Introduction
Orbital rhabdomyosarcoma (RMS) as a prevalent
malignancy, and is the most common malignant tumor of the orbit
amongst children (1,2). Malignant tumors require a sufficient
blood supply in order to support their growth. It was previously
believed that blood vessels were only able to be formed by
endothelial cells. However, the rigid cellular identity of the
blood vessel has since been questioned, following the observation
that tumor cells are also capable of forming extravascular networks
in high-grade malignancies (3). The
process of vasculogenic mimicry (VM), describes the mechanism by
which highly aggressive tumor cells are able to form vessel-like
structures, as a result of their high plasticity. It has therefore
been suggested that tumors may develop vascularization via
angiogenesis, and that this process is associated with poor
prognosis (4–6).
Orbital RMS is a solid tumor with the morphological
characteristic of bidirectional differentiation, which is the
cellular basis of the formation of VM (7). However, to the best of our knowledge, no
specific study regarding the novel blood-supplying pattern in
orbital RMS has previously been published. Furthermore, the precise
molecular mechanism of VM formation remains to be elucidated.
Epithelial cell kinase (EphA2) and matrix metalloproteinase-2
(MMP-2) have been found to be a crucial inducers of VM (8,9), therefore
it is of significant interest to examine the role of EphA2 and
MMP-2 in VM formation in orbital RMS. The present pilot study was
designed to investigate whether VM occurs in orbital RMS and if so,
to evaluate the correlation between VM and clinicopathological
disease features. Furthermore, the present study aimed to elucidate
the potential mechanisms underlying VM formation.
Materials and methods
Ethics statement
The present study adhered to the conditions of the
Declaration of Helsinki. Ethical approval was obtained from the
institutional Medical Research Ethics Committee, and informed
consent was obtained from all patients prior to treatment. The
study was performed following approval by the ethics committee of
the Second Affiliated Hospital of Tianjin Medical University
(Tianjin, China).
Patients and samples
In the present cross-sectional study, tissue
specimens were obtained from 55 patients who underwent surgery for
orbital RMS between 1997 and 2009 through the Tumor Tissue Bank of
the Second Affiliated Hospital of Tianjin University (Tianjin,
China). The diagnoses of these orbital RMS samples were verified by
pathologists. Thirteen cases were excluded due to poor fixation or
processing. Detailed pathological and clinical data of the
remaining 32 cases were collected, including age, gender, tumor
location and size, mitotic rate, histological type, recurrence and
survival duration. The paraffin-embedded tumor tissue samples were
obtained from patients who had not been subjected to therapy prior
to surgery.
Definition of VM and patient
grouping
VM was defined as tumor-cell-surrounded channels,
within which red blood cells (RBCs) were detectable. In hematoxylin
and eosin-stained slides, VM was observed to be formed by tumor
cells, but not endothelial cells, without hemorrhage, necrosis or
inflammatory cells infiltrating proximal to these structures. In
CD31/periodic acid Schiff (PAS) double-stained slides, VM was
defined as tumor cells (rather than endothelial cells) forming
channels with PAS-positive material and RBCs. No VM-positive cells
were detected in CD31-stained slides. The cells forming VM were
confirmed to be orbital RMS cells by actin staining.
Laminin-stained slides confirmed the topography of the basement
membrane of the vessels and vessel-like structures, which were
positively stained. The 32 patients evaluated possessed sufficient
follow-up data for evaluation and were divided into 2 groups
according to VM-positivity.
Immunohistochemical analysis
Reagents
The primary antibodies used were as follows:
Monoclonal mouse antiserum against human Actin (dilution, 1:500;
cat. no. M087401, clone Alpha-Sr-1; Dako Cytomation, Glostrup,
Denmark); monoclonal mouse anti-human CD31 (dilution, 1:100; cat.
no. M082301, clone JC70A; Dako Cytomation); polyclonal rabbit
anti-mouse IgG against EphA2 (dilution, 1:100; cat. no. sc-924;
clone no. C-20; Santa Cruz Biotechnology, Inc., Dallas, TX, USA);
and monoclonal mouse anti-human MMP-2 (dilution, 1:100; cat. no.
MAB9554; clone no. 17B11; Abnova, Tapei City, Taiwan). All slides
were subjected to heat-induced epitope retrieval at 92°C in citrate
buffer (0.01 mol/l; pH 6.0) prior to immunohistochemical staining.
The 0.5% PAS solutions were produced in the Pathology Department of
Tianjin Eye Hospital (Tianjin, China) and confirmed to be effective
in previous experiments (10).
Immunohistochemical assay
Staining with primary antibodies against Actin,
CD31, EphA2 and MMP-2 was performed on formalin-fixed,
paraffin-embedded tissues using the SP-9000 kit (Zhongshan Chemical
Co.). Briefly, the tumor tissues were formalin-fixed and embedded
in paraffin. The tissues were cut into 4-µm sections and mounted
onto slides, dried overnight at 65°C and deparaffinized in xylene.
Next, the sections were rehydrated through graded alcohol into
water. Endogenous peroxidase activity was blocked with 3% hydrogen
peroxide in 50% methanol for 10 min at room temperature. The
sections were washed with phosphate-buffered saline (PBS; Zhongshan
Chemical Co.) and pretreated with citrate buffer (0.01 M citric
acid; pH 6.0) for 20 min at 100°C in a microwave oven. After
rinsing with PBS, the slides were incubated overnight at 4°C with
primary polyclonal antibodies against EphA2 (dilution, 1:100),
MMP-2 (dilution, 1:100) and MMP-9 (dilution, 1:100). The sections
were then washed with PBS and incubated with polyclonal
HRP-conjugated goat anti-rabbit IgG (dilution, 1:1,000; cat. no.
ab6721; Abcam, Cambridge, UK) secondary antibody for 30 min at
37°C. Visualization was performed using a 2-Solution DAB Kit (cat.
no. 88–2014; Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer's instructions. The sections were
then incubated in 95% alcohol with agitation for 30 min to remove
formalin granules. After washing with tap water, the sections were
incubated with potassium permanganate for 3 h and bleached with 2%
oxalic acid for 5 min. The nuclei were counterstained with
hematoxylin, followed by dehydration and cover-slip mounting. The
slides were visualized using a light microscope (Leica DM4000 B
LED; Leica Microsystems GmbH, Wetzlar, Germany). Paraffin-embedded
gastric mucosa samples obtained from gastric carcinoma patients at
Tianjin Medical University Cancer Institute and Hospital (Tianjin,
China), and PBS were used as the positive and negative controls,
respectively.
CD31 staining and PAS double-staining
CD31/PAS double-staining of the samples was
performed as previously described by Sun et al (11). Following CD31 immunostaining, the
sections were treated with 0.5% periodic acid solution for 10 min
at room temperature, prior to rinsing in distilled water for 3 min.
These sections were then treated with periodic acid solution for
15–30 min in the dark at room temperature. After rinsing in
distilled water, sections were counterstained with hematoxylin
(Zhongshan Chemical Co.). Finally, the sections were counterstained
with hematoxylin or PAS. PBS replaced the primary antibodies for
the negative control, while normal gastric mucosa tissue, obtained
from the Department of Pathology, Tianjin Medical University Cancer
Institute and Hospital, was used as a positive control. VM channels
and mosaic vessels (MVs; in which both endothelial cells and tumor
cells form the luminal surface) were counted. The microvessel
density (MVD) was established by light microscopic examination
(Leica DM4000 B LED; Leica Microsystems GmbH) of CD31-stained
sections at the location with the highest quantity of capillaries
and small venules. The mean vessel count of three fields
(magnification, x400) with the greatest neovascularization was
considered to indicate the MVD. Counts were conducted blindly in a
minimum of three randomly selected sections from each group. The
mean value from 10 fields observed in each section for each type of
microvessel comprised the final outcome.
Assessment methods
The staining index was evaluated to investigate
differences in the expression of EphA2 and MMP-2 between the
VM-positive and -negative lesions. Tumor cells with brown
cytoplasmic staining were considered to be positive. Ten randomly
selected visual fields were examined, and 100 cells were counted
within each of these fields. The mean percentage of positive cells
was considered to indicate the expression of the two proteins
within each section. The results were quantified as previously
described (12).
Statistical analysis
All data in the study were evaluated using SPSS
software version 11.5 (SPSS Inc., Chicago, IL, USA). Survival rates
were calculated using the Kaplan-Meier method, statistically
significant differences were identified using the log-rank test and
potential prognostic factors were analyzed using the Cox
proportional hazards model. The Wilcoxon-Mann-Whitney test was
performed to analyze differences in EphA2 and MMP-2 expression
between the VM and non-VM groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Evidence of VM in orbital RMS
VM structures were identified in a subset of orbital
RMS specimens. VM was identified by the formation of a tubular,
fracture-like structure by tumor cells (not endothelial cells),
with RBCs within the cavities, observed with structural integrity,
no incidence of hemorrhage and an absence of necrosis or
inflammatory cells infiltrating the channels. Such structures were
detected in 11 orbital RMS samples (34%; Fig. 1). Clinical data are presented in
Table I.
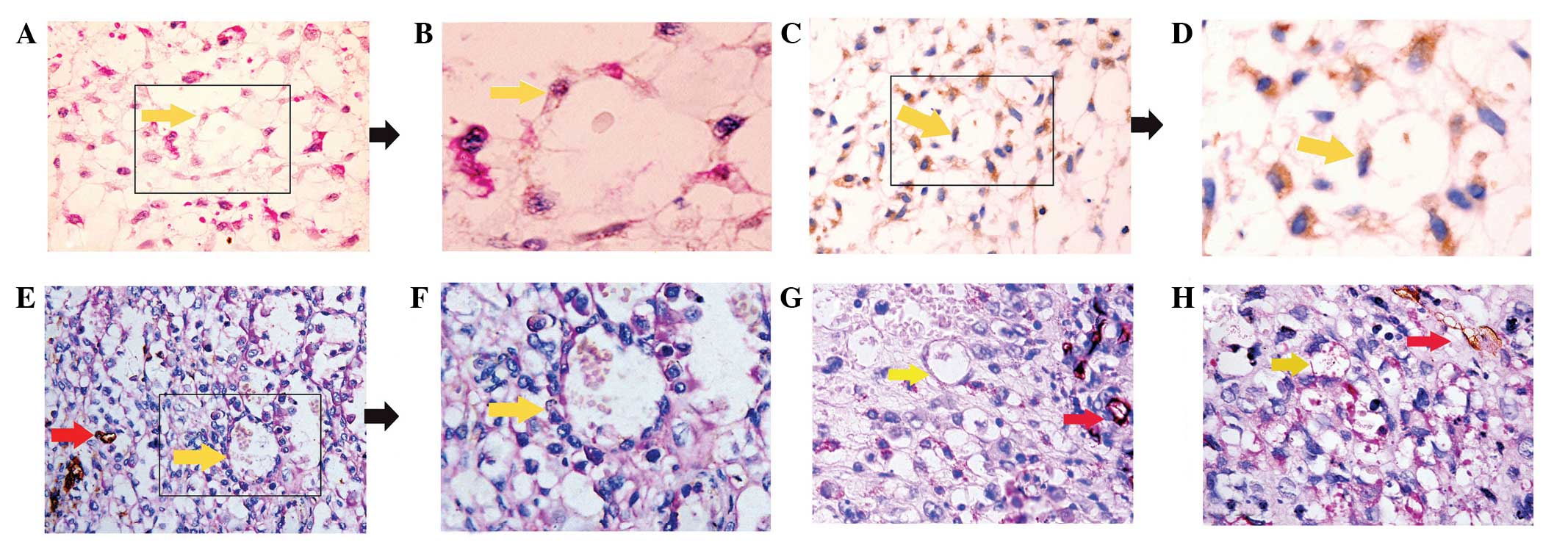 | Figure 1.Evidence of VM in orbital RMS. (A)
Morphological observation with hematoxylin and eosin staining. The
channels (yellow arrow) lined with tumor cells contained red blood
cells, without shuttle-like endothelial cells in the VM wall
(magnification, x400). (B) Enlargement of box outlined in A
(magnification, x1,000). (C) Orbital RMS tumor cells arranged in
tubular structure, indicated by actin immunohistochemical staining.
Actin positivity was observed as brown-colored cytoplasmic and
membrane staining in the specimen. Serial sections from one block,
as in the panel, indicated that the cells lining the VM were
orbital RMS tumor cells (magnification, x200). (D) Enlargement of
box outlined in C (magnification, x400). (E) CD31/PAS
double-staining of VM. The wall of the VM channel was positive for
PAS staining, while tumor cells lining the external wall were
negative for CD31 staining (yellow arrow). The
endothelial-dependent microvessels were positive for CD31 and PAS
(red arrows) (magnification, x400). (F) Enlargement of box outlined
in E (magnification, x1,000). (G and H) VM was identified in
various cases of orbital RMS tissue by CD31/PAS double-staining
(magnification, x400). Yellow arrows, VM; red arrows, microvessel
co-existence in the same field. VM, vasculogenic mimicry; RMS,
rhabdomyosarcoma; PAS, periodic acid Schiff. |
 | Table I.Association between vasculogenic
mimicry and clinicopathological data. |
Table I.
Association between vasculogenic
mimicry and clinicopathological data.
| Factor | Patients, n | VM, n (%) | Non-VM, n (%) | χ2 | P-value |
|---|
| Gender |
|
|
|
1.948 | 0.163 |
| Male | 21 | 9
(42.9) | 12 (57.1) |
|
|
|
Female | 11 | 2
(18.2) | 9
(81.8) |
|
|
| Age, years |
|
|
|
0.204 | 0.652 |
|
<12 | 22 | 7
(31.8) | 15 (68.2) |
|
|
| ≥12 | 10 | 4
(40.0) | 6
(60.0) |
|
|
| Tumor location |
|
|
|
2.586 | 0.108 |
| Right
orbit | 17 | 8
(47.1) | 9
(52.9) |
|
|
| Left
orbit | 15 | 3
(20.0) | 12 (80) |
|
|
| Recurrence |
|
|
| 10.891 | 0.008a |
| Yes | 17 | 13 (76.5) | 4
(23.5) |
|
|
| No | 15 | 7
(46.7) | 8
(53.3) |
|
|
| Survival |
|
|
|
6.362 | 0.012a |
| Dead | 11 | 7
(63.6) | 4
(36.4) |
|
|
|
Alive | 21 | 4
(19.0) | 17 (81.0) |
|
|
| Cell type |
|
|
|
7.469 | 0.024a |
|
Embryonal | 19 | 6
(31.6) | 13 (68.4) |
|
|
|
Alveolar | 7 | 5
(71.4) | 2
(28.6) |
|
|
|
Multiformity | 6 | 0(0) |
6(100) |
|
|
| Mitotic rate/50
HPF |
|
|
| 11.23 | 0.001a |
| ≥5 | 10 |
7(70.0) |
3(30.0) |
|
|
|
<5 | 22 |
3(13.63) | 19(86.37) |
|
|
Association between VM and
clinicopathological data
The clinicopathological data regarding the 32
patients are summarized in Table I.
The incidence of VM was significantly greater in lesions with a
mitotic rate of ≥5/50 high-power fields (HPF) (7/10; 70.0%), than
in lesions with a lower mitotic rate (3/22; 13.6%). The incidence
of VM was also significantly greater in lesions associated with
necrosis (2/8, 25.0%), than in those without necrosis (7/24,
29.2%). The VM positive rate was higher for alveolar cell types
(5/7; 71.4%) than for embryonal cell types (6/19; 31.6%), which
exhibited greater incidence than that of the multiformity cell type
(0/6; 0%). The VM-positive group also had a lower survival rate
(7/11; 63.6%) than that of the VM-negative group (4/21; 19.0%;
P=0.012). The incidence of VM did not differ with respect to
patient gender, age or tumor location.
VM is associated with poor
prognosis
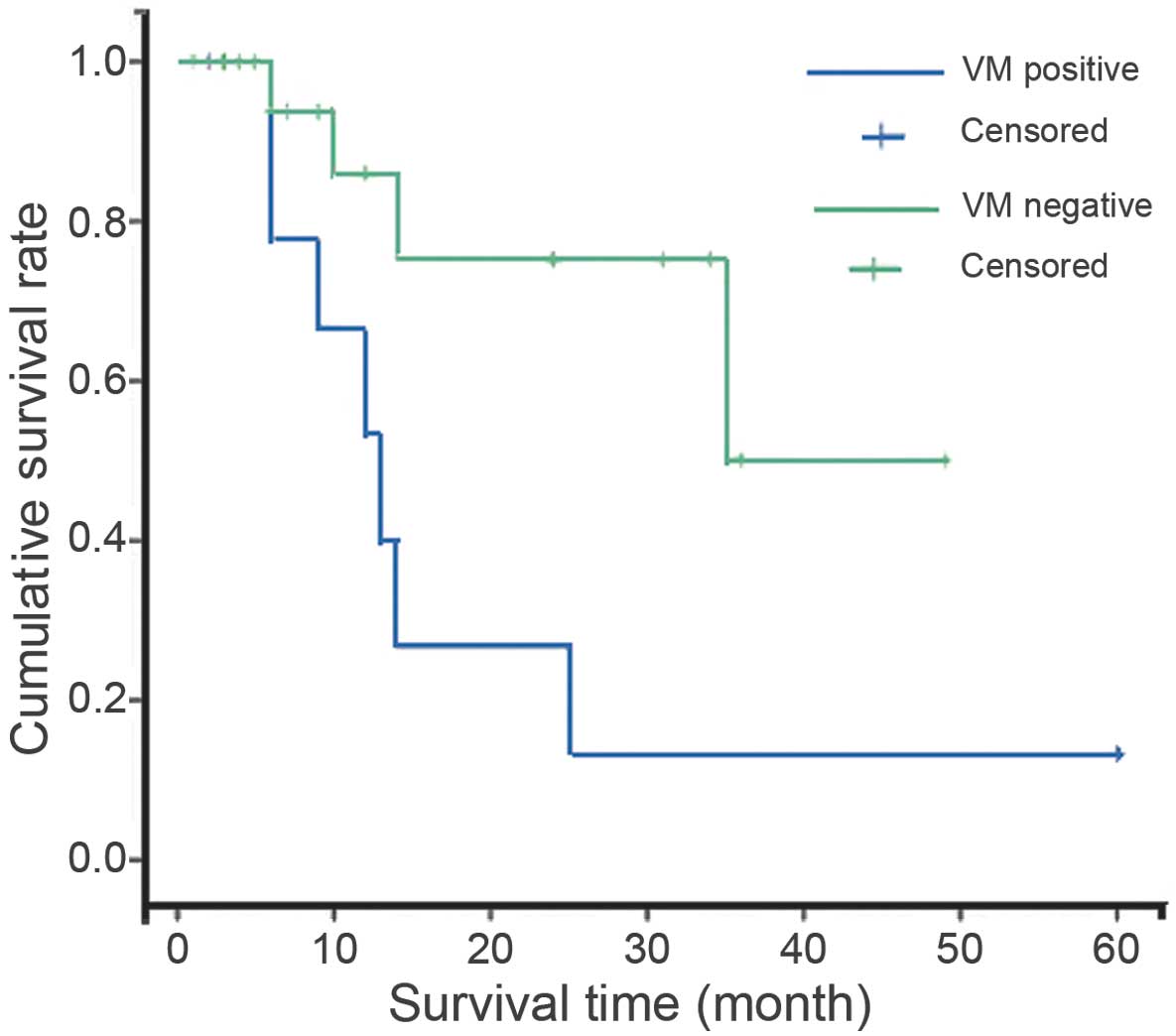
VM-positive patients exhibited a lower survival rate
(36.4%) and shorter survival period than that of patients without
VM (Table II). Kaplan-Meier survival
analysis indicated that the survival rate for patients with tumors
exhibiting VM was significantly poorer than that of patients
without VM (P=0.001; Fig. 2). Cox
proportional hazards model analysis was performed according to VM
and mitotic rate (Table II). This
multivariate analysis revealed that the presence of VM and mitotic
rate were independent indicators of poor prognosis (P=0.001 and
P=0.001, respectively).
 | Table II.Multivariate analysis of factors
influencing survival. |
Table II.
Multivariate analysis of factors
influencing survival.
|
| Cox proportional
hazards analysis |
|
|---|
|
|
|
|
|---|
| Factor | Hazard ratio | 95% CI | P-value |
|---|
| VM, positive | 6.521 | 3.136–12.800 | 0.001a |
| Mitotic rate | 2.910 | 1.632–6.116 | 0.001a |
VM is associated with enhanced
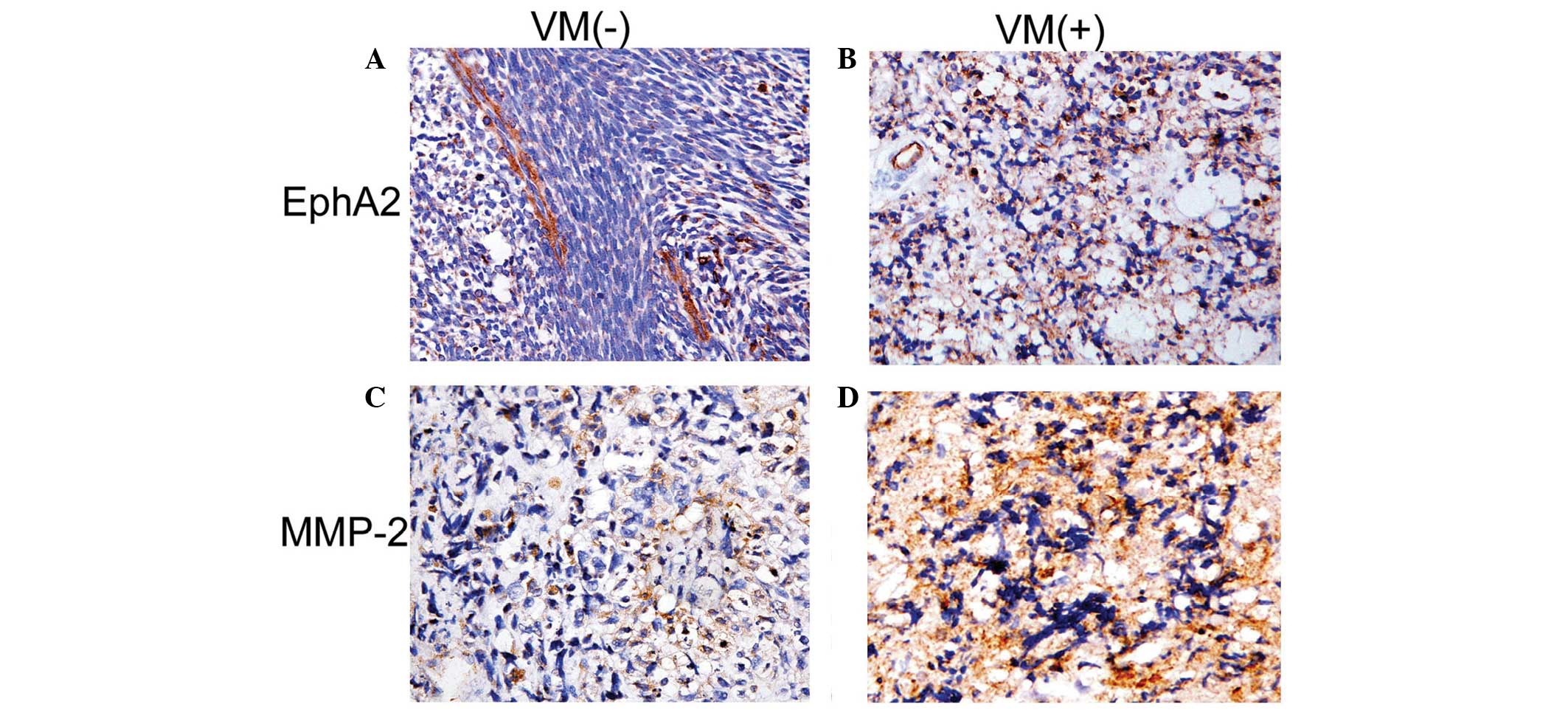
expression of EphA2 and MMP-2
EphA2 was expressed in the cytoplasm of orbital RMS
cells (Fig. 3); and expression was
markedly greater in the VM-positive group than that of the
VM-negative group (P=0.005). MMP-2 was also expressed in the
cytoplasm of orbital RMS cells, and the expression levels of MMP-2
were greater in VM-positive tumors than those in the VM-negative
group (P=0.001; Table III).
 | Table III.Comparison of the expression of EphA2
and MMP-2 in the VM and non-VM groups. |
Table III.
Comparison of the expression of EphA2
and MMP-2 in the VM and non-VM groups.
|
| VM, mean rank |
|
|
|---|
|
|
|
|
|
|---|
| Factor | Positive, %
(±SD) | Negative, %
(±SD) | Z | P-value |
|---|
| EphA2 |
49.3±5.02 |
34.20±3.21 | 2.600 | 0.005a |
| MMP-2 |
69.02±2.22 |
45.36±1.28 | 3.416 | 0.001a |
Discussion
RMS is a highly malignant tumor, and orbital RMS is
one of the few life-threatening diseases initially presented to
ophthalmologists. However there are certain differences between the
orbital RMS and RMS in other locations; for example, the majority
of orbital RMS tumors (60.6%) present with localized disease and
are consequently more favorable when compared with such tumors at
sites in other parts of the body. Orbital RMS is associated with an
excellent survival rate of >85%, whereas the survival rate for
RMS at other sites is 63–76.9% (2).
Furthermore, orbital RMS patients have a 5-year survival rate of
~84.3%, which is associated with low-grade malignancy (13–15). Tumor
cells must acquire an adequate blood supply in order to facilitate
their survival, proliferation and metastasis. Angiogenesis
describes the process of the formation of novel vessels from the
existing vasculature. The identification of non-endothelialized
vessel-like channels in malignant tumors, termed VM, has provided
insight into underlying tumor behaviors and presents potential
targets for drug therapy (10,16). The
formation of VM by tumor cells requires a genetic reversion of
cells to a pluripotent embryonic-like genotype, a change known as
‘cancer plasticity’ (6,17,18).
Orbital RMS is a solid tumor with the capacity for bidirectional
differentiation, which encompasses features of the endothelial
cells lining the vessels, as well as the mesenchymal cells that
secrete extracellular matrix (ECM) proteins, associated with the
remodeling of the matrix for the cellular basis of the formation of
VM (19).
To the best of our knowledge, the present study
provides the first evidence of the pattern of VM in human orbital
RMS. This hypothesis is based on the principle that the walls of VM
channels are similar to those of blood vessels, possessing an ECM
and glycosaminoglycans that make them PAS positive. However, by
definition, these VM channels have no endothelial lining and are
not stained by endothelial markers, including CD31 (20,21). In
2004, Sun et al confirmed that VM channels were an indicator
of poor prognosis in alveolar RMS (12), although their study was principally
based on virtual patient samples. In the present study, CD31/PAS
double-staining combined with actin immunohistochemical staining
were performed, to validate the existence of VM in orbital RMS for
the first time. Serial sections were obtained for each VM-positive
lesion, and VM channels were identified at random in each section.
Based on CD31 and PAS staining, VM was detected in 11 patients out
of 32 (34.4%). These 11 VM-positive lesions confirmed that the VM
channels were not generated accidentally. Based on the results
discussed above, it was concluded that VM tissues were present in
orbital RMS. VM was indicated by matrix-associated channels, which
contained RBCs and were not lined by endothelium.
VM in orbital RMS, which generates lumens containing
RBCs, is similar to the pattern of VM observed in alveolar RMS
(12), but not identical to that seen
in uveal melanomas, in which a higher number of lumens are observed
(3). A potential reason for this is
that uveal melanoma cells are rich in reticular fibers, while
orbital RMS cells are less reticular and collagen fiber-rich. This
point is determined by the enhanced reticular fiber content in
tumor cells, rather than the distribution of tumor cells.
In the present retrospective study of 32 orbital RMS
patients, it was demonstrated that VM was correlated with tumor
cell type, high mitosis-risk, recurrence and short survival time.
These results indicated that VM may be a potential indicator of
poor prognosis for orbital RMS. The VM-positive rate was also found
to be significantly greater in high mitosis-risk patients than that
in the low mitosis-risk group. This likely reflects the more
complex genotype and higher growth rate of high proliferation-risk
orbital RMS tumors. Further analysis suggested that the survival
rate was shorter in the VM group than that in the non-VM group.
Kaplan-Meier and multivariate analyses revealed that VM negatively
affected patient prognosis; and therefore VM may be used for the
assessment of orbital RMS patient prognosis. Due to the specialized
structure of VM, namely the lack of a barrier formed by endothelial
cells to prevent tumor cells from accessing the microcirculation
(3,22), tumor cells are able to migrate easily
into the blood flow. Patients with orbital RMS with VM are
therefore particularly vulnerable to metastasis, explaining why VM
is associated with shorter survival in many malignances. Another
valuable finding from the present study is that VM may be used as a
predictor of decreased response to clinical therapy. This is due to
the fact that certain chemotherapeutic strategies may only target
traditional angiogenesis-associated factors, hence would be
ineffective for VM-positive cases. Therefore, identification of the
molecular markers of VM may provide an improved approach for the
selection of appropriate cancer therapeutic strategies.
VM describes the ability of highly aggressive tumor
cells to express endothelial cell-associated genes, including EphA2
and VE-cadherin, enabling them to form ECM-rich, tubular networks
when cultured on a three-dimensional (3D) matrix (9). However, the exact mechanism underlying
VM remains to be elucidated. To date, several molecules have been
identified which have functional roles in VM. Hess et al
(23) demonstrated that the
expression of epithelial cell marker EphA2 by highly aggressive
melanoma tumor cells, facilitated their ability to mimic
endothelial cells, generating VM in 3D culture. Furthermore,
transient knockout of EphA2 attenuated the ability of these tumor
cells to generate tubular structures. These results indicated that
EphA2 may be involved in the formation of tubular networks by
orbital RMS tumor cells, and therefore may represent a novel
therapeutic target for the treatment of orbital RMS, requiring
further clinical investigation.
The present study and previous studies have
suggested that EphA2 expression occurs with respect to MMP-2
upregulation. The MMP-2 protein is considered to have a significant
role in VM formation in melanomas (24). EphA2 and VE-cadherin may activate
MMP-2 via the PI3K pathway, and the activated MMP-2 may
subsequently promote VM formation (25). In the present study, a difference in
the staining index of MMP-2 expression was identified; with higher
expression in the VM group than that of the non-VM group. It was
hypothesized that orbital RMS cells secrete and activate MMP-2, and
then assist the formation of VM. In the tissue samples evaluated in
the present study, MMP2 expression was markedly higher in the
VM-positive group than that of the VM-negative group, and was
correlated with EphA2 expression. Notably, the results revealed
that EphA2 and the associated molecular pathways may represent
novel therapeutic targets for the inhibition of orbital RMS
recurrence and angiogenesis.
VM is a predictor of poor prognosis for orbital RMS.
In the present pilot study, VM was identified in orbital RMS, and
was found to be indicative of poor prognosis. VM was observed in
RMS at the protein levels, significantly influencing progression,
metastasis and targeting therapy. Furthermore, EphA2 and MMP-2 may
have critical roles in VM formation. These two proteins form a
complex in vivo which results in the development of VM and
tumor promotion (26,27). However, the present study was
retrospective in nature and comprised a relatively small sample
size. Further research regarding the underlying molecular
mechanisms of VM may identify novel therapeutic strategies for
orbital RMS.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81201643), the
Science and Technology Foundation of the Health-Bureau of Tianjin
City (no. 2010k101) and the Tianjin Natural Science Foundation (no.
09ZCZDSF04400). The funders provided financial support without
interference in the ongoing work.
References
|
1
|
Morax S and Desjardins L: Orbital tumor
emergencies in childhood. J Fr Ophtalmol. 32:357–367. 2009.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boutroux H, Levy C, Mosseri V, Desjardins
L, Plancher C, Helfre S, Freneaux P, Cellier C and Orbach D:
Long-term evaluation of orbital rhabdomyosarcoma in children. Clin
Experiment Ophthalmol. 43:12–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun B, Zhang S, Zhang D, Du J, Guo H, Zhao
X, Zhang W and Hao X: Vasculogenic mimicry is associated with high
tumor grade, invasion and metastasis and short survival in patients
with hepatocellular carcinoma. Oncol Rep. 16:693–698.
2006.PubMed/NCBI
|
|
5
|
Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW,
Che N, Wang XH, Du J, Liu YX and Sun BC: Expression and functional
significance of Twist1 in hepatocellular carcinoma: Its role in
vasculogenic mimicry. Hepatology. 51:545–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hendrix MJ, Seftor EA, Hess AR and Seftor
RE: Vasculogenic mimicry and tumour-cell plasticity: Lessons from
melanoma. Nat Rev Cancer. 3:411–421. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hao X, Sun B, Zhang S and Zhao X:
Microarray study of vasculogenic mimicry in bi-directional
differentiation malignant tumor. Zhonghua Yi Xue Za Zhi.
82:1298–1302. 2002.(In Chinese). PubMed/NCBI
|
|
8
|
Wang W, Lin P, Sun B, Zhang S, Cai W, Han
C, Li L, Lu H and Zhao X: Epithelial-mesenchymal transition
regulated by EphA2 contributes to vasculogenic mimicry formation of
head and neck squamous cell carcinoma. Bio Med Res Int.
2014:8039142014.
|
|
9
|
Kirschmann DA, Seftor EA, Hardy KM, Seftor
RE and Hendrix MJ: Molecular pathways: Vasculogenic mimicry in
tumor cells: Diagnostic and therapeutic implications. Clin Cancer
Res. 18:2726–2732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen LX, He YJ, Zhao SZ, Wu JG, Wang JT,
Zhu LM, Lin TT, Sun BC and Li XR: Inhibition of tumor growth and
vasculogenic mimicry by curcumin through down-regulation of the
EphA2/PI3K/MMP pathway in a murine choroidal melanoma model. Cancer
Biol Ther. 11:229–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun B, Qie S, Zhang S, Sun T, Zhao X, Gao
S, Ni C, Wang X, Liu Y and Zhang L: Role and mechanism of
vasculogenic mimicry in gastrointestinal stromal tumors. Hum
Pathol. 39:444–451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun B, Zhang S, Zhao X, Zhang W and Hao X:
Vasculogenic mimicry is associated with poor survival in patients
with mesothelial sarcomas and alveolar rhabdomyosarcomas. Int J
Oncol. 25:1609–1614. 2004.PubMed/NCBI
|
|
13
|
Turner JH and Richmon JD: Head and neck
rhabdomyosarcoma: A critical analysis of population-based incidence
and survival data. Otolaryngol Head Neck Surg. 145:967–973. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chung EM, Smirniotopoulos JG, Specht CS,
Schroeder JW and Cube R: From the archives of the AFIP: Pediatric
orbit tumors and tumorlike lesions: Nonosseous lesions of the
extraocular orbit. Radiographics. 27:1777–1799. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rustemeyer J, Günther L and Junker K:
Limits and chances in an unfortunate course of recurrent orbital
rhabdomyosarcoma. Nepal J Ophthalmol. 3:202–205. 2011.PubMed/NCBI
|
|
16
|
Seftor RE, Hess AR, Seftor EA, Kirschmann
DA, Hardy KM, Margaryan NV and Hendrix MJ: Tumor cell vasculogenic
mimicry: From controversy to therapeutic promise. Am J Pathol.
181:1115–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Schaft DW, Hillen F, Pauwels P,
Kirschmann DA, Castermans K, Egbrink MG, Tran MG, Sciot R, Hauben
E, Hogendoorn PC, et al: Tumor cell plasticity in Ewing sarcoma, an
alternative circulatory system stimulated by hypoxia. Cancer Res.
65:11520–11528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mourad-Zeidan AA, Melnikova VO, Wang H,
Raz A and Bar-Eli M: Expression profiling of galectin-3-depleted
melanoma cells reveals its major role in melanoma cell plasticity
and vasculogenic mimicry. Am J Pathol. 173:1839–1852. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Desch A, Strozyk EA, Bauer AT, Huck V,
Niemeyer V, Wieland T and Schneider SW: Highly invasive melanoma
cells activate the vascular endothelium via an MMP-2/integrin
αvβ5-induced secretion of VEGF-A. Am J Pathol. 181:693–705. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Maniotis AJ, Majumdar D, Pe'er J
and Folberg R: Uveal melanoma cell staining for CD34 and assessment
of tumor vascularity. Invest Ophthalmol Vis Sci. 43:2533–2539.
2002.PubMed/NCBI
|
|
21
|
Hendrix MJ, Seftor EA, Hess AR and Seftor
RE: Molecular plasticity of human melanoma cells. Oncogene.
22:3070–3075. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Folberg R and Maniotis AJ: Vasculogenic
mimicry. APMIS. 112:508–525. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hess AR, Seftor EA, Gardner LM,
Carles-Kinch K, Schneider GB, Seftor RE, Kinch MS and Hendrix MJ:
Molecular regulation of tumor cell vasculogenic mimicry by tyrosine
phosphorylation: Role of epithelial cell kinase (Eck/EphA2). Cancer
Res. 61:3250–3255. 2001.PubMed/NCBI
|
|
24
|
Seftor RE, Seftor EA, Koshikawa N, Meltzer
PS, Gardner LM, Bilban M, Stetler-Stevenson WG, Quaranta V and
Hendrix MJ: Cooperative interactions of laminin 5 gamma2 chain,
matrix metalloproteinase-2 and membrane
type-1-matrix/metalloproteinase are required for mimicry of
embryonic vasculogenesis by aggressive melanoma. Cancer Res.
61:6322–6327. 2001.PubMed/NCBI
|
|
25
|
Hess AR, Seftor EA, Seftor RE and Hendrix
MJ: Phosphoinositide 3-kinase regulates membrane type 1-matrix
metalloproteinase (MMP) and MMP-2 activity during melanoma cell
vasculogenic mimicry. Cancer Res. 63:4757–4762. 2003.PubMed/NCBI
|
|
26
|
Chen LX, Sun BC, Li XR, He YJ and Song GX:
Overexpression of the receptor tyrosine kinase EphA2 in choroidal
melanoma: Correlation with vasculogenic mimicry and prognosis.
Zhonghua Yan Ke Za Zhi. 48:985–990. 2012.(In Chinese). PubMed/NCBI
|
|
27
|
Qie S, Sun BC, Zhao XL, Zhang SW, Sun T,
Gao SY and Wang XH: Correlation between expressions of matrix
metalloproteinase-2 & 9 and vasculogenic mimicry in
gastrointestinal stromal tumors. Zhonghua Yi Xue Za Zhi.
89:1106–1109. 2009.(In Chinese). PubMed/NCBI
|