Introduction
Protein tyrosine phosphorylation is a key regulatory
mechanism in eukaryotic cell physiology (1–3). The
aberrant expression or function of protein tyrosine kinases (PTKs)
and/or protein tyrosine phosphatases (PTPs) may result in the
development of severe human diseases, including cancer and
cardiovascular disease. SHP2 is a ubiquitously expressed cytosolic
tyrosine phosphatase (4,5), which contains two tandem Src homology 2
(SH2) domains and a PTP domain. The SH2 domains are responsible for
its role in cellular signalling. SHP2 binds to the intracellular
domains of various growth factor and cytokine receptors (6,7), thereby
influencing cell proliferation, differentiation and migration
(8,9).
A considerable body of evidence supports an
oncogenic role for SHP2, and since 2001, mutant and overexpressed
SHP2 have been detected in patients with solid tumours, Noonan
syndrome and myeloproliferative disease (1,10–12). Further research has suggested that
SHP2 overexpression and gain-of-function mutations in SHP2 are
present in almost all types of solid tumours and in 50% of Noonan
syndrome, 35% of juvenile myelomonocytic leukaemia, 10% of
myelodysplastic syndrome, 6% of B-cell acute lymphoblastic
leukaemia and 3% of acute myelogenous leukaemia (10–12). SHP2
was the first identified oncogenic tyrosine phosphatase. Although
PTPN11 mutations are infrequent in human solid tumours, an
oncogenic role for SHP2 has been reported in several types of
tumour. Evidence suggests that somatic gain-of-function mutations
of the PTPN11 gene are present in certain lung adenocarcinomas
(13,14), breast cancer (15–17),
gastric cancer (18), laryngeal
carcinoma (19) and oral cancer
(20). These observations suggest
that PTPN11/SHP2 may have a tumourigenic function. Recently,
investigators have also found that SHP2 is downregulated and may
exert a tumour suppressor role in hepatocellular (21) and colon (22) carcinogenesis.
The present study aimed to evaluate whether SHP2
expression is altered in thyroid cancer, and investigate the
mechanisms and clinical significance of SHP2 in human thyroid
cancer. SHP2 protein expression was investigated in 65 patients
with thyroid cancer, and paired adjacent normal tissue samples were
collected from 40 patients. Associations between protein
expression, patient clinical characteristics and prognostic
outcomes were also investigated.
Materials and methods
Clinical samples and human thyroid
cancer cell lines
Sixty-five thyroid cancer tissues were included in
the present study. Patients ages ranged from 23–70 years, with a
mean age of 43 years. Thyroid tissue was excised, and the diagnosis
was confirmed at the Department of Pathology, Zhongda Hospital
(Nanjing, China). In addition, 40 normal thyroid samples were
collected at the Department of Pathology for comparison. Enrolled
patients had not previously been treated for thyroid cancer.
Informed consent was obtained from each patient. The study design
was approved by the ethics review board of the Southeast University
(Nanjing, China) and patients provided written informed consent.
Human thyroid cancer cells, including SW579, IHH-4, FTC-133, TPC-1,
DRO, TA-K, and ML-1, and the human normal thyroid cell line
Nthy-ori3-1 were obtained from the Institute of Biochemistry and
Cell Biology, Chinese Academy of Sciences (Shanghai, China). All
cell lines were cultured in RPMI-1640 (Gibco-BRL, Carlsbad, CA,
USA) medium containing 10% foetal bovine serum (FBS; Hyclone, GE
Healthcare Life Sciences, Logan, UT, USA), penicillin (100 U/ml),
streptomycin (100 U/ml) and amphotericin B (0.25 mg/ml) at 37°C
with 5% CO2 (GBCBIO™ Technologies, Guangzhou,
China).
Immunohistochemistry (IHC)
Sections (4 µm) were blocked with 1% bovine serum
albumin (BSA) and 0.01% Triton X-100 (Amresco LLC, Solon, OH, USA)
for 1 h at room temperature, and then incubated with the primary
monoclonal rabbit anti-human SHP2 (1:500; cat. no. 20145-1-AP;
ProteinTech Group, Inc., Chicago, IL, USA) antibody overnight at
4°C. The negative controls were obtained by omitting the primary
antibody or incubating the sections with normal rabbit
immunoglobulin G (Vector Laboratories Inc., Burlingame, CA, USA) or
phosphate-buffered saline (PBS). Detection was performed by
incubation with horseradish peroxidase-conjugated polyclonal goat
anti-rabbit secondary antibody (1:5,000; cat. no. A21020; Dako
North America, Inc., Carpinteria, CA, USA) for 1 h at room
temperature. The sections were incubated with the
avidin-biotin-peroxidase complex (Vector Laboratories Inc.; 1:100
in PBS) for 1 h and developed in 0.05% 3,3′-diaminobenzidine
(Sigma-Aldrich, St. Louis, MO, USA) containing 0.003%
H2O2 in PBS.
SHP2 staining was independently evaluated by two
experimenters who were blinded to the clinical and follow-up
information. The IHC score was determined using the H-score system
(22), which considers the staining
intensity and the percentage of cells stained at a specific range
of intensity. Consistent with the known intracellular localisation
of SHP2, thyroid cancer specimens with positive staining
demonstrated a cytoplasmic distribution of SHP2. Using the scoring
system described previously, strong cytoplasmic reactivity of
>10% of all tumour cells was designated as 3+, moderate
reactivity as 2+ and faint reactivity as 1+. Faint reactivity not
discernible above background or the absence of staining was
considered negative for SHP2 expression. A reactivity of 2+ or 3+
was considered positive for SHP2 expression.
Microscopically-confirmed sections of normal thyroid specimens were
used as the controls (22).
Western blotting
The immunoblotting procedure was conducted as
described previously (16). In brief,
proteins (10 µg) were separated on 10% SDS-PAGE (Sigma-Aldrich) by
electrophoresis. The separated proteins were transferred onto
nitrocellulose membranes, and the membranes were blocked with 5%
BSA or 5% nonfat milk in 0.01 M PBS (pH 7.4) and 0.05% PBS-Tween-20
(PBS-T; Amresco LLC) at room temperature for 1 h. Subsequently, the
membranes were probed with primary SHP2 antibody overnight at 4°C.
Following three rapid washes in PBS-T, the membranes were incubated
with horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (cat. no. A21020; Dako North America, Inc.). The immune
complexes were detected using an enhanced chemiluminescence kit (GE
Healthcare Life Sciences, Chalfont, UK). The same membranes were
then stripped in stripping buffer for 15 min at room temperature,
and re-probed with a rabbit anti-mouse actin monoclonal antibody
(1:5,000; cat. no. sc-47778; Santa Cruz Biotechnology, Inc.). The
images were scanned and the signal intensity was quantified with
Image J software version 1.43g (National Institutes of Health,
Bethesda, MD, USA).
Growth inhibition assay
Sense S-oligonucleotides (Roche Diagnostics
Corporation, Indianapolis, IN, USA) were synthesised to nucleotides
1–18 of the SHP2 messenger RNA (5′-ATGACATCGCGGAGATGG-3′), and
antisense S-oligonucleotides were synthesised to the complementary
strand (5′-CCATCTCCGCGATGTCAT-3′). One day prior to transfection,
thyroid cancer cells (1×105 cells/well) were plated in
6-well tissue-culture plates. Thyroid cancer cells were incubated
with SHP2 antisense S-oligonucleotides at various concentrations
(0, 1, 3 and 5 µM), according to the manufacturer's instructions.
Following 48 h of culture, the cells were collected for analysis of
apoptosis using flow cytometry. The cells were washed with cold PBS
and resuspended in binding buffer according to the instruction of
the Annexin V-PI Apoptosis detection Kit (Beyotime Institute of
Biotechnology, Shanghai, China). Fluorescein isothiocyanate
(FITC)-Annexin V and propidium iodide (PI) were added to the fixed
cells for 20 min in darkness at room temperature. Then, Annexin V
binding buffer was added to the mixture prior to the measurement of
fluorescence using a flow cytometer (FACSCaliber™, BD Biosciences,
San Jose, CA, USA). Cell apoptosis was analyzed using CellQuest
software (BD Biosciences). SHP2 expression was also analysed via
western blotting with a SHP2 antibody, as described previously.
The cells were resuspended in 1 ml complete medium
containing 0.7% agar (Sigma-Aldrich) (1×103 cells/well
in 6-well plates) and then plated on top of a bottom layer
containing 1.2% agar (Sigma-Aldrich) with complete medium.
Following 24 h, the cells were incubated with SHP2 antisense
S-oligonucleotides at various concentrations (0 and 3 µM) for 4
days, with fresh medium and oligonucleotides added each day.
Subsequently, the oligonucleotide-containing medium was removed,
and the plates were cultured under normal conditions for 21 days,
and the colonies (>40 cells) were then counted and images were
captured under an inverted microscope (Olympus CX31; Olympus
Corporation, Tokyo, Japan). The results are expressed as the mean
percentage of clonal growth in the plates. Each experiment was
performed at least three times.
Statistical analysis
All experiments were performed at least three times,
and all data are presented as the mean ± standard deviation. Groups
were compared by one-way analysis of variance, followed by
Pearson's coefficient analysis for bivariate correlation. A
two-tailed probability value P≤0.05 was considered to indicate a
statistically significant difference.
Results
SHP2 expression is increased in
thyroid cancer patient samples and cell lines
SHP2 expression was detected in thyroid cancer
cells, including SW579, IHH-4, FTC-133, TPC-1, DRO, TA-K, and ML-1
cells, and the normal human thyroid cell line Nthy-ori3-1 by
western blotting. Significantly enhanced SHP2 expression was
observed in the thyroid cancer cells compared with that of the
normal thyroid epithelium (Fig. 1A and
B). In addition, SHP2 expression was evaluated in human thyroid
cancer samples. IHC analysis revealed that SHP2 protein was
expressed in the cytoplasm and nuclei (Fig. 1C). Further measurements using the
H-score system revealed that SHP2 expression in tumour tissues was
significantly higher than that in paired normal tissues
(P<0.001; Fig. 1C). No significant
difference was observed between SHP2 expression in the normal
thyroid and peritumour tissues (P=0.8994; Table I). Similar results were obtained from
western blot analysis (Fig. 1D and
E). In conclusion, SHP2 protein expression was significantly
increased in human thyroid carcinoma.
 | Figure 1.SHP2 is overexpressed in human thyroid
cancer cell lines and tumour tissues. (A) Expression of SHP2 in
normal human thyroid and thyroid cancer cell lines. Lanes: 1,
Mthy-ori3-1; 2, IHH-4; 3, DRO; 4, TPC-1; 5, FTC-133; 6, TA-K; 7,
SW579 and 8, ML-1. (B) Quantitative relative band intensities of
the western blot presented in A. *P<0.05 and **P<0.01 vs.
normal human thyroid cells (lane 1). (C) Representative
immunohistochemical photomicrographs of human thyroid tissues (a-c:
Magnification, x400, d: Magnification, x200). Increased SHP2
expression was detected in human thyroid cancers by
immunohistochemical staining of 65 human thyroid cancer samples
(3+), 40 self-paired peritumour tissues (1+) and 40 normal tissues
(1+). (D) Western blot analysis of SHP2 protein expression in human
thyroid specimens (N, normal; P, peritumour; T, tumour). (E)
Semi-quantitative western blotting of human thyroid samples
revealed significantly increased SHP2 protein levels in tumour
tissues compared with those of normal and peritumour tissues. Actin
was used as an internal control. **P<001 vs. normal tissue. |
 | Table I.SHP2 expression in normal, peritumour
and thyroid cancer tissues. |
Table I.
SHP2 expression in normal, peritumour
and thyroid cancer tissues.
|
|
| SHP2 expression,
n |
|
|---|
|
|
|
|
|
|---|
| Pathological
diagnosis | Cases, n | – | + | ++ | +++ | Positive rate,% |
|---|
| Thyroid cancer | 65 | 3 | 7 | 34 | 21 | 84.6 |
| Peritumour | 40 | 20 | 19 | 1 | 0 | 2.5 |
| Normal | 40 | 33 | 6 | 0 | 0 | 0.0 |
SHP2 expression is positively
correlated with the degree of tumour differentiation and TNM
stage
All thyroid cancer specimens were subjected to IHC
analysis, and staining intensity was measured by the H-score
system. SHP2 expression did not differ between genders. High SHP2
expression was significantly correlated with tumour
differentiation, high TNM stage and lymph node metastasis
(P<0.05; Table II). Therefore,
SHP2 may have a positive role in tumour initiation and
progression.
 | Table II.Correlations between SHP2 expression
in human thyroid cancer and clinicopathological parameters. |
Table II.
Correlations between SHP2 expression
in human thyroid cancer and clinicopathological parameters.
| Parameter | Cases, n | SHP2 overexpression,
n | χ2 | P-value |
|---|
| Age, years |
|
| 0.64 | >0.05 |
|
<40 | 36 | 29 |
|
|
| ≥40 | 29 | 22 |
|
|
| Gender |
|
| 0.71 | >0.05 |
| Male | 18 | 14 |
|
|
|
Female | 47 | 37 |
|
|
| Tumour, cm |
|
| 0.48 | >0.05 |
|
<1 | 12 | 9 |
|
|
| 1–4 | 45 | 35 |
|
|
|
>4 | 8 | 7 |
| Pathological
type |
|
| 0.56 | >0.05 |
| Papillary
thyroid carcinoma | 31 | 24 |
|
|
| Follicle
carcinoma | 22 | 17 |
|
|
| Medullary
carcinoma | 11 | 9 |
|
|
|
Undifferentiated
carcinoma | 1 | 1 |
|
|
| TNM stage |
|
| 6.39 | <0.01 |
| I–II | 39 | 27 |
|
|
|
III | 26 | 24 |
|
|
| Differentiation
grade |
|
| 7.08 | <0.01 |
| I | 20 | 11 |
|
|
| II | 26 | 21 |
|
|
|
III | 19 | 19 |
|
|
| Lymph node
metastasis |
|
| 8.17 | <0.01 |
|
Present | 23 | 22 |
|
|
|
Absent | 42 | 29 |
|
|
| Capsular
invasion |
|
| 9.25 | <0.01 |
|
Present | 20 | 19 |
|
|
|
Absent | 45 | 32 |
|
|
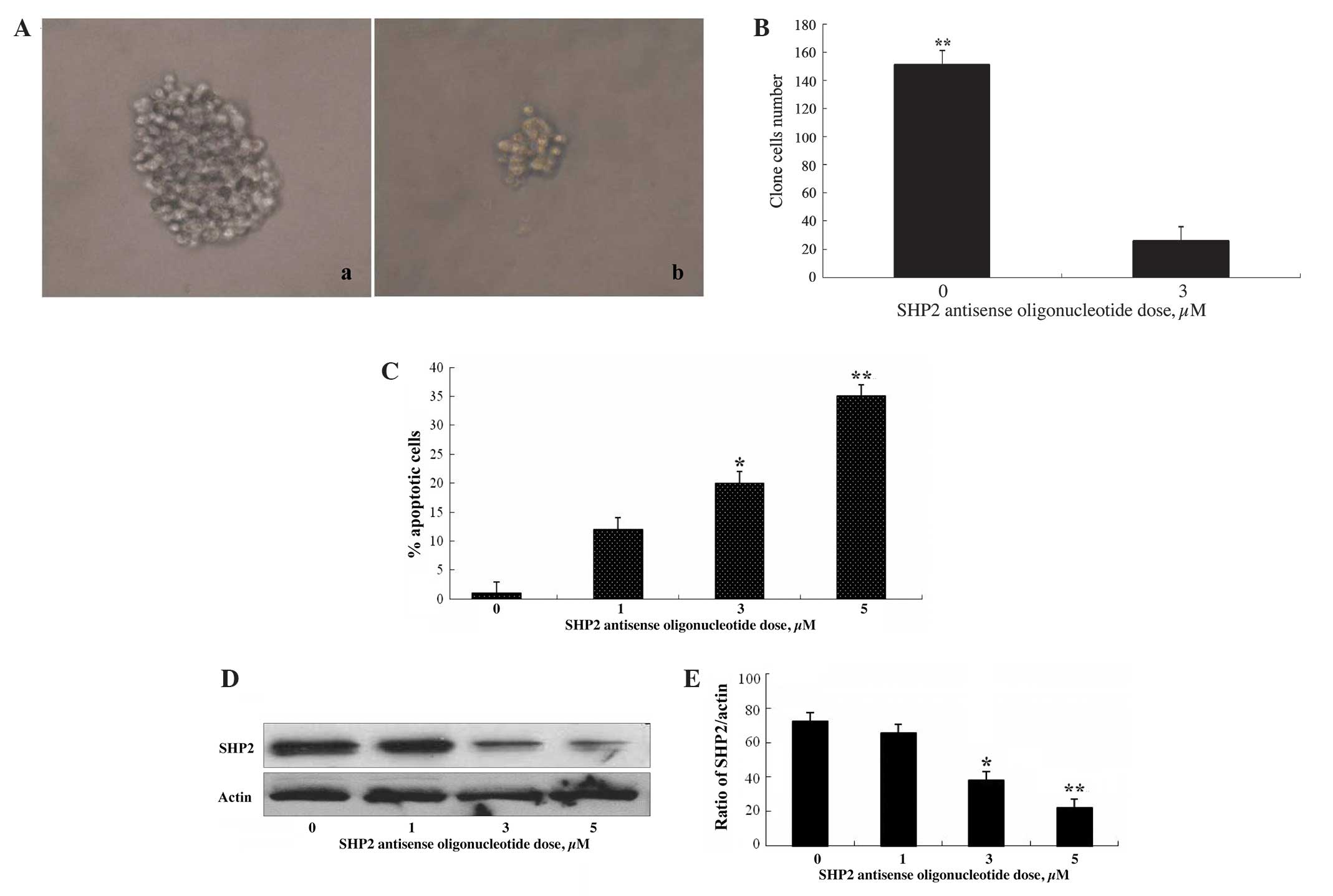
Suppression of SHP2 expression induces
apoptosis and inhibits growth of thyroid cancer cells
To evaluate the role of SHP2 in the proliferation of
thyroid cancer cells, SHP2 expression was blocked with SHP2
antisense oligonucleotides and the effects on thyroid cancer cell
clone growth were evaluated. SW579 thyroid cancer cells, which
express high levels of SHP2 protein, were treated with SHP2
antisense oligonucleotides at various concentrations. As shown in
Fig. 2A and B, the growth of thyroid
cancer cell clones was markedly suppressed following exposure to
SHP2 antisense oligonucleotides for 4 days. In addition, enhanced
apoptosis of thyroid cancer cells was observed, accompanied by
decreased SHP2 protein expression, following SHP2 antisense
treatment (Fig. 2C–E), demonstrating
a positive role for SHP2 in thyroid cancer cell survival and
proliferation.
Discussion
With more in-depth research regarding tumour
molecular biology, the molecular mechanisms of malignancy and the
process of tumour evolution in recent years, it is well understood
that cancer is a disease characterised by abnormal cellular
proliferation and differentiation (23,24). SHP2
is a member of the PTP family and has been shown to be ubiquitously
expressed in mammalian tissues. PTPs regulate several cellular
processes, including cell growth, cellular differentiation, mitotic
cycles and oncogenic transformation (8,9). PTPs also
regulate the phosphorylation state of numerous signalling molecules
with PTKs, including the MAP kinase family and signal transducer
and activator of transcription family proteins (25,26). In
recent years, there has been expanding interest in the role of SHP2
in the genesis, development and prognosis of thyroid cancer.
To date, studies have suggested that SHP2 activation
may be a critical step in the initiation and continued development
of multiple types of malignant tumour (27,28).
However, studies evaluating the expression of SHP2 in thyroid
cancer are scarce. To the best of our knowledge, the results of the
present study demonstrate, for the first time, that SHP2 expression
is enhanced in thyroid carcinoma tissues, compared with that in
normal thyroid and paracancerous tissues. SHP2 expression was
demonstrated to be associated with the metastasis of thyroid
cancer, suggesting its involvement in the initiation and
development of thyroid cancer, and indicating that SHP2 may be
useful for evaluating prognostic outcome. Based on the findings of
the present study, it was speculated that SHP2 may exist in a
relatively stable state in normal thyroid tissue and that its
expression is increased during the development of lesions, to
maintain cellular homeostasis and facilitate adjustment to the
disorder. As malignant tumours form, SHP2 is further activated, and
the overexpression of SHP2 promotes cell proliferation, growth and
metastasis. The association between SHP2 protein expression and the
clinicopathological parameters of thyroid carcinoma were analysed,
and the results revealed that positive expression of SHP2 and
cancer staging, histological differentiation, lymph node
metastasis, tumour size and capsule invasion were closely
associated (P<0.01); while patient age and gender were not
significantly associated with SHP2 expression (P>0.05).
Increased SHP2 expression is not only involved in the initiation of
thyroid cancer but also has roles in invasion and metastasis, as
well as tumour evolution. In addition, the results of the present
study suggest that SHP2 may be a novel marker for predicting the
malignant biological behaviour of thyroid cancer. Finally, SHP2 may
represent a novel therapeutic target for the treatment of thyroid
cancer. Such a therapy may potentially promote tumour cell
apoptosis, thus inhibiting cancer cell growth and metastasis, and
it may delay tumour recurrence and improve the overall survival
rate of patients with thyroid cancer.
Acknowledgements
The present study was supported by the Fundamental
Research Funds for the Central Universities (2242014K40004).
References
|
1
|
Grossmann KS, Rosário M, Birchmeier C and
Birchmeier W: The tyrosine phosphatase SHP2 in development and
cancer. Adv Cancer Res. 106:53–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrari E, Tinti M, Costa S, et al:
Identification of new substrates of the protein-tyrosine
phosphatase PTP1B by Bayesian integration of proteome evidence. J
Biol Chem. 286:4173–4185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Araki T, Chan G, Newbigging S, et al:
Noonan syndrome cardiac defects are caused by PTPN11 acting in
endocardium to enhance endocardial-mesenchymal transformation. Proc
Natl Acad Sci USA. 106:4736–4741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hagihara K, Zhang EE, Ke YH, et al: Shp2
acts downstream of SDF-1alpha/CXCR4 in guiding granule cell
migration during cerebellar development. Dev Biol. 334:276–284.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niihori T, Aoki Y, Ohashi H, et al:
Functional analysis of PTPN11/SHP-2 mutants identified in Noonan
syndrome and childhood leukemia. J Hum Gene. 50:192–202. 2005.
View Article : Google Scholar
|
|
6
|
Ogata T and Yoshida R: PTPN11 mutations
and genotype-phenotype correlations in Noonan and LEOPARD
syndromes. Pediatr Endocrinol Rev. 2:669–674. 2005.PubMed/NCBI
|
|
7
|
Oh ES, Gu H, Saxton TM, et al: Regulation
of early events in integrin signaling by protein tyrosine
phosphatase SHP-2. Mol Cell Biol. 19:3205–3215. 1999.PubMed/NCBI
|
|
8
|
Bowen ME, Ayturk UM, Kurek KC, et al: SHP2
regulates chondrocyte terminal differentiation, growth plate
architecture and skeletal cell fates. PLoS Genet. 10:e10043642014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartman ZR, Schaller MD and Agazie YM: The
tyrosine phosphatase SHP2 regulates focal adhesion kinase to
promote EGF-induced lamellipodia persistence and cell migration.
Mol Cancer Res. 11:651–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu W, Wang X, Romanov V, et al:
Structural insights into Noonan/LEOPARD syndrome-related mutants of
protein-tyrosine phosphatase SHP2 (PTPN11). BMC Struct Biol.
14:102014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oishi K, Zhang H, Gault WJ, et al:
Phosphatase-defective LEOPARD syndrome mutations in PTPN11 gene
have gain-of-function effects during Drosophila development.
Hum Mol Genet. 18:193–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan G, Kalaitzidis D, Usenko T, et al:
Leukemogenic Ptpn11 causes fatal myeloproliferative disorder via
cell-autonomous effects on multiple stages of hematopoiesis. Blood.
113:4414–4424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schneeberger VE, Luetteke N, Ren Y, et al:
SHP2E76K mutant promotes lung tumorigenesis in transgenic mice.
Carcinogenesis. 35:1717–1725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Furcht CM, Muñoz Rojas AR, Nihalani D and
Lazzara MJ: Diminished functional role and altered localization of
SHP2 in non small cell lung cancer cells with EGFR-activating
mutations. Oncogene. 32:2346–2355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sausgruber N, Coissieux MM, Britschgi A,
et al: Tyrosine phosphatase SHP2 increases cell motility in
triple-negative breast cancer through the activation of SRC-family
kinases. Oncogene. 34:2272–2278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Z, Fang H, Wang X, et al:
Overexpression of SHP2 tyrosine phosphatase promotes the
tumorigenesis of breast carcinoma. Oncol Rep. 32:205–212.
2014.PubMed/NCBI
|
|
17
|
Aceto N, Sausgruber N, Brinkhaus H, et al:
Tyrosine phosphatase SHP2 promotes breast cancer progression and
maintains tumor-initiating cells via activation of key
transcription factors and a positive feedback signaling loop. Nat
Med. 18:529–537. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang J, Jin MS, Kong F, et al: Increased
expression of tyrosine phosphatase SHP-2 in Helicobacter
pylori-infected gastric cancer. World J Gastroenterol.
19:575–580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong LB, Li GQ, Tian ZH, et al:
Expressions of Src homology 2 domain-containing phosphatase and its
clinical significance in laryngeal carcinoma. Genet Mol Res.
12:4207–4212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang HC, Chiang WF, Huang HH, et al:
Src-homology 2 domain-containing tyrosine phosphatase 2 promotes
oral cancer invasion and metastasis. BMC Cancer. 14:4422014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bard-Chapeau EA, Li S, Ding J, et al:
PTPN11/SHP2 acts as a tumor suppressor in hepatocellular
carcinogenesis. Cancer Cell. 19:629–639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai P, Guo W, Yuan H, et al: Expression
and clinical significance of tyrosine phosphatase SHP-2 in colon
cancer. Biomed Pharmacother. 68:285–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma N, Everingham S, Ramdas B, et al:
SHP2 phosphatase promotes mast cell chemotaxis toward stem cell
factor via enhancing activation of the Lyn/Vav/Rac signaling axis.
J Immunol. 192:4859–4866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Princen F, Bard E, Sheikh F, et al:
Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated
cardiomyopathy, insulin resistance and premature death. Mol Cell
Biol. 29:378–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edwards JJ, Martinelli S, Pannone L, et
al: A PTPN11 allele encoding a catalytically impaired SHP2 protein
in a patient with a Noonan syndrome phenotype. Am J Med Genet A.
164A:2351–2355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Müller PJ, Rigbolt KT, Paterok D, et al:
Protein tyrosine phosphatase SHP2/PTPN11 mistargeting as a
consequence of SH2-domain point mutations associated with Noonan
Syndrome and leukemia. J Proteomics. 84:132–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo H, Tang C, Yang X and Zhou X: The
tyrosine phosphatase SHP2: A key molecule linked both type 2
diabetes and cancers? Med Chem. 4:435–438. 2014.
|
|
28
|
Meng F, Zhao X and Zhang S: SHP-2
phosphatase promotes cervical cancer cell proliferation through
inhibiting interferon-β production. J Obstet Gynaecol Res.
39:272–279. 2013. View Article : Google Scholar : PubMed/NCBI
|