Introduction
The number of patients with cervical cancer has
decreased as a result of cytological screening and DNA testing for
the high-risk human papilloma virus. However, cervical cancer
remains a considerable burden, with 500,000 new cases and 250,000
mortalities each year worldwide (1).
The important prognostic factors for cervical carcinoma are
represented by the International Federation of Gynecology and
Obstetrics (FIGO) stage (2), lymph
node metastasis and the pathological features of the primary tumor,
including tumor size, depth of stromal invasion, histological type
and lymph-vascular space involvement. Of these factors, lymph node
metastasis has demonstrated the most marked association with
disease recurrence in early-stage cases (3–5). An
increasing requirement exists to identify biomarkers that may be
able to predict treatment responses and patient survival. In
addition, biological variables and gene profiles associated with
aggressive clinical behavior may aid in establishing optimal
therapeutic strategies for early-stage cervical cancers that
present with high-risk factors.
Hypoxia is an important process in tumor biology, as
it induces an aggressive phenotype with increased invasiveness,
leads to the formation of metastases and results in poorer patient
survival (6,7). In addition, hypoxic malignant cells
exhibit increased resistance to chemotherapy and radiotherapy
(8,9).
Cells react to hypoxic conditions by altering their metabolism and
activating specific survival genes. Hypoxia inducible factor-1
(HIF-1) has an important role in the adaptive cellular response to
hypoxia (10). HIF-1 is a
transcription factor composed of the basic helix-loop-helix
DNA-binding proteins, HIF-1α and HIF-1β. Under normoxia, HIF-1α is
hydroxylated by prolyl hydroxylases. Hydroxylated HIF-1α is then
recognized by the von Hippel Lindau protein, ubiquitinated and
targeted to the proteasome for degradation. However, during
hypoxia, this process is inhibited (11). Instead, following nuclear
translocation, the stabilized HIF-1α heterodimerizes with HIF-1β to
transactivate target genes (12).
Furthermore, glycolytic enzymes, glucose transporters, growth
factors and genes that are involved in gluconeogenesis are
activated under hypoxia. These molecules enable the cell to survive
hypoxic stress by increasing oxygen delivery through angiogenesis
and by inducing a switch to anaerobic glycolysis (10,12–14).
HIF-1α expressed under hypoxic conditions has a significant role in
these processes, as it activates the expression of target genes,
such as carbonic anhydrase-IX (CA-IX), which has a role in pH
regulation (15), glucose
transporter-1 (GLUT-1), which is a transmembrane glucose
transporter (16), and vascular
endothelial growth factor (VEGF), which is involved in angiogenesis
(17).
CA-IX is a transmembrane glycoprotein that catalyzes
the reversible hydration of carbonic dioxide to carbonic acid.
CA-IX is therefore important for pH regulation and the elimination
of the hypoxia-generated acid load during glycolysis. Previous data
has established that CA-IX is overexpressed in a number of human
cancers (18). Further studies have
demonstrated that elevated levels of CA-IX are predictive of
hypoxia in a variety of cancers and are associated with a poorer
prognosis (19,20).
The membrane-bound glycoprotein, GLUT-1, is a
high-affinity glucose transporter responsible for the regulation of
glucose uptake (21). The expression
of GLUT-1 is upregulated during hypoxia and other conditions that
induce an increased dependence on the use of anaerobic glycolysis
as an energy source (22). GLUT-1 is
undetectable in the majority of normal epithelial tissues and
benign epithelial tumors, but is expressed at a significantly
higher level in a range of human carcinomas (21).
VEGF is a highly-specific mitogen for vascular
endothelial cells. In response to hypoxia, the expression of VEGF
is upregulated by activated oncogenes and a number of cytokines.
VEGF initiates endothelial cell proliferation and angiogenesis, as
well as the permeabilization of tumor blood vessel (23).
The majority of locally advanced cervical cancers
can be cured with radical surgery and chemo-radiotherapy. However,
patients with persistent or recurrent disease have limited
treatment options, and novel therapeutic strategies, including
immunotherapy, are required for such patients (24).
It has been reported that vaccination with
CA-IX-derived peptides is an effective immunotherapy for renal cell
carcinoma patients (25), and that
the addition of bevacizumab, a humanized anti-VEGF monoclonal
antibody, to conventional therapy for patients with cervical cancer
improves survival rates (26).
In anticipation of the development of novel
therapeutic strategies for locally advanced cervical cancer, the
present study aimed to determine whether HIF-1α, CA-IX, GLUT-1 or
VEGF were associated with the clinicopathological characteristics,
lymph node metastasis or progression-free survival of patients with
cervical carcinoma.
Materials and methods
Clinical samples
Formalin-fixed, paraffin-embedded tumor tissues were
obtained from 54 patients with locally advanced cervical carcinoma.
All patients had attended the Gynecology Clinic at the Aichi
Medical University Hospital (Nagakute, Japan), were diagnosed with
cervical carcinoma and had undergone a radical hysterectomy. The
mean age of the patients was 49.4±11.9 years old (range, 25 to 72
years old). The clinicopathological characteristics of the patients
and the adjuvant therapies used following radical surgery are shown
in Table I. The present study was
approved by the regional ethics committee of Aichi Medical
University, School of Medicine (Nagakute, Japan). Written informed
consent was obtained from all participants prior to study
enrollment.
 | Table I.Characteristics of the 54 cervical
cancer patients who underwent radical hysterectomy followed by
adjuvant radiotherapy or chemo-radiotherapy. |
Table I.
Characteristics of the 54 cervical
cancer patients who underwent radical hysterectomy followed by
adjuvant radiotherapy or chemo-radiotherapy.
|
|
| Adjuvant therapy
following radical hysterectomy (n) |
|---|
|
|
|
|
|---|
| Characteristic | n | None | Radiotherapy |
Chemo-radiotherapy |
|---|
| FIGO stage |
|
|
|
|
| Ib1 | 24 | 18 | 4 | 2 |
| Ib2 | 15 | 1 | 5 | 9 |
| IIa1 | 1 | 0 | 0 | 1 |
| IIa2 | 5 | 0 | 2 | 3 |
| IIb | 9 | 0 | 2 | 7 |
| Histology |
|
|
|
|
| Squamous
cell carcinoma | 34 | 9 | 9 | 16 |
|
Adenocarcinoma | 20 | 10 | 4 | 6 |
| Tumor size, cm |
|
|
|
|
|
<4 | 27 | 18 | 5 | 4 |
| ≥4 | 27 | 1 | 8 | 18 |
| Lymph node
metastasis |
|
|
|
|
|
Negative | 36 | 18 | 9 | 9 |
|
Positive | 18 | 1 | 4 | 13 |
| Lymph-vascular space
involvement |
|
|
|
|
|
Negative | 38 | 19 | 8 | 11 |
|
Positive | 16 | 0 | 5 | 11 |
| Microvessel density,
vessels/×400 field |
|
|
|
|
| ≤5 | 25 | 13 | 4 | 8 |
|
>5 | 29 | 6 | 9 | 14 |
| Total | 54 | 19 | 13 | 22 |
Immunohistochemistry
The 3-µm thick tumor sections were first
deparaffinized and rehydrated. Subsequent to microwave processing
in 10 mM citrate buffer (pH 6.0) for 25 min, the sections were
incubated for 30 min in methanol containing 0.5%
H2O2. Following incubation in normal goat
serum for 1 h at room temperature to block non-specific binding,
the slides were incubated at 4°C overnight with the following
primary antibodies: Mouse anti-HIF-1α antiserum (dilution, 1:100;
product no. ab1; Abcam, Tokyo, Japan), rabbit anti-CA-IX antiserum
(dilution, 1:1000; product no. ab15086; Abcam), rabbit anti-VEGF
antiserum (dilution, 1:200; product no. ab46154; Abcam) and rabbit
anti-GLUT-1 antiserum (dilution, 1:200; product no. ab14683;
Abcam). Following incubation with the primary antibody, the
Envision Polymer Component (ChemMate ENVISION kit; Dako, Kyoto,
Japan) was added to the slides for 30 min at room temperature. The
horseradish peroxidase reaction was then developed using
3,3′-diaminobenzidine tetrahydrochloride (Katayama Chemical
Industries Co., Ltd., Osaka, Japan). Finally, for the microscopic
examination, sections were counterstained with hematoxylin
(magnification, ×200; Olympus BX43; Olympus Corporation, Tokyo,
Japan). The tissues were defined as having positive expression when
>50% of the tumor cells demonstrated intense staining.
Examination of microvessel density and
lymph-vascular space involvement
Blood vessels were identified by immunohistochemical
staining of the endothelial cells using mouse anti-cluster of
differentiation (CD)34 antiserum (dilution, 1:25; product no. ICO
115; Cell Signaling Technology Japan, Tokyo, Japan), according to
the aforementioned procedure. The total number of vessels in each
case was taken to be the total sum of vessels counted in each of 10
microscopic fields. The vessels were analyzed at ×400
magnification. The microvessel density was defined as the average
number of microvessels per field, calculated from the total number
of microvessels in 10 fields.
For the assessment of lymph-vascular space
involvement, lymph and blood vessels were immunohistochemically
stained with the already diluted mouse monoclonal antibody against
D2-40 (dilution, 1:25; product no. 413451; Nichirei Biosciences
Inc., Tokyo, Japan) and the mouse anti-CD34 antiserum (dilution,
1:25; Cell Signaling Technology Japan) according to the
aforementioned procedure. The presence of carcinoma cells in the
lymph and blood vessels indicated positive lymph-vascular space
involvement.
Statistical analysis
Stat View-J version 5 (Apple Inc., Cupertino, CA,
USA) was used for the statistical analyses. The statistical
significance of the differences between categories of expression
was analyzed using Fisher's exact test. The potential significance
of plural risk factors for lymph node metastasis was analyzed using
a logistic regression test. Progression-free survival was analyzed
by the Kaplan-Meier method and a log-rank test. P<0.05 was used
to indicate a statistically significant difference.
Results
The expression of HIF-1α, CA-IX, GLUT-1 and VEGF in
cervical squamous cell carcinomas was analyzed
immunohistochemically. HIF-1α was observed in the cell nuclei and
cytoplasm of the tumor cells, whereas CA-IX, GLUT-1 and VEGF were
predominantly localized in the cell membrane and cytoplasm. HIF-1α
and VEGF stained homogenously throughout the cancer nest, whereas
CA-IX and GLUT-1 were localized in the center (Fig. 1).
Of the 54 cases, 28 were positive for HIF-1α
expression, 35 for CA-IX, 40 for GLUT-1 and 23 for VEGF. Analysis
of the correlation between the expression of these different
factors indicated that HIF-1α was significantly associated with
CA-IX, but not with GLUT-1 or VEGF. In addition, CA-IX expression
was correlated with GLUT-1 and VEGF, but no association was
identified between GLUT-1 and VEGF (Table II).
 | Table II.Co-expression of HIF-1α, CA-IX,
GLUT-1 and VEGF. |
Table II.
Co-expression of HIF-1α, CA-IX,
GLUT-1 and VEGF.
| A, HIF-1α |
|---|
|
|---|
| Parameter | Negative
(n=26) | Positive
(n=28) | P-value |
|---|
| CA-IX |
|
| 0.0096 |
|
Negative (n=19) | 14 | 5 |
|
|
Positive (n=35) | 12 | 23 |
|
| GLUT-1 |
|
| 0.4399 |
|
Negative (n=14) | 8 | 6 |
|
|
Positive (n=40) | 18 | 22 |
|
| VEGF |
|
| 0.5541 |
|
Negative (n=31) | 16 | 15 |
|
|
Positive (n=23) | 10 | 13 |
|
|
| B, CA-IX |
|
| Parameter | Negative
(n=19) | Positive
(n=35) | P-value |
|
| GLUT-1 |
|
| 0.0081 |
|
Negative (n=14) | 9 | 5 |
|
|
Positive (n=40) | 10 | 30 |
|
| VEGF |
|
| 0.0033 |
|
Negative (n=31) | 16 | 15 |
|
|
Positive (n=23) | 3 | 20 |
|
|
| C, GLUT-1 |
|
| Parameter | Negative
(n=14) | Positive
(n=40) | P-value |
|
| VEGF |
|
| 0.347 |
|
Negative (n=31) | 10 | 21 |
|
|
Positive (n=23) | 4 | 19 |
|
The expression of these factors was then correlated
with the tumor parameters. A higher expression level of HIF-1α,
CA-IX and GLUT-1 was observed in stage II cases compared with stage
I cases. By contrast, VEGF was not associated with the tumor stage.
HIF-1α was the only factor to demonstrate a higher expression level
in adenocarcinomas compared with the squamous cell carcinomas. None
of the other factors exhibited an association with expression
levels and tumor histology. CA-IX was the only factor to
demonstrate an association with tumor size. CA-IX was more highly
expressed in tumors measuring ≥4 cm compared with those measuring
<4 cm. Furthermore, CA-IX was also the only factor to be
correlated with lymph node metastasis, being more highly expressed
in these cases. Only CA-IX and GLUT-1 exhibited an association with
lymph-vascular space involvement, and only VEGF was correlated with
microvessel density. A higher expression level of VEGF was observed
in tumors with high microvessel density than in those with low
microvessel density (Table
III).
 | Table III.Association of HIF-1α, CA-IX, GLUT-1
and VEGF expression levels with FIGO stage, histological type,
tumor size, lymph node metastasis, lymph-vascular space involvement
and microvessel density. |
Table III.
Association of HIF-1α, CA-IX, GLUT-1
and VEGF expression levels with FIGO stage, histological type,
tumor size, lymph node metastasis, lymph-vascular space involvement
and microvessel density.
|
|
| Positive
immunohistochemical expression [n, (%)] |
|---|
|
|
|
|
|---|
| Characteristic | n | HIF-1α | CA-IX | GLUT-1 | VEGF |
|---|
| FIGO stage |
|
|
|
|
|
| I | 39 | 16 (41%) | 22 (56%) | 25 (64%) | 15 (38%) |
| II | 15 | 12 (80%) | 13 (87%) | 15 (100%) | 8 (53%) |
|
P-value |
| 0.0102 | 0.0370 | 0.0070 | 0.3222 |
| Histology |
|
|
|
|
|
|
SCC | 34 | 13 (38%) | 20 (59%) | 28 (82%) | 13 (38%) |
|
Adenocarcinoma | 20 | 15 (75%) | 15 (75%) | 12 (60%) | 10 (50%) |
|
P-value |
| 0.0090 | 0.2293 | 0.0703 | 0.3985 |
| Tumor size, cm |
|
|
|
|
|
|
<4 | 27 | 13 (48%) | 13 (48%) | 17 (63%) | 8 (30%) |
| ≥4 | 27 | 15 (56%) | 22 (81%) | 23 (85%) | 15 (56%) |
|
P-value |
| 0.5863 | 0.0103 | 0.0624 | 0.0525 |
| Lymph-node
metastasis |
|
|
|
|
|
|
Negative | 36 | 18 (50%) | 20 (56%) | 24 (67%) | 17 (47%) |
|
Positive | 18 | 10 (56%) | 15 (83%) | 16 (89%) | 6 (33%) |
|
P-value |
| 0.7001 | 0.0439 | 0.0790 | 0.3306 |
| Lymph-vascular
space involvement |
|
|
|
|
|
|
Negative | 38 | 18 (47%) | 21 (55%) | 25 (66%) | 15 (39%) |
|
Positive | 16 | 10 (63%) | 14 (88%) | 15 (94%) | 8 (50%) |
|
P-value |
| 0.3095 | 0.0235 | 0.0323 | 0.4750 |
| Microvessel
density, vessels/×400 field |
|
|
|
|
|
| ≤5 | 25 | 11 (44%) | 13 (52%) | 16 (64%) | 6 (24%) |
|
>5 | 29 | 17 (59%) | 22 (76%) | 24 (83%) | 17 (59%) |
| P-value |
| 0.2836 | 0.0671 | 0.1168 | 0.0103 |
The association between the co-expression levels of
these factors and the tumor parameters was then investigated.
Higher co-expression of HIF-1α and CA-IX was observed in stage II
cases compared with stage I cases. In addition, higher
co-expression of CA-IX and GLUT-1 was observed in stage II cases
compared with stage I cases, as well as in tumors ≥4 cm, in cases
with lymph node metastasis and in tumors with lymph-vascular space
involvement. Finally, a higher co-expression level of CA-IX and
VEGF was observed in tumors ≥4 cm and in tumors with a higher
microvessel density (Table IV).
 | Table IV.Association of HIF-1α, CA-IX, GLUT-1
and VEGF co-expression with the FIGO stage, histological type,
size, lymph node metastasis, lymph-vascular space involvement and
microvessel density of the tumors. |
Table IV.
Association of HIF-1α, CA-IX, GLUT-1
and VEGF co-expression with the FIGO stage, histological type,
size, lymph node metastasis, lymph-vascular space involvement and
microvessel density of the tumors.
|
|
| Immunohistochemical
co-expression [n, (%)] |
|---|
|
|
|
|
|---|
| Characteristic | n | HIF-1α + CA-IX | CA-IX + GLUT-1 | CA-IX + VEGF |
|---|
| FIGO stage |
|
|
|
|
| I | 39 | 13 (33%) | 17 (44%) | 12 (31%) |
| II | 15 | 10 (67%) | 13 (87%) | 8 (53%) |
|
P-value |
| 0.0349 | 0.0056 | 0.2074 |
| Histology |
|
|
|
|
|
SCC | 34 | 11 (32%) | 20 (59%) | 11 (32%) |
|
Adenocarcinoma | 20 | 12 (60%) | 10 (50%) | 9 (45%) |
|
P-value |
| 0.0861 | 0.5798 | 0.3934 |
| Tumor size, cm |
|
|
|
|
|
<4 | 27 | 10 (37%) | 9 (33%) | 6 (22%) |
| ≥4 | 27 | 13 (48%) | 21 (78%) | 14 (52%) |
|
P-value |
| 0.5826 | 0.0023 | 0.0473 |
| Lymph-node
metastasis |
|
|
|
|
|
Negative | 36 | 14 (39%) | 16 (44%) | 14 (39%) |
|
Positive | 18 | 9 (50%) | 14 (78%) | 6 (33%) |
|
P-value |
| 0.5615 | 0.0239 | 0.7712 |
| Lymph-vascular
space involvement |
|
|
|
|
|
Negative | 38 | 14 (37%) | 17 (45%) | 12 (32%) |
|
Positive | 16 | 9 (56%) | 13 (81%) | 8 (50%) |
|
P-value |
| 0.2350 | 0.0176 | 0.2301 |
| Microvessel
density, vessels/×400 field |
|
|
|
|
| ≤5 | 25 | 9 (36%) | 11 (44%) | 4 (16%) |
|
>5 | 29 | 14 (48%) | 19 (66%) | 16 (55%) |
|
P-value |
| 0.4170 | 0.1700 | 0.0044 |
The multivariate regression analysis revealed that
CA-IX expression and lymph-vascular space involvement were
independent variables associated with lymph node metastasis in
patients with cervical cancer (Table
V).
 | Table V.Multivariate analyses of variables
associated with lymph node metastasis in 54 patients with cervical
cancer. |
Table V.
Multivariate analyses of variables
associated with lymph node metastasis in 54 patients with cervical
cancer.
| Variables | Odds ratio | 95% CI | P-value |
|---|
| FIGO stage (II vs.
I) | 8.486 | 0.794–90.749 | ns |
| Histology
(adenocarcinoma vs. squamous cell carcinoma) | 0.289 | 0.034–2.427 | ns |
| Tumor size (≥4 cm
vs. <4 cm) | 0.16 | 0.012–2.181 | ns |
| Lymph-vascular
space involvement (positive vs. negative) | 39.413 | 2.792–556.363 | 0.0065 |
| Microvessel density
(>5 vs. ≤5, vessels/×400 field) | 6.531 | 0.648–65.781 | ns |
| HIF-1α expression
(positive vs. negative) | 0.247 | 0.031–1.952 | ns |
| CA-IX expression
(positive vs. negative) | 33.217 | 1.016–1085.947 | 0.0489 |
| GLUT-1 expression
(positive vs. negative) | 0.421 | 0.019–9.189 | ns |
| VEGF expression
(positive vs. negative) | 0.134 | 0.002–1.574 | ns |
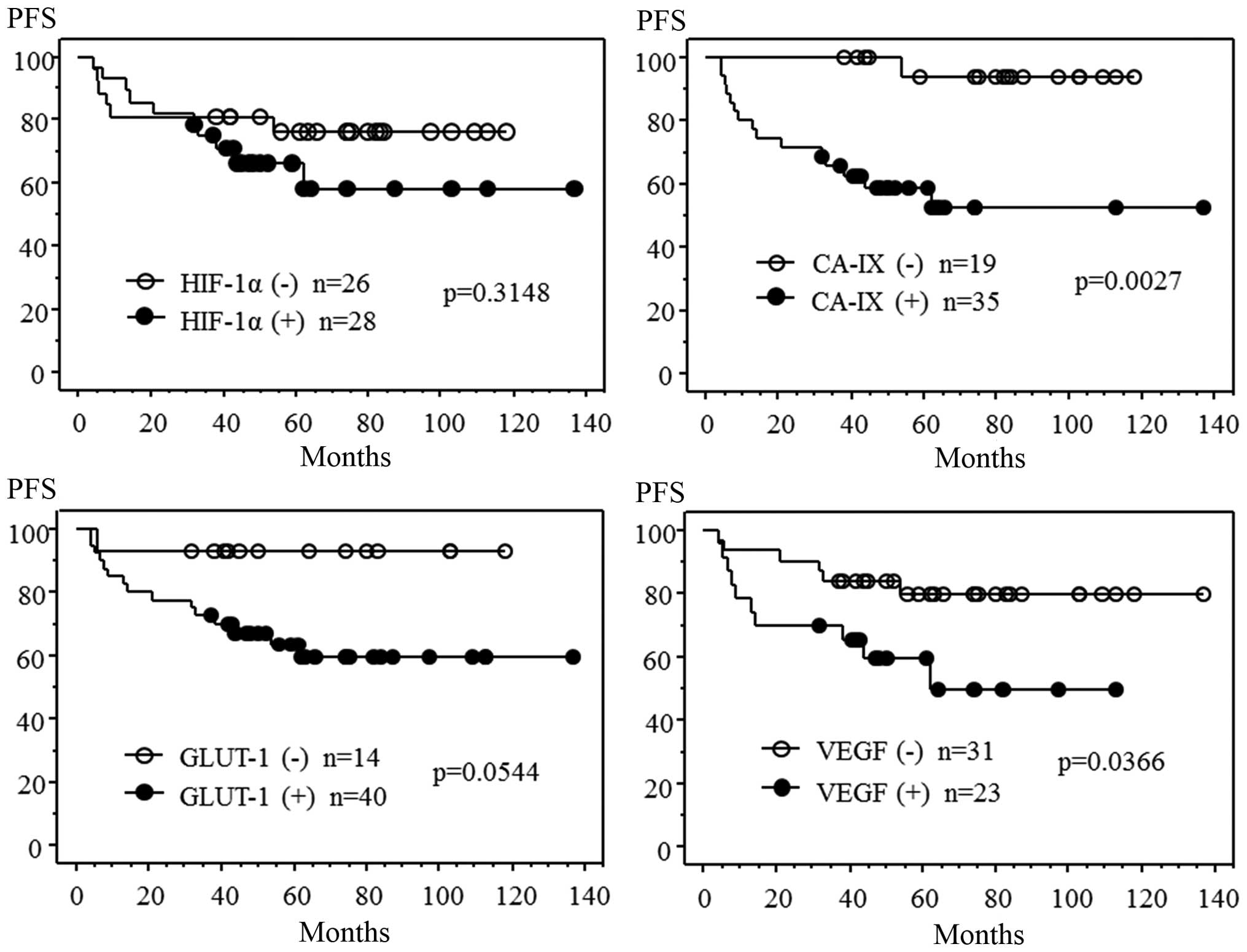
The Kaplan-Meier analyses indicated that
progression-free survival time was shorter in patients with a
larger tumor size, positive lymph node metastasis, positive
lymph-vascular space involvement and a higher tumor microvessel
density (data not shown). Progression-free survival time was also
shorter in patients positive for CA-IX or VEGF expression than in
those negative for CA-IX or VEGF expression. However,
progression-free survival time demonstrated no association with the
expression of HIF-1α or GLUT-1 (Fig.
2). In the 35 patients treated with radiotherapy or
chemo-radiotherapy following radical hysterectomy, progression-free
survival time was also shorter for those individuals positive for
CA-IX expression compared with those negative for CA-IX expression
(Fig. 3), and for patients with
positive lymph-vascular space involvement compared with those
negative for lymph-vascular space involvement (data not shown).
However, progression-free survival time exhibited no correlation
with tumor size, lymph node metastasis, microvessel density (data
not shown) or VEGF expression (Fig.
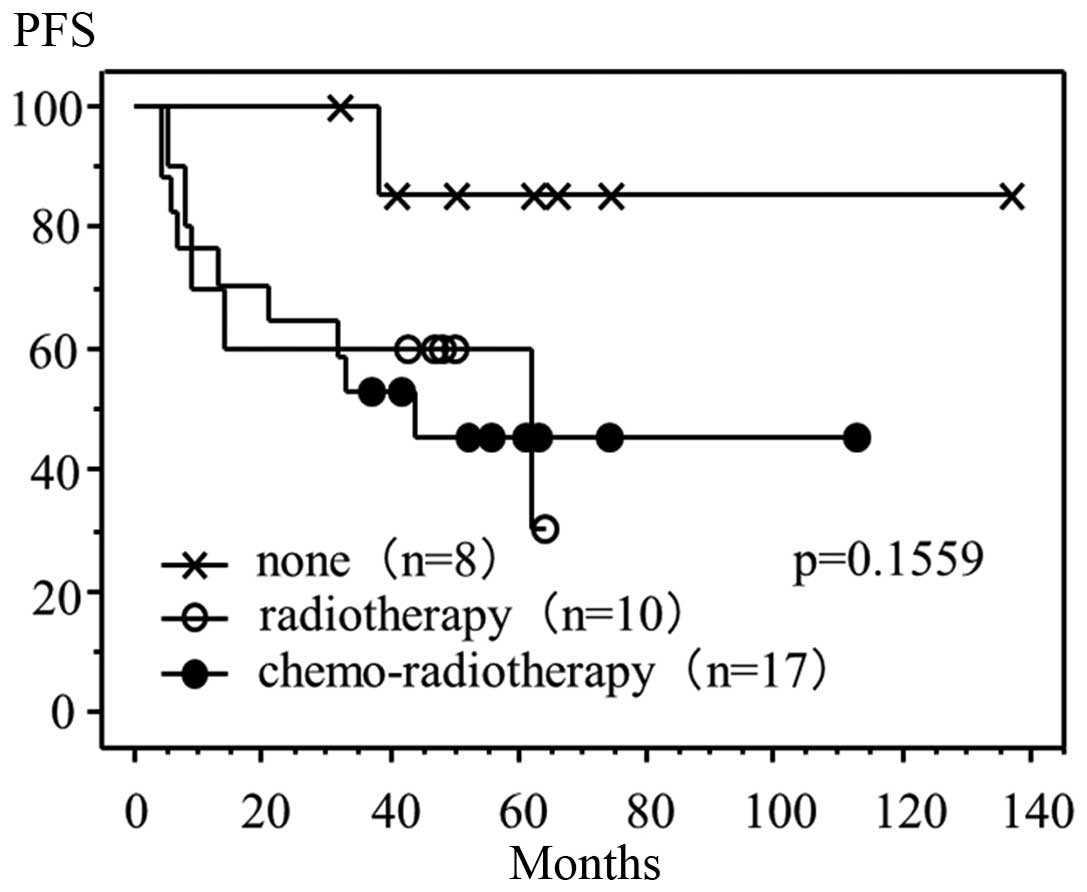
3). Finally, of the 35 cases positive for CA-IX expression,
progression-free survival time demonstrated no association with the
performance of adjuvant therapies following radical hysterectomy
(Fig. 4).
Discussion
Tumor hypoxia is a factor known to be associated
with genetic instability, resistance to apoptosis, invasive growth
and metastatic spread. In the present study, the expression of
HIF-1α, CA-IX, GLUT-1 and VEGF was examined in order to determine
whether these molecules may be useful tissue biomarkers of tumor
hypoxia. The expression of these potential markers was examined
immunohistochemically in biopsies obtained from patients with
locally advanced cervical carcinoma who had undergone radical
hysterectomy followed by post-surgical radiotherapy or
chemo-radiotherapy. The most important findings of this study were
that the expression of CA-IX and the presence of lymph-vascular
space involvement were associated with lymph node metastasis, and
that the expression of CA-IX was clearly associated with disease
recurrence, regardless of the treatment modality.
A previous retrospective study that analyzed 130
squamous cell cervical carcinoma biopsies revealed that the
expression of CA-IX was an independent prognostic indicator of poor
overall survival and metastasis-free survival following definitive
radiotherapy (20). In a further
study by the Gynecological Oncology Group, the expression of CA-IX
was immunohistochemically analyzed in tumor biopsies obtained from
166 women who had undergone a radical hysterectomy for stage
Ia2-IIa cervical cancer that had presented with pathological
findings of lymph node metastases, parametrial involvement or
positive surgical margins. The patients in the study received
either adjuvant pelvic radiotherapy alone, or adjuvant pelvic
radiotherapy combined with concomitant cisplatin- and
5-fluorouracil-based chemotherapy (27,28). A
high expression of CA-IX has been identified to be significantly
associated with tumor size and depth of stromal invasion in
patients with cervical cancer, and is also an independent predictor
of poor survival (28). In addition,
it has been demonstrated that the absence of GLUT-1 immunostaining
is associated with improved metastasis-free survival in patients
who receive definitive radiotherapy (29). VEGF immunostaining has also been
reported to be significantly correlated with disease-free survival
and overall survival in patients treated with neoadjuvant
chemotherapy or primary radiotherapy (30,31). Of
the VEGF isoforms, it has been established that VEGF-C is essential
for lymphangiogenesis and the lymphatic spread of tumors (32). A previous study revealed that VEGF-C
expression is higher in patients positive for lymph node metastasis
than in those negative for lymph node metastasis. Furthermore, the
results indicated that this variable was an independent predictor
of lymph node status, and that the univariate, but not the
multivariate analysis of patients whose tumors were positive for
VEGF-C mRNA revealed a shorter disease-free survival time (33).
The present study revealed that CA-IX was associated
with the expression of HIF-1α, GLUT-1 and VEGF. However, no
correlation was identified between the expression of HIF-1α and
GLUT-1, between HIF-1α and VEGF, or between GLUT-1 and VEGF. The
results also demonstrated an association between these markers and
specific tumor parameters. HIF-1α expression was associated with
the FIGO stage and histological type, but not with tumor size,
lymph node metastasis, lymph-vascular involvement or microvessel
density. CA-IX expression was associated with the FIGO stage, tumor
size, lymph node metastasis and lymph-vascular space involvement.
GLUT-1 expression was associated with the FIGO stage and
lymph-vascular involvement, and VEGF expression was associated with
microvessel density. The co-expression of CA-IX and GLUT-1 was
associated with the FIGO stage, tumor size, lymph node metastasis
and lymph-vascular space involvement, and the co-expression of
CA-IX and VEGF was associated with tumor size and microvessel
density.
These findings suggest that lymph node metastasis
following lymph-vascular space involvement may be associated with
pH regulation and anaerobic glycolysis, which under the hypoxic
conditions of the tumor, is assisted by CA-IX and GLUT-1. In
addition, the results indicate that lymph node metastasis is
possibly associated with VEGF-induced angiogenesis.
The multivariate regression analysis revealed that
CA-IX expression and lymph-vascular space involvement were
independent variables associated with lymph node metastasis in
patients with cervical cancer. These findings suggest: i) That the
pH regulation induced by CA-IX under hypoxic conditions may be
associated with lymph node metastasis; ii) that CA-IX can function
as a biomarker with the ability to predict lymph node metastasis;
and iii) that CA-IX is potential molecular target for the treatment
of cervical cancer.
The Kaplan-Meier analyses indicated that the
progression-free survival time was shorter for patients with
positive CA-IX or VEGF expression than for those with negative
CA-IX or VEGF expression. A similar correlation between CA-IX
expression and progression-free survival time was identified in
patients treated with radiotherapy or chemo-radiotherapy following
radical surgery. However, no association was established between
progression-free survival time and the performance of adjuvant
therapies in patients positive for CA-IX expression following
radical surgery.
These results suggest that CA-IX expression, as well
as lymph node metastasis, larger tumor size and lymph-vascular
space involvement, are important predictive factors associated with
disease recurrence in locally advanced cervical cancer. Therefore,
it is hoped that novel therapeutic approaches that target CA-IX can
be developed, as at present, no improvement in progression-free
survival time in cases positive for CA-IX expression has been
observed, even when adjuvant radiotherapy or chemo-radiotherapy is
administered following radical surgery.
Although extremely few targeted therapies have been
evaluated for cervical carcinoma, the findings of the present study
indicate a possibility for molecular therapies targeted at CA-IX or
VEGF. It was previously reported that vaccination with
CA-IX-derived peptides was an effective immunotherapy for renal
cell carcinoma patients (25). The
results of the present study suggest that vaccination with
CA-IX-derived peptides may also present a novel form of therapy for
cervical cancer patients. Additionally, it has been reported that
the addition of bevacizumab, a humanized anti-VEGF monoclonal
antibody, to the conventional therapy of patients with cervical
cancer improves survival (26). The
results of the present study demonstrate that VEGF expression in
cervical cancer is an important risk factor associated with disease
recurrence. Therefore, anti-VEGF immunotherapy may also be a useful
therapeutic approach for the treatment of patients with cervical
cancer.
In conclusion, the findings of the present study
indicate that CA-IX is a possible risk factor for lymph node
metastasis and disease recurrence in locally advanced cervical
cancer patients. The pH regulation induced by CA-IX expression
under hypoxic conditions may be associated with lymph node
metastasis and a poor progression-free survival time. Therefore, it
is hypothesized that the vaccination of cervical carcinoma patients
whose tumors express CA-IX with CA-IX-derived peptides may prove to
be an effective therapy.
References
|
1
|
Tewari KS and Monk BJ: Invasive cervical
cancerClinical Gynecologic Oncology. DiSaia PJ and Creaseman WT:
8th. Mosby; Philadelphia, PA: pp. 51–120. 2012, View Article : Google Scholar
|
|
2
|
Pecorelli S: Revised FIGO staging for the
vulva, cervix and endometrium. Int J Gynaecol Obstet. 105:103–104.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Creasman WT and Kohler MF: Is lymph
vascular space involvement an independent prognostic factor in
early cervical cancer? Gynecol Oncol. 92:525–529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Narayan K, Fisher R and Bernshaw D:
Significance of tumor volume and corpus uteri invasion in cervical
cancer patients treated by radiotherapy. Int J Gynecol Cancer.
16:623–630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Biewenga P, van der Velden J, Mol BW, et
al: Validation of existing prognostic models in patients with
early-stage cervical cancer. Gynecol Oncol. 115:277–284. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kurokawa T, Miyamoto M, Kato K, et al:
Overexpression of hypoxia-inducible-factor 1alpha (HIF-1alpha) in
oesophageal squamous cell carcinoma correlates with lymph node
metastasis and pathologic stage. Br J Cancer. 89:1042–1047. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schindl M, Schoppmann SF, Samonigg H, et
al: Austrian Breast and Colorectal Cancer Study Group:
Overexpression of hypoxia-inducible factor 1alpha is associated
with an unfavorable prognosis in lymph node-positive breast cancer.
Clin Cancer Res. 8:1831–1837. 2002.PubMed/NCBI
|
|
8
|
Unruh A, Ressel A, Mohamed HG, et al: The
hypoxia-inducible factor-1 alpha is a negative factor for tumor
therapy. Oncogene. 22:3213–3220. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Greijer AE, de Jong MC, Scheffer GL,
Shvarts A, van Diest PJ and van der Wall E: Hypoxia-induced
acidification causes mitoxantrone resistance not mediated by drug
transporters in human breast cancer cells. Cell Oncol. 27:43–49.
2005.PubMed/NCBI
|
|
10
|
Greijer AE, van der Groep P, Kemming D, et
al: Up-regulation of gene expression by hypoxia is mediated
predominantly by hypoxia-inducible factor 1 (HIF-1). J Pathol.
206:291–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang LE, Arany Z, Livingston DM and Bunn
HF: Activation of hypoxia-inducible transcription factor depends
primarily upon redox-sensitive stabilization of its alpha subunit.
J Biol Chem. 271:32253–32259. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semenza GL: Regulation of mammalian O2
homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol.
15:551–578. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Semenza GL: HIF-1: mediator of
physiological and pathophysiological responses to hypoxia. J Appl
Physiol (1985). 88:1474–1480. 2000.PubMed/NCBI
|
|
14
|
Ratcliffe PJ, O'Rourke JF, Maxwell PH and
Pugh CW: Oxygen sensing, hypoxia-inducible factor-1 and the
regulation of mammalian gene expression. J Exp Biol. 201:1153–1162.
1998.PubMed/NCBI
|
|
15
|
VaughanJones RD and Spitzer KW: Role of
bicarbonate in the regulation of intracellular pH in the mammalian
ventricular myocyte. Biochem Cell Biol. 80:579–596. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Behrooz A and Ismail-Beigi F: Stimulation
of glucose transport by hypoxia: Signals and mechanisms. News
Physiol Sci. 14:105–110. 1999.PubMed/NCBI
|
|
17
|
Pedersen MW, Holm S, Lund EL, Højgaard L
and Kristjansen PE: Coregulation of glucose uptake and vascular
endothelial growth factor (VEGF) in two small-cell lung cancer
(SCLC) sublines in vivo and in vitro. Neoplasia. 3:80–87. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robertson N, Potter C and Harris AL: Role
of carbonic anhydrase IX in human tumor cell growth, survival and
invasion. Cancer Res. 64:6160–6165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, et al: Expression of hypoxia-inducible carbonic anhydrase-9
relates to angiogenic pathways and independently to poor outcome in
non-small cell lung cancer. Cancer Res. 61:7992–7998.
2001.PubMed/NCBI
|
|
20
|
Loncaster JA, Harris AL, Davidson SE, et
al: Carbonic anhydrase (CA IX) expression, a potential new
intrinsic marker of hypoxia: correlations with tumor oxygen
measurements and prognosis in locally advanced carcinoma of the
cervix. Cancer Res. 61:6394–6399. 2001.PubMed/NCBI
|
|
21
|
Younes M, Lechago LV, Somoano JR, Mosharaf
M and Lechago J: Wide expression of the human erythrocyte glucose
transporter Glut1 in human cancers. Cancer Res. 56:1164–1167.
1996.PubMed/NCBI
|
|
22
|
Clavo AC, Brown RS and Wahl RL:
Fluorodeoxyglucose uptake in human cancer cell lines is increased
by hypoxia. J Nucl Med. 36:1625–1632. 1995.PubMed/NCBI
|
|
23
|
Obermair A, BancherTodesca D, Bilgi S, et
al: Correlation of vascular endothelial growth factor expression
and microvessel density in cervical intraepithelial neoplasia. J
Natl Cancer Inst. 89:1212–1217. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Monk BJ, Tewari KS and Koh WJ:
Multimodality therapy for locally advanced cervical carcinoma:
state of the art and future directions. J Clin Oncol. 25:2952–2965.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uemura H, Fujimoto K, Tanaka M, et al: A
phase I trial of vaccination of CA9-derived peptides for
HLA-A24-positive patients with cytokine-refractory metastatic renal
cell carcinoma. Clin Cancer Res. 12:1768–1775. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tewari KS, Sill MW, Long HJ III, et al:
Improved survival with bevacizumab in advanced cervical cancer. N
Engl J Med. 370:734–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peters WA III, Liu PY, Barrett RJ II, et
al: Concurrent chemotherapy and pelvic radiation therapy compared
with pelvic radiation therapy alone as adjuvant therapy after
radical surgery in high-risk early-stage cancer of the cervix. J
Clin Oncol. 18:1606–1613. 2000.PubMed/NCBI
|
|
28
|
Liao SY, Darcy KM, Randall LM, et al:
Prognostic relevance of carbonic anhydrase-IX in high-risk,
early-stage cervical cancer: a Gynecologic Oncology Group study.
Gynecol Oncol. 116:452–458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Airley R, Loncaster J, Davidson S, et al:
Glucose transporter glut-1 expression correlates with tumor hypoxia
and predicts metastasis-free survival in advanced carcinoma of the
cervix. Clin Cancer Res. 7:928–934. 2001.PubMed/NCBI
|
|
30
|
Loncaster JA, Cooper RA, Logue JP,
Davidson SE, Hunter RD and West CM: Vascular endothelial growth
factor (VEGF) expression is a prognostic factor for radiotherapy
outcome in advanced carcinoma of the cervix. Br J Cancer.
83:620–625. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng WF, Chen CA, Lee CN, Wei LH, Hsieh
FJ and Hsieh CY: Vascular endothelial growth factor and prognosis
of cervical carcinoma. Obstet Gynecol. 96:721–726. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alitalo A and Detmar M: Interaction of
tumor cells and lymphatic vessels in cancer progression. Oncogene.
31:4499–4508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hashimoto I, Kodama J, Seki N, Hongo A,
Yoshinouchi M, Okuda H and Kudo T: Vascular endothelial growth
factor-C expression and its relationship to pelvic lymph node
status in invasive cervical cancer. Br J Cancer. 85:93–97. 2001.
View Article : Google Scholar : PubMed/NCBI
|