P<0.01). No correlations were observed between CXCR4 or
CXCL12/SDF-1 and gender, age, tumor grade or differentiation. In
addition, there were no significant differences for CXCL12/SDF-1 in
different clinicopathological grouping.
 | Table II.Correlation of chemokine-chemokine
receptor expression with clinicopathological characteristics in
lung carcinoma. |
Table II.
Correlation of chemokine-chemokine
receptor expression with clinicopathological characteristics in
lung carcinoma.
| A, CXCL12/SDF-1 and
CXCR4 expression |
|
|
|
|
|
|
|
|---|
|
|---|
|
|
|
| CXCL12/SDF-1 |
| CXCR4 |
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | N |
| Mean ± Std | IHC (%) |
| Mean ± Std | IHC (%) |
|---|
| Gender |
|
|
|
|
|
|
|
|
Male | 86 |
|
231.11±67.27a | 58.1 |
| 52.59±9.01 | 34.9 |
|
Female | 34 |
| 157.67±40.89 | 70.6 |
| 50.30±10.41 | 11.8 |
| Histological
type |
|
|
|
|
|
|
|
|
Adc | 30 |
| 237.16±54.54 | 80.0 |
|
116.70±15.61b | 20.0 |
|
Scc | 54 |
| 175.72±39.93 | 59.3 |
| 28.88±2.70 | 33.3 |
|
Asc | 24 |
| 235.09±47.14 | 58.3 |
| 58.23±7.25 | 33.3 |
|
Csc | 12 |
|
305.56±62.66a | 33.3 |
| 32.19±5.96 | 16.7 |
| TNM staging |
|
|
|
|
|
|
|
|
I–II | 66 |
|
196.42±43.99b | 57.6 |
| 50.65±5.96 | 30.3 |
|
III–IV | 54 |
| 242.72±39.16 | 66.7 |
| 54.41±5.69 | 25.9 |
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
N=0 | 48 |
| 222.60±64.77 | 45.8b |
|
42.54±4.25b | 16.7a |
|
N>0 | 72 |
| 207.13±56.69 | 72.2 |
| 65.65±8.28 | 36.1 |
| Vascular
invasion |
|
|
|
|
|
|
|
|
Negative | 48 |
|
320.82±63.17b | 66.7 |
|
20.90±5.85b | 29.2 |
|
Positive | 72 |
| 212.94±40.34 | 58.3 |
| 84.87±18.79 | 27.8 |
|
| B, CXCL8/IL-8,
CXCR1 and CXCR2 expression |
|
|
|
|
|
|
|
|
| CXCL8/IL-8 | CXCR1 | CXCR2 |
|
|
|
|
|
|
|
| Characteristic | N | Mean ± Std | IHC (%) | Mean ± Std | IHC (%) | Mean ± Std | IHC (%) |
|
| Gender |
|
|
|
|
|
|
|
|
Male | 86 | 41.39±13.92 | 37.2 | 5.17±3.93 | 32.6 |
45.96±15.76b | 39.5 |
|
Female | 34 | 44.92±12.44 | 47.1 | 3.86±2.50 | 35.3 | 14.43±6.03 | 58.8 |
| Histological
type |
|
|
|
|
|
|
|
|
Adc | 30 |
63.27±9.19b | 46.7 | 2.03±1.14 | 53.3 |
64.02±18.74a | 73.3b |
|
Scc | 54 | 38.54±8.72 | 37.0 | 1.98±0.85 | 18.5 | 39.58±14.99 | 37.0 |
|
Asc | 24 | 36.43±8.22 | 33.3 | 4.62±2.43 | 50.0 | 15.92±7.25 | 41.7 |
|
Csc | 12 | 29.61±6.43 | 50.0 |
21.20±8.38a | 16.7 | 24.47±9.39 | 16.7 |
| TNM staging |
|
|
|
|
|
|
|
|
I–II | 66 |
35.53±10.13a | 39.4 | 5.61±2.66 | 36.4 |
43.87±15.33a | 42.4 |
|
III–IV | 54 | 52.97±8.35 | 40.7 | 3.76±1.41 | 29.6 | 30.95±13.70 | 48.1 |
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
N=0 | 48 |
38.21±9.45a | 25.0b | 4.73±2.49 | 33.3 |
32.64±13.65a | 33.3b |
|
N>0 | 72 | 44.96±13.71 | 50.0 | 5.00±3.60 | 33.3 | 43.39±15.49 | 52.8 |
| Vascular
invasion |
|
|
|
|
|
|
|
|
Negative | 48 | 45.37±6.64 | 50.0 | 5.14±1.23 | 50.0b | 51.06±14.23 | 50.0 |
|
Positive | 72 | 43.13±5.44 | 33.3 | 3.88±1.71 | 22.2 | 46.02±10.04 | 41.7 |
|
| C, CCL19, CCL21 and
CCR7 expression |
|
|
|
|
|
|
|
|
|
|
| CCL19 | CCL21 | CCR7 |
|
|
|
|
|
|
| Characteristic | N | Mean ± Std | IHC (%) | Mean ± Std | IHC (%) | Mean ± Std | IHC (%) |
|
| Gender |
|
|
|
|
|
|
|
|
Male | 86 | 47.55±6.97 | 43.0 | 9.52±3.66 | 16.3 | 30.16±13.37 | 39.5 |
|
Female | 34 | 49.99±5.11 | 47.1 | 10.44±3.78 | 20.6 | 26.31±9.03 | 35.3 |
| Histological
type |
|
|
|
|
|
|
|
|
Adc | 30 |
143.50±20.55b | 66.7b | 9.76±2.81 | 20.0 |
18.08±4.92a | 40.0 |
|
Scc | 54 | 25.08±3.40 | 31.5 | 7.85±2.58 | 16.7 | 33.38±13.27 | 40.7 |
|
Asc | 24 | 12.99±1.27 | 33.3 |
13.32±3.95a | 20.8 | 23.40±9.93 | 33.3 |
|
Csc | 12 | 11.47±0.81 | 25.0 | 6.55±2.44 | 25.0 | 21.17±11.97 | 33.3 |
| TNM staging |
|
|
|
|
|
|
|
|
I–II | 66 |
39.75±15.29a | 40.9 | 8.59±2.56 | 15.2 |
30.79±13.29a | 36.4 |
|
III–IV | 54 | 55.81±18.73 | 48.1 | 9.52±4.89 | 20.4 | 21.09±12.09 | 40.7 |
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
N=0 | 48 |
52.79±17.21a | 41.7 | 8.49±3.60 | 12.5 |
36.42±14.35a | 37.5 |
|
N>0 | 72 | 41.01±15.99 | 45.8 | 9.27±4.75 | 20.8 | 20.53±11.89 | 38.9 |
| Vascular
invasion |
|
|
|
|
|
|
|
|
Negative | 48 |
12.01±4.24b | 37.5 | 10.61±3.03 | 16.7 | 13.84±2.07 | 54.2 |
|
Positive | 72 | 70.52±22.86 | 48.6 | 10.75±4.55 | 18.1 |
44.31±10.97b | 27.8b |
In 120 lung carcinoma patients, the average relative
expression of CXCR4 and CXCL12/SDF-1 messenger (m)RNA in tumor
tissues was 56.24 and 212.63 respectively, which was significantly
higher than those in normal lung tissues (6.81 and 78.02,
respectively; P<0.001). In the four histological subtypes, Csc
exhibited increased CXCL12/SDF-1 mRNA expression (P<0.05),
whereas Adc expressed higher CXCR4 mRNA levels compared with normal
tissues (P<0.01) (Table II).
CXCL12/SDF-1 mRNA expression was significantly associated with
higher TNM staging (P<0.01), which was coincident with the
immunohistochemical results. Higher CXCL12/SDF-1 mRNA expression
was found in the patients without vascular invasion (P<0.01),
while higher CXCR4 mRNA levels were correlated with lymph node
metastasis and vascular invasion (P<0.01) (Table II).
Expression of CXCL8/IL-8, CXCR1 and
CXCR2 in lung carcinoma
Of the 120 tumor samples, 48 (40%), 40 (33%) and 54
(45%) were positively stained for CXCL8/IL-8, CXCR1 and CXCR2 in
tumor cells, respectively. Similarly in the neutrophilic
granulocytes of the stroma, weak cytoplasmic staining was observed
in certain normal alveolar epithelial cells (Fig. 1C and D). As shown in Table II, the CXCL8/IL-8-positive percentage
was significantly higher in lung carcinoma with lymph node
metastasis (50 vs. 25%; χ2=7.50; P<0.01).
Furthermore, no significant associations were observed between
other clinicopathologic characteristics and CXCL8/IL-8, CXCR1 or
CXCR2 immunoreactivity.
In all patients, the average relative expression
levels of CXCL8/IL-8 and CXCR2 mRNA in tumor tissues were 46.21 and
40.69, respectively, which was significantly increased compared
with those in normal lung tissues (13.7 and 19.67, respectively;
P<0.01). However, CXCR1 mRNA expression was lower in lung
carcinomas compared with normal tissues (4.98 vs. 27.7; P<0.01).
In different histological subtypes, Csc exhibited higher CXCR1 mRNA
expression, whereas Adc demonstrated higher CXCL8/IL-8 and CXCR2
mRNA expression (P<0.05) (Table
II). CXCL8/IL-8 or CXCR2 mRNA expression levels, but not CXCR1,
were associated with a high incidence of lymph node metastasis
(P<0.05), which was coincident with the immunohistochemical
staining results. A strong positive correlation was substantiated
between CXCL8/IL-8 mRNA expression with TNM staging (P<0.05)
(Table II). By contrast, a negative
correlation was identified between CXCR2 mRNA expression and TNM
staging (P<0.05) (Table II).
These results suggested the crucial role of CXCL8/IL-8 and CXCR2 in
tumor progression of lung carcinoma, without the involvement of
CXCR1.
Expression of CCL19, CCL21 and CCR7 in
lung carcinoma
The majority of CCL21 immunostaining in the tumor
islets was weak in inflammatory cells and tumor epithelial cells.
Moderate immunostaining of CCL19 and CCR7 was detected in the
cytoplasm of tumor cells and stromal inflammatory cells, whereas
weak expression was evident in normal bronchial and alveolar
epithelial cells. Furthermore, CCR7 was found to be expressed on
the membrane of certain tumor cells (Fig.
1E and F). A total of 44.2% (53/120), 28% (21/120) and 38.3%
(46/120) tumor samples were positively stained with CCL19, CCL21
and CCR7, respectively. As shown in Table II, staining intensity and positive
percentage of CCL19 were higher in adenocarcinoma (66.7%;
χ2=12.09; P<0.01). However, no significant
correlations were identified between the clinicopathologic
characteristics and the positivity of CCR7 or CCL21.
The average relative expression levels of CCL19 and
CCR7 mRNA in tumor tissues were 48.31 and 36.24, respectively,
which was significantly higher compared with those in normal lung
tissues (10.22 and 10.29, respectively; P<0.001). However, CCL21
mRNA expression was low in lung carcinoma and normal tissues (9.48
and 11.09, respectively). Compared with other histological
subtypes, Adc exhibited the highest CCL19 mRNA expression and the
lowest CCR7 mRNA expression (P<0.05). By contrast, Asc had
markedly increased CCL21 mRNA expression compared with the other
subtypes (P<0.05) (Table II).
Tumor staging was inversely correlated with CCR7 mRNA expression
and positively correlated with CCL19 mRNA expression (P<0.05).
Furthermore, the mRNA expression of CCL19 and CCR7 was negatively
correlated with lymph node metastasis (P<0.05) and positively
correlated with vessel invasion (P<0.01) (Table II). No correlation was observed
between CCR7 expression and gender, histology, staging or
metastasis (Table II).
Discussion
The human chemokines superfamily consists of 48
chemoattractant cytokines, which are known to form interactions
with 19 different G protein-coupled chemokine receptors (2). Chemokine ligands are small secreted
proteins that are released in response to cell activation by
various cytokines or pathological stimuli. There are four
sub-families of chemokines, C, C-C, C-X-C and C-X3-C),
which are classified according to cysteine residue spacing proximal
to the N terminus of these chemokines. It has been reported that
certain chemokines and their receptors may have important functions
in tumorigenesis and/or metastasis (4). It was suggested that chemokine receptors
may enable tumor dissemination at numerous stages during
metastasis, including tumor cell adherence to the endothelium,
blood vessels extravasation, metastatic colonization, angiogenesis,
proliferation and immune response evasion via activation of key
survival pathways (3,4).
The most prevalent chemokine receptor, CXCR4, has
been reported to be overexpressed in human cancers. CXCL12/SDF-1,
the only ligand of CXCR4, is a homeostatic chemokine and is
continuously produced in numerous types of tissues, including those
where metastases commonly occur (6).
CXCL12/SDF-1 interactions with CXCR4 initiate divergent downstream
signaling pathways, which may lead to various responses, including
chemotaxis, cell survival and/or proliferation, increased
intracellular calcium levels and gene transcription. A previous
in vitro and in vivo study reported that CXCL12-CXCR4
interactions in the tumor microenvironment may promote local tumor
growth; in addition, elevated CXCR4 expression in tumor cells was
suggested to enhance the invasive and metastatic potential of these
cells (13). A previous preclinical
trial demonstrated that anti-SDF-1 or anti-CXCR4 monoclonal
antibodies have been reported to neutralize SDF-1 in vivo
and result in a significant decrease in non-small-cell lung cancer
(NSCLC) metastases (14). At present,
>15 novel drugs are being developed, which target the
CXCL12/CXCR4 axis; one of these drugs, AMD3100 (also known as
Plerixafor and Mozobil), has gained Food and Drug Administration
approval (15). In the present study,
the expression of CXCR4 and CXCL12/SDF-1 mRNA in tumor tissues was
significantly increased compared with that in normal lung tissues,
although the latter demonstrated low-level constitutive
CXCL12/SDF-1 expression.
As demonstrated in retrospective studies of human
metastatic cancers, elevated CXCL12/CXCR4 expression was associated
with advanced diseases stages and with poorer prognoses (13,16).
Franco et al (17) reported
that increased CXCR4 expression in lung carcinoma cells was
associated with a marked elevation in the microvascular structure
density of tumors, which was in turn associated with increased
microvessel invasion by tumor cells. In the present study, it was
observed that higher CXCR4 mRNA expression occurred in lung
carcinomas with lymph node metastasis or with vascular invasion,
which was in concurrence with the previous consensus of the role of
CXCR4 in promotion of invasion and metastasis (13,17,18).
Previous studies have reported an association between high
CXCL12/SDF-1 expression and high T scores as well as an increased
tendency to form lymph node metastases (18,19). In
the present study, CXCL12/SDF-1 mRNA expression was significantly
associated with higher TNM staging, but inversely correlated with
vascular invasion. This indicated that CXCL12/SDF-1 was a potential
marker of tumor progression. Although these are preliminary results
and require further validation, they indicated that the
CXCL12-CXCR4 axis was activated in lung carcinoma and may have
important roles in tumor differentiation, invasion and
metastasis.
CXCL8/IL-8 is critical for the neovascularization
required for the initiation and maintenance of tumor growth, which
is associated with metastasis (8). A
previous study reported a 4-fold increase in IL-8 levels in human
tissue homogenates of non-small-cell bronchogenic carcinoma
compared with normal lung tissue (20). In addition, Yuan et al
(21) demonstrated that IL-8 mRNA
expression in NSCLC exhibited a marked association with tumor
progression, tumor angiogenesis, survival and time to relapse,
which suggested IL-8 may be used as a prognostic indicator
(21). In the present study, weak
cytoplasmic staining was observed in certain normal alveolar
epithelial cells, while CXCL8/IL-8 mRNA expression was
significantly greater in tumor tissue; in particular, in lung
adenocarcinomas. High expression of IL-8 was highly associated with
TNM staging and lymph node metastasis, as determined using
immunohistochemistry and RT-qPCR; however, no correlation was
identified between the expression of IL-8 and vessel invasion.
CXCR1 and CXCR2 are two closely associated receptors
that regulate the biological activity of CXCL8/IL-8. CXCR1 only
interacts with IL-8 and CXCL6, whereas CXCR2 is able to interact
with all known angiogenic CXC chemokines containing the Glu-Leu-Arg
motif, including CXCL8/IL-8. The presence of CXCR1 and CXCR2 has
been detected on numerous types of normal cells, including
inflammatory and endothelial cells. However, the function of CXCR1
and CXCR2 in IL-8-mediated activity remains to be fully elucidated
as previous results were controversial. Zhu et al (8) reported a significant reduction in cell
proliferation due to the presence of anti-CXCR1 antibodies,
although this was not observed with anti-CXCR2 antibodies; this
therefore suggested that CXCR1 was the primary receptor involved in
mediating the mitogenic function of IL-8 in lung cancer (8). By contrast, a murine model was used to
demonstrate that the attenuation of CXCR2 inhibited tumor growth
and angiogenesis in lung cancer (22). In the present study, the simultaneous
expression of IL-8 and its receptors was investigated in a large
number of lung carcinoma tissue samples. The results revealed that
CXCR2 mRNA expression was significantly elevated in tumor tissues
compared with normal lung tissues, while lung carcinomas produced
low or undetectable levels of CXCR1 mRNA. In addition, it was
demonstrated that the expression of CXCR1 mRNA was increased in Csc
and CXCR2 mRNA expression was elevated in Adc. This therefore
indicated that CXCL8/IL-8 was able to mediate different receptor
activation in different types of lung carcinoma; however, more
samples of Csc are required to confirm this conclusion. A previous
study reported evidence of crosstalk between the IL-8 and epidermal
growth factor receptor (EGFR); in addition, EGFR-induced Ras
activation promoted the expression of CXCL8/IL-8 and CXCL1 in tumor
cells (23). Therefore, these
chemokines were found to promote tumor cell proliferation via
autocrine loops and promote tumor-associated angiogenesis through
CXCR2 in a paracrine manner (24). In
the present study, CXCR2 mRNA expression levels, but not CXCR1,
were found to be associated with a high incidence of lymph node
metastasis and high TNM staging. This may indicate that IL-8
functions as an autocrine and/or paracrine growth factor in lung
carcinoma and promoted lymphatic metastasis via the mediation of
CXCR2.
The CCR7-CCL19/CCL21 axis has been
well-characterized for its crucial role in the formation of
secondary lymphoid structures under physiological conditions
(25). In cancer, the
CCR7-CCL19/CCL21 axis is primarily responsible for lymph node
metastasis formation by recruiting tumor cells to the T cell zone
of lymph nodes (25). CCR7
overexpression has been observed in a large number of malignant
tumors, including esophageal squamous cell carcinoma, gastric
carcinoma, colon carcinoma and NSCLC, which was confirmed to be
associated with lymph node metastasis, tumor growth, angiogenesis
and invasion (6,26,27).
However, the underlying mechanisms of these associations remained
to be elucidated, although they were thought to involve integrins
or phosphoinositide 3-kinase (26,28). In
present study, higher mRNA expression of CCR7 was observed in tumor
tissues compared with normal lung tissues. In addition, CCR7
expression was positively correlated with vascular invasion and
negatively correlated with tumor staging and lymph node metastasis.
These results seemed to be discordant with those of previous
studies (10,27). One previous study reported that high
CCR7 expression improved the postoperative prognosis of lung
adenocarcinoma patients (29). The
role of CCR7 in lung cancer appears to be complex and
post-transcription regulation of CCR7 may be important in the
progression and metastasis of lung cancer. For example, Su et
al (30) reported that CCR7 was a
sialylated protein; sialylation has a critical role in paracrine
stimulation via the endogenous ligand CCL19. In addition, it was
demonstrated that the inhibition of aberrant sialylation of CCR7
attenuated proliferation and invasion as well as promoted apoptosis
in breast cells (30).
Cancer cells of several tumors have been reported to
upregulate CCR7 and disseminate from the primary tumor, which was
suggested to occur through sensing the immobilized CCL21 gradient
and actively migrating towards the next lymphatic vessel (25). Shields et al (31) demonstrated that numerous types of
cancer secreted CCL21, which may mediate lymphoid tissue
neogenesis; in addition, it was proposed that CCL21-secreting
tumors were able to shift the host immune response from immunogenic
to tolerogenic, a phenomenon that facilitates tumor progression
(31). Koizumi et al (32) demonstrated CCL21-induced migration
in vitro and the metastatic behavior of human NSCLC in an
animal model. In the present study, 28% of lung cancer specimens
were positively stained for CCL21, which was primarily presented in
inflammatory cells. CCL21 mRNA expression was at a relatively low
level in lung carcinomas and normal tissues. In addition,
constitutive expression of CCL21 was detected in interstitial
inflammatory cells and endothelial cells.
Zhang et al (33) reported that CCL19/CCR7 upregulated the
expression of heparanase via specificity protein-1 and contributed
to the invasion of A549 cells. In addition, other previous studies
have demonstrated that CCL19 had an antitumor role in colorectal
cancer and acted as a promising clinical prognostic factor for lung
adenocarcinoma, as well as CCR7 (29,34). As a
potential immune stimulator, the antitumor activity of CCL19 was
determined in a transplantable model for lung cancer through
injecting recombinant CCL19 intratumorally, which led to
significant systemic reduction in tumor volumes (35,36). The
present study observed increased expression of CCL19 in lung cancer
and an association between CCL19 expression and high TNM staging or
vascular invasion. By contrast, a negative correlation was
identified between CCL19 expression and lymph node metastasis. This
indicated that, as the predominant ligand of CCR7, CCL19 may serve
as an indicator of tumor progression and the CCL19/CCR7 axis may
have an important role in hematogenous metastasis, instead of
lymphatic metastasis in lung cancer.
In conclusion, the chemotactic interaction between
CXCR4 and CXCL12/SDF-1, CXCR2 and CXCL8/IL8 as well as CCR7 and
CCL19, may be potent mechanisms for the induction of tumor
differentiation, lymph node metastasis and vascular invasion in
lung carcinoma. Chemokines, including CXCL12/SDF-1, CXCL8/IL8 and
CCL19, may function as autocrine and/or paracrine growth factors,
which are indicative of tumor progression and higher TNM staging.
Furthermore, CXCR4 and CXCR2 promoted lymphatic metastasis through
the activation of their specific ligands, while CCL19 and its
receptor CCR7 had an essential role in hematogenous metastasis of
lung carcinoma. These findings suggested that chemokine receptors
may be useful antitumor targets in controlling lymph node
metastasis and vascular invasion for lung cancer therapy.
Acknowledgements
The present study was supported by a grant awarded
to Dr Yan Liu by the Science & Technology Foundation of Tianjin
Health Bureau, China (no. ky0702).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saintigny P and Burger JA: Recent advances
in non-small cell lung cancer biology and clinical management.
Discov Med. 13:287–297. 2012.PubMed/NCBI
|
|
3
|
Zlotnik A, Burkhardt AM and Homey B:
Homeostatic chemokine receptors and organ-specific metastasis. Nat
Rev Immunol. 11:597–606. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kakinuma T and Hwang ST: Chemokines,
chemokine receptors and cancer metastasis. J Leukoc Biol.
79:639–651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keeley EC, Mehrad B and Strieter RM: CXC
chemokines in cancer angiogenesis and metastases. Adv Cancer Res.
106:91–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wald O, Shapira OM and Izhar U:
CXCR4/CXCL12 axis in non small cell lung cancer (NSCLC) pathologic
roles and therapeutic potential. Theranostics. 3:26–33. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu YM, Webster SJ, Flower D and Woll PJ:
Interleukin-8/CXCL8 is a growth factor for human lung cancer cells.
Br J Cancer. 91:1970–1976. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wiley HE, Gonzalez EB, Maki W, Wu MT and
Hwang ST: Expression of CC chemokine receptor-7 and regional lymph
node metastasis of B16 murine melanoma. J Natl Cancer Inst.
93:1638–1643. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mashino K, Sadanaga N, Yamaguchi H, et al:
Expression of chemokine receptor CCR7 is associated with lymph node
metastasis of gastric carcinoma. Cancer Res. 62:2937–2941.
2002.PubMed/NCBI
|
|
11
|
Tsim S, O'Dowd CA, Milroy R and Davidson
S: Staging of non-small cell lung cancer (NSCLC): A review. Respir
Med. 104:1767–1774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su L, Zhang J, Xu H, Wang Y, Chu Y, Liu R
and Xiong S: Differential expression of CXCR4 is associated with
the metastatic potential of human non-small cell lung cancer cells.
Clin Cancer Res. 11:8273–8280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Villena V, López-Encuentra A, García-Luján
R, Echave-Sustaeta J and Martínez CJ: Clinical implications of
appearance of pleural fluid at thoracentesis. Chest. 125:156–159.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brave M, Farrell A, Ching Lin S, et al:
FDA review summary: Mozobil in combination with granulocyte
colony-stimulating factor to mobilize hematopoietic stem cells to
the peripheral blood for collection and subsequent autologous
transplantation. Oncology. 78:282–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burger JA, Stewart DJ, Wald O and Peled A:
Potential of CXCR4 antagonists for the treatment of metastatic lung
cancer. Expert Rev Anticancer Ther. 11:621–630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Franco R, Pirozzi G, Scala S, et al:
CXCL12-binding receptors expression in non-small cell lung cancer
relates to tumoral microvascular density and CXCR4 positive
circulating tumoral cells in lung draining venous blood. Eur J
Cardiothorac Surg. 41:368–375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wagner PL, Hyjek E, Vazquez MF, et al:
CXCL12 and CXCR4 in adenocarcinoma of the lung: association with
metastasis and survival. J Thorac Cardiovasc Surg. 137:615–621.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wald O, Izhar U, Amir G, et al:
Interaction between neoplastic cells and cancer-associated
fibroblasts through the CXCL12/CXCR4 axis: Role in non-small cell
lung cancer tumor proliferation. J Thorac Cardiovasc Surg.
141:1503–1512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yatsunami J, Tsuruta N, Ogata K, et al:
Interleukin-8 participates in angiogenesis in non-small cell, but
not small cell carcinoma of the lung. Cancer Lett. 120:101–108.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan A, Yang PC, Yu CJ, Chen WJ, Lin FY,
Kuo SH and Luh KT: Interleukin-8 messenger ribonucleic acid
expression correlates with tumor progression, tumor angiogenesis,
patient survival and timing of relapse in non-small-cell lung
cancer. Am J Respir Crit Care Med. 162:1957–1963. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keane MP, Belperio JA, Xue YY, Burdick MD
and Strieter RM: Depletion of CXCR2 inhibits tumor growth and
angiogenesis in a murine model of lung cancer. J Immunol.
172:2853–2860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sparmann A and Bar-Sagi D: Ras-induced
interleukin-8 expression plays a critical role in tumor growth and
angiogenesis. Cancer Cell. 6:447–458. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gerber PA, Hippe A, Buhren BA, Müller A
and Homey B: Chemokines in tumor-associated angiogenesis. Biol
Chem. 390:1213–1223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Legler DF, Uetz-von Allmen E and Hauser
MA: CCR7: Roles in cancer cell dissemination, migration and
metastasis formation. Int J Biochem Cell Biol. 54:78–82. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Zhang X, Thomas SM, Grandis JR,
Wells A, Chen ZG and Ferris RL: Chemokine receptor 7 activates
phosphoinositide-3 kinase-mediated invasive and prosurvival
pathways in head and neck cancer cells independent of EGFR.
Oncogene. 24:5897–5904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takanami I: Overexpression of CCR7 mRNA in
nonsmall cell lung cancer: Correlation with lymph node metastasis.
Int J Cancer. 105:186–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li P, Liu F, Sun L, et al: Chemokine
receptor 7 promotes cell migration and adhesion in metastatic
squamous cell carcinoma of the head and neck by activating integrin
α vβ 3. Int J Mol Med. 27:679–687. 2011.PubMed/NCBI
|
|
29
|
Itakura M, Terashima Y, Shingyoji M, et
al: High CC chemokine receptor 7 expression improves postoperative
prognosis of lung adenocarcinoma patients. Br J Cancer.
109:1100–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Su ML, Chang TM, Chiang CH, Chang HC, Hou
MF, Li WS and Hung WC: Inhibition of chemokine (C-C motif) receptor
7 sialylation suppresses CCL19-stimulated proliferation, invasion
and anti-anoikis. PLoS One. 9:e988232014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shields JD, Kourtis IC, Tomei AA, Roberts
JM and Swartz MA: Induction of lymphoidlike stroma and immune
escape by tumors that express the chemokine CCL21. Science.
328:749–752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koizumi K, Kozawa Y, Ohashi Y, et al:
CCL21 promotes the migration and adhesion of highly lymph node
metastatic human non-small cell lung cancer Lu-99 in vitro. Oncol
Rep. 17:1511–1516. 2007.PubMed/NCBI
|
|
33
|
Zhang Q, Sun L, Yin L, Ming J, Zhang S,
Luo W and Qiu X: CCL19/CCR7 upregulates heparanase via specificity
protein-1 (Sp1) to promote invasion of cell in lung cancer. Tumour
Biol. 34:2703–2308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu J, Zhao J, Feng H, et al: Antitumor
efficacy of CC motif chemokine ligand 19 in colorectal cancer. Dig
Dis Sci. 59:2153–2162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hillinger S, Yang SC, Zhu L, et al:
EBV-induced molecule 1 ligand chemokine (ELC/CCL19) promotes
IFN-gamma-dependent antitumor responses in a lung cancer model. J
Immunol. 171:6457–6465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hillinger S, Yang SC, Batra RK, Strieter
RM, Weder W, Dubinett SM and Sharma S: CCL19 reduces tumour burden
in a model of advanced lung cancer. Br J Cancer. 94:1029–1034.
2006. View Article : Google Scholar : PubMed/NCBI
|