Introduction
Esophageal carcinoma ranks in the forefront of
cancers in terms of incidence and mortality rate in males and
females in developing countries (1).
Moreover, almost 50% of the world's cases of esophageal cancer
occur in China. As the most common histological subtype, esophageal
squamous cell carcinoma (ESCC) accounts for around 90% of all
esophageal cancers that are diagnosed in China each year. To date,
the molecular pathogenesis of ESCC remains unclear. The current
focus of biological studies is the transition from novel gene
cloning to the characterization of protein product function
(2). As a result, a considerable
research effort has been directed at novel specific esophageal
cancer-associated proteins in order to identify their functions and
identify the relevant molecular mechanisms for carcinogenesis in
ESCC.
Human chromosome 2 open reading frame 40
(C2ORF40; also known as ECRG4) has been shown to be
expressed in a variety of tissues, including the heart, placenta,
brains, lungs, skeletal muscle, liver, kidneys and pancreas
(3). Recent studies showed that
C2ORF40 was important in a number of processes, including cell
differentiation, senescence, apoptosis, inflammation and
neuroendocrine hormone regulation (4–6). Moreover,
a variety of studies revealed that C2ORF40 was a candidate
tumor suppressor gene that is associated with prognosis in
multi-tumors (7–9). C2ORF40 was an independent prognostic
factor for ESCC, and low C2ORF40 expression in ESCC patients
was associated with a poor prognosis (9,10). Our
previous results demonstrated that C2ORF40 was highly
expressed in the adult esophageal epithelia, but that it was
downregulated in ESCC tissues and cell lines (9). Furthermore, C2ORF40 transfection
inhibited cancer cell growth and invasion (9,11–13). Bioinformatics analysis indicated that
pro-C2ORF40 protein was a secreted protein with a signal peptide.
Therefore, the C2ORF40 protein could be secreted into cell medium
to function biologically. However, the exact biological function of
secreted C2ORF40 (sC2ORF40) protein in carcinogenesis has not been
thoroughly researched in ESCC thus far.
The present study initially cloned and expressed
soluble recombinant human C2ORF40 protein (rhC2ORF40), validated
the tumor-suppressing biological activities of rhC2ORF40 protein
and investigated the possible molecular mechanism for growth
inhibition in ESCC.
Materials and methods
Production and purification of soluble
recombinant human C2ORF40 protein
Cut-short rhC2ORF40 complementary (c)DNA (1–28
signal peptide sequence was deleted) was excised from preserved
pGEM-T-C2ORF40 (constructed and preserved by the State Key
Laboratory of Molecular Oncology and Department of Etiology and
Carcinogenesis, Cancer Institute and Hospital, Chinese Academy of
Medical Sciences and Peking Union Medical College, Beijing, China)
and subcloned into the pET30a(+) plasmid, producing an inducible
expression vector, pET30a-C2ORF40, coding for His-tagged soluble
rhC2ORF40 protein (without signal peptide). The primers were as
follows: Forward, 5′-TCGGATCCATAAGTGGAAATAACTC-3′; and reverse,
5′-TCAAGCTTTTAGTAGTCATCGTAGTT-3′. Thermal cycles were as follows:
95°C for 5 min, then 35 cycles at 95°C for 30 sec, 55°C for 30 sec
and 72°C for 45 sec, followed by extension at 72°C for 7 min. The
polymerase chain reaction (PCR) product was digested by
BamHI and HindIII. Subsequently, the recombinant
plasmids were transformed into Escherichia coli BL21 (DE3)
cells to produce N-terminal His-tagged soluble rhC2ORF40 protein.
Recombinant rhC2ORF40 protein expression in Escherichia coli
BL21 cells was induced with 0.3 mM isopropyl
β-D-1-thiogalactopyranoside and detected by SDS-PAGE and western
blotting. Next, the rhC2ORF40 protein was purified and renatured by
affinity chromatography with nickel-nitrilotriacetic acid resin
(Novagen, Merck Millipore, Darmstadt, Germany) according to the
manufacturer's instructions. The purified recombinant rhC2ORF40
protein was dialyzed in phosphate-buffered saline [PBS; 0.1M sodium
phosphate and 0.15M sodium chloride (pH 7.4)] to remove the
denaturant. The recombinant soluble rhC2ORF40 protein was used for
functional experiments.
Cell proliferation assay
The human esophageal squamous EC9706 cell line was
studied. The EC9706 cells were seeded into 96-well plates
(1.5×103 cells/well). Different concentrations of
rhC2ORF40 protein (1.5, 3.0, 4.5, 6.0, 7.5, 9.0 and 10.5 µg/ml)
were added to each well and cultured for 48h, and cell
proliferation inhibition was evaluated by thiazolyl blue
tetrazolium bromide (MTT) assay, according to the manufacturer's
instructions (Sigma-Aldrich, St. Louis, MO, USA). In brief, 10 µl
MTT solution (5 mg/ml) was added to each well, then the cells were
cultured for another 4 h at 37°C, and 100 µl DMSO was added to each
well and mix vigorously to solubilize colored crystals produced
within the living cells. The absorbance at 570 nm was measured by
using a multi-well scanning spectrophotometer (Victor 3;
PerkinElmer, Waltham, MA, USA). Cell growth curves for treatment
with 10 µg/ml rhC2ORF40 protein or PBS for various durations (1, 2,
3, 4 and 5 days) were also plotted by MTT assay. The mean values
for statistical analysis represented the mean of three independent
experiments.
Flow cytometric analysis of the cell
cycle
The EC9706 cells were seeded at a density of
106 cells/100-mm dish in RPMI-1640 medium with 10% FBS
and treated with 10 µg/ml rhC2ORF40 or PBS for 48 h. Next, the
cells were washed with ice-cold PBS, harvested and fixed in 70%
ethanol for 30 min. The cells were treated with RNase A and stained
with 25 µg/ml propidium iodide. Samples were analyzed using a
FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA),
according to the manufacturer's instructions. Experiments were
performed three times in triplicate.
Statistical analysis
All statistical analyses were performed with the
SPSS statistical program (version 16.0; SPSS, Inc., Chicago, IL,
USA). Statistical significance was determined using Student's
t-test and an analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
C2ORF40 protein is a secreted
protein
C2ORF40 protein is a secreted protein with a signal
peptide upon Swiss-Prot Protein Sequence Database Bioinformatics
analysis (http://web.expasy.org/docs/swiss-prot_guideline.html).
It was discovered that the sC2ORF40 protein existed in EC9706
cancer cell medium transfected with C2ORF40 plasmid compared
with the control group, as determined by western blot analysis
(data not shown). Therefore, C2ORF40 protein could be secreted into
the extracelluar environment to function biologically.
Production of purified rhC2ORF40
protein
In the present study, the signal peptide sequence of
the C2ORF40 cDNA was cut off to produce secreted rhC2ORF40
protein. The constructed plasmid, pET30a-C2ORF40, was identified by
PCR restrictive enzyme digestion (Fig.
1A) and DNA sequencing (data not shown). Next, recombinant BL21
strains that expressed rhC2ORF40 protein were obtained (Fig. 1B). The rhC2ORF40 protein was
specifically recognized by the anti-His and anti-C2ORF40 antibodies
(Fig. 1C). The rhC2ORF40 protein was
purified, with a purity of >95% (Fig.
1D).
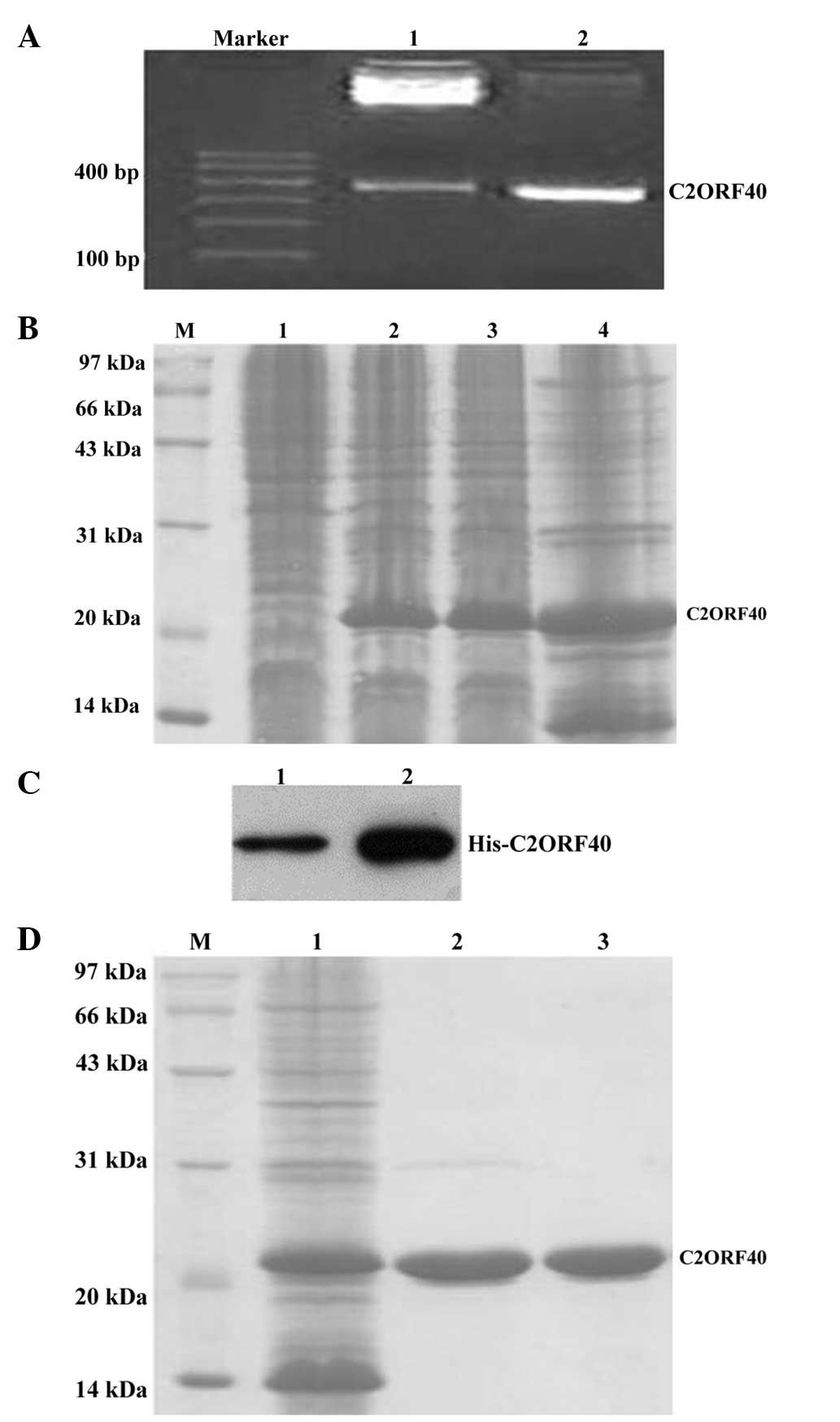 | Figure 1.Production and purification of
recombinant human chromosome 2 open reading frame 40 (rhC2ORF40)
protein. (A) Production of rhC2ORF40 DNA and electrophoresis
analysis of rhC2ORF40 complementary (c)DNA by polymerase chain
reaction (PCR) and restrictive enzyme digestion. Lane 1,
pET30a-C2ORF40 was digested by BamHI and HindIII;
lane 2, PCR product of C2ORF40 cDNA from pET30a-C2ORF40. (B)
SDS-PAGE analysis of the total proteins of recombinant BL21. Lane
1, BL21 transfected with empty plasmid pET30a; lane 2, BL21
transfected with pET30a-C2ORF40; lane 3, the lysate deposit of BL21
transfected with pET30a-C2ORF40; lane 4, the lysate supernatant of
BL21 transfected with pET30a-C2ORF40. (C) Recombinant rhC2ORF40
protein was detected in BL21 transfected with pET30a-C2ORF40 by
western blotting (lane 1, anti-C2ORF40; lane 2, anti-His). The BL21
transfected with empty plasmid pET30a was used as a negative
control, and it demonstrated no C2ORF40 protein expression. (D)
SDS-PAGE analysis of the purified rhC2ORF40 protein. Lane 1, the
supernatant protein of BL21 transfected with pET30a-C2ORF40; lane
2, rhC2ORF40 protein was purified; lane 3, final purified rhC2ORF40
protein with a purity of >95%. M, marker. |
rhC2ORF40 inhibits tumor cell
proliferation
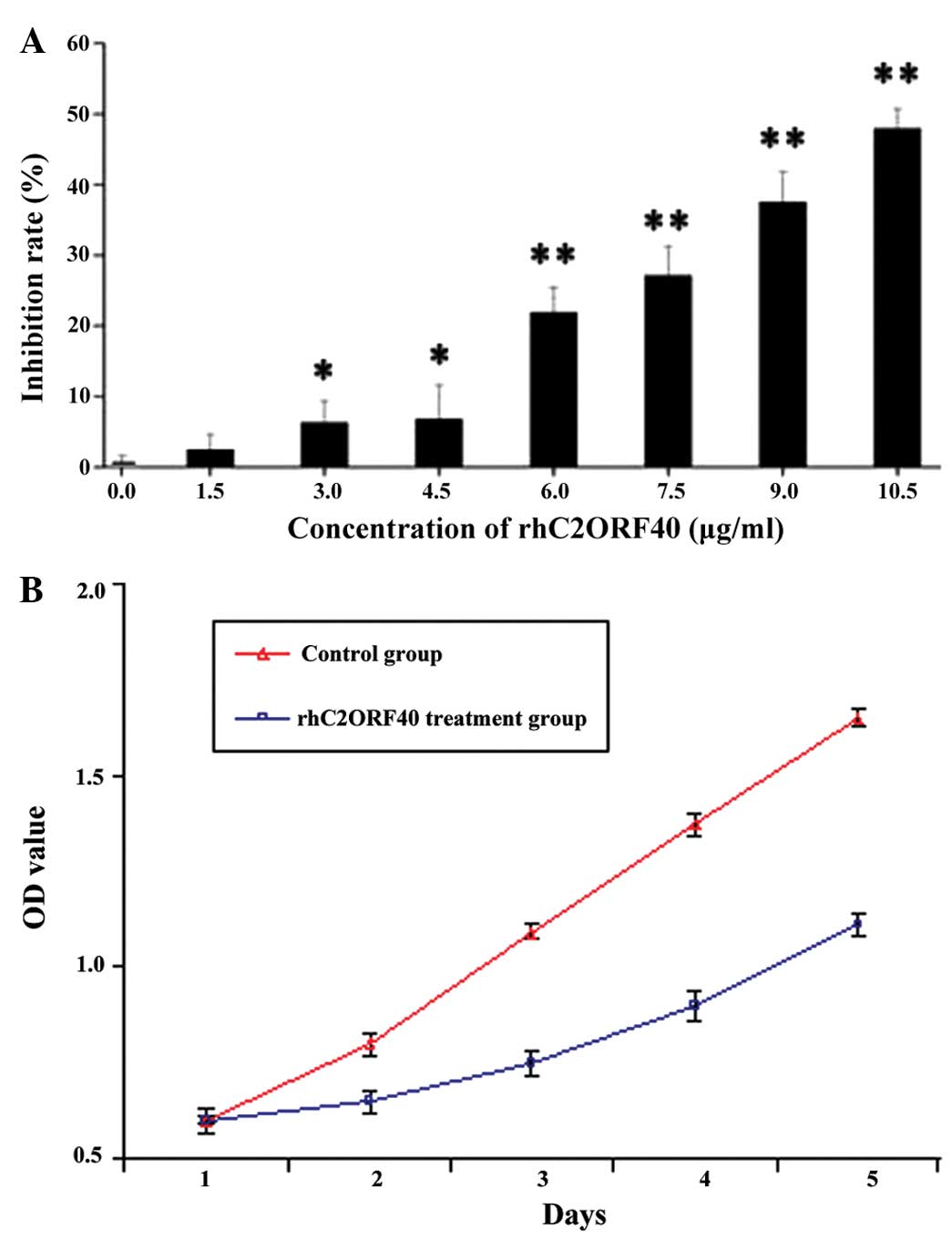
The purified rhC2ORF40 protein was produced to test
its biological tumor-suppressing activity in ESCC. MTT assay showed
that rhC2ORF40 protein markedly inhibited EC9706 cell proliferation
with high potency in a dose-dependent manner (Fig. 2A). The half maximal inhibitory
concentration (IC50) of the rhC2ORF40 protein was ~10
µg/ml, and the growth curves demonstrated that the EC9706 cells
treated with 10µg/ml rhC2ORF40 grew more slowly than those in the
PBS control group (P<0.05), as shown by MTT assay (Fig. 2B).
rhC2ORF40 blocks cell cycle
progression
Cell cycle analysis was performed by flow cytometry,
and the result suggested that rhC2ORF40 could arrest the ESCC cells
at the G1/S checkpoint and delay the transition of the
cell cycle into the S phase (Table
I), which was consistent with our previous C2ORF40 gene
transfection result (4).
Consequently, rhC2ORF40 protein slowed down cell cycle progression,
and caused cell cycle G1 phase block.
 | Table I.rhC2ORF40 causes cell cycle
G1 phase block. |
Table I.
rhC2ORF40 causes cell cycle
G1 phase block.
|
| Relative cell number
ratio |
|---|
|
|
|
|---|
| Group | G1 | S | G2/M |
|---|
|
rhC2ORF40-treateda | 73.6±1.79 | 14.7±1.20 | 11.7±0.98 |
| Control | 60.1±2.11 | 24.5±1.53 | 15.4±1.67 |
Discussion
ESCC is a highly invasive and clinically challenging
cancer. Although advances have been made towards a clinically
comprehensive treatment, the prognosis of ESCC remains poor due to
its diffuse and invasive nature (1).
Novel biological therapy drugs with high antitumor efficacy are
being constantly sought to improve the survival of patients with
ESCC. In the present study, it was observed that rhC2ORF40 protein
inhibited tumor cell growth by inducing cell cycle G1
phase block in vitro, which was consistent with the results
of our previous C2ORF40 gene transfection study in ESCC
(9,11,14).
Therefore, the soluble rhC2ORF40 protein with high purity and
biological activity was obtained. In vivo functional
experiments require further study to prove that the soluble
rhC2ORF40 protein could be a candidate biological therapy drug for
esophageal carcinoma.
Transformed cells acquire a series of additional
malignant traits during ESCC development and progression. Among
them, the alteration to the cell cycle plays a major role in
carcinogenesis. Tumorigenesis can be a consequence of an imbalance
in cell cycle regulation. Moreover, it has been demonstrated that
numerous oncogenes and tumor suppressor genes are directly involved
in the regulation of cell cycle events. Of these, the p21 and p16
genes, which are critical cyclin-dependent kinase inhibitors, were
believed to hold functional relevance with regard to the regulation
of the cell cycle G1 phase. Our previous study result
demonstrated that C2ORF40 transfection induced upregulation
of p21 expression in ESCC cells (11,14).
However, there was no significant upregulation of p16
expression level in the ESCC cells (data not shown). Therefore, the
increased expression of p21, but not p16, was likely
to be responsible for the cell cycle G1 phase block
induced by C2ORF40 in ESCC (11,14).
Transmembrane protease serine 11A (TMPRSS11A;
also known as ECRG1) is a novel candidate tumor suppressor gene
that has been shown to be important in the control of gene
expression for those genes involved in cell cycle G1
phase arrest in ESCC. In pervious studies, TMPRSS11A gene
overexpression inhibited tumor cell growth in vitro and
in vivo (15), and induced
cell cycle G1 phase arrest and cell senescence (16) in ESCC. As C2ORF40 is a putative
pro-hormone protein (also known as augurin) (17,18), it
may be a good candidate substrate for the transmembrane serine
protease TMPRSS11A in ESCC. We previously found that C2ORF40 could
directly bind to TMPRSS11A in ESCC cells, as determined by binding
affinity assay and co-immunoprecipitation experiments (14). C2ORF40 was able to cause cell cycle
G1 phase block by interaction with TMPRSS11A in ESCC
(14). However, the detailed
molecular mechanism remains to be clarified.
In the present study, the soluble rhC2ORF40 protein
with high purity and biological activity was obtained, which
suppressed tumor cell growth by inducing cell cycle G1
phase block in vitro in ESCC. These results indicate that
soluble rhC2ORF40 protein could be a potential biological
therapeutic drug for ESCC.
Acknowledgements
This study was supported by the Chinese National
Natural Science Foundation (grant no. U1304817) and the Zhengzhou
City Science Research Project (grant no. 141PPTGHG298).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eisenberg D, Marcotte EM, Xenarios I and
Yeates TO: Protein function in the post-genomic era. Nature.
405:823–826. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steck E, Breit S, Breusch SJ, Axt M and
Richter W: Enhanced expression of the human chitinase 3-like 2 gene
(YKL-39) but not chitinase 3-like 1 gene (YKL-40) in osteoarthritic
cartilage. Biochem Biophys Res Commun. 299:109–115. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kujuro Y, Suzuki N and Kondo T: Esophageal
cancer-related gene 4 is a secreted inducer of cell senescence
expressed by aged CNS precursor cells. Proc Natl Acad Sci USA.
107:8259–8264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huh YH, Ryu JH, Shin S, Lee DU, Yang S, Oh
KS, Chun CH, Choi JK, Song WK and Chun JS: Esophageal cancer
related gene 4 (ECRG4) is a marker of articular chondrocyte
differentiation and cartilage destruction. Gene. 448:7–15. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuzaki J, Torigoe T, Hirohashi Y,
Kamiguchi K, Tamura Y, Tsukahara T, Kubo T, Takahashi A, Nakazawa
E, Saka E, et al: ECRG4 is a negative regulator of
caspase-8-mediated apoptosis in human T-leukemia cells.
Carcinogenesis. 33:996–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu J, Wen M, Huang Y, He X, Wang Y, Wu Q,
Li Z, Castellanos-Martin A, Abad M, Cruz-Hernandez JJ, et al:
C2ORF40 suppresses breast cancer cell proliferation and invasion
through modulating expression of M phase cell cycle genes.
Epigenetics. 8:571–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Götze S, Feldhaus V, Traska T, Wolter M,
Reifenberger G, Tannapfel A, Kuhnen C, Martin D, Müller O and
Sievers S: ECRG4 is a candidate tumor suppressor gene frequently
hypermethylated in colorectal carcinoma and glioma. BMC Cancer.
9:4472009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li LW, Yu XY, Yang Y, Zhang CP, Guo LP and
Lu SH: Expression of esophageal cancer related gene 4 (ECRG4), a
novel tumor suppressor gene, in esophageal cancer and its
inhibitory effect on the tumor growth in vitro and in vivo. Int J
Cancer. 125:1505–1513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mori Y, Ishiguro H, Kuwabara Y, Kimura M,
Mitsui A, Kurehara H, Mori R, Tomoda K, Ogawa R, Katada T, et al:
Expression of ECRG4 is an independent prognostic factor for poor
survival in patients with esophageal squamous cell carcinoma. Oncol
Rep. 18:981–985. 2007.PubMed/NCBI
|
|
11
|
Li LW, Zhang CP, Li XY, Lu S and Zhou Y:
The candidate tumor suppressor gene ECRG4 inhibits cancer cells
migration and invasion in esophageal carcinoma. J Exp Clin Cancer
Res. 29:1332010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu T, Xiao D and Zhang X: ECRG4 inhibits
growth and invasiveness of squamous cell carcinoma of the head and
neck in vitro and in vivo. Oncol Lett. 5:1921–1926.
2013.PubMed/NCBI
|
|
13
|
Li W, Liu XR, Zhang B, Qi D, Zhang L, Jin
Y and Yang H: Overexpression of candidate tumor suppressor ECRG4
inhibits glioma proliferation and invasion. J Exp Clin Cancer Res.
29:892010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li LW, Li YY, Li XY, Zhang CP, Zhou Y and
Lu SH: A novel tumor suppressor gene ECRG4 interacts directly with
TMPRSS11A (ECRG1) to inhibit cancer cell growth in esophageal
carcinoma. BMC Cancer. 11:522011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yueying W1, Jianbo W, Hailin L, Huaijing
T, Liping G and Shih-Hsin L: ECRG1, a novel esophageal gene, cloned
and identified from human esophagus and its inhibition effect on
tumors. Carcinogenesis. 29:157–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao N, Huang G, Guo L and Lu SH: ECRG1, a
novel candidate of tumor suppressor gene in the esophageal
carcinoma, triggers a senescent program in NIH3T3 cells. Exp Biol
Med (Maywood). 231:84–90. 2006.PubMed/NCBI
|
|
17
|
Baird A, Coimbra R, Dang X, Lopez N, Lee
J, Krzyzaniak M, Winfield R, Potenza B and Eliceiri BP: Cell
surface localization and release of the candidate tumor suppressor
Ecrg4 from polymorphonuclear cells and monocytes activate
macrophages. J Leukoc Biol. 91:773–781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dang X, Podvin S, Coimbra R, Eliceiri B
and Baird A: Cell-specific processing and release of the
hormone-like precursor and candidate tumor suppressor gene product,
Ecrg4. Cell Tissue Res. 348:505–514. 2012. View Article : Google Scholar : PubMed/NCBI
|