Introduction
Neuroendocrine tumors (NETs) originate from cells
that produce peptide hormones and amines, and express common
neuroendocrine markers, including synaptophysin and chromogranin A.
According to the widely accepted concept of the diffuse
neuroendocrine system (1), NETs can
develop in any organ that has cells with such neuroendocrine
signatures. Recently, the incidence of NET has increased worldwide
(2,3).
The gastrointestinal (GI) tract is the major site of NET
development in humans; 56.3% of carcinoid tumors, a former name for
NETs, were found in the GI tract in a large-scale NET study
analyzing 11,842 patients (4). The
clinical course of NETs is diverse; certain NETs are slow growing,
like benign tumors, while others behave as aggressive cancers and
are associated with a poor prognosis. It was reported that distant
metastasis occurred in >70% of NETs when the primary tumor size
was >21 mm in diameter, and even 5.5% of NETs if the size was
<10 mm (4). Colorectal NETs of
>10 mm in diameter showed lymph node metastasis as frequently as
colorectal cancers of similar size. Furthermore, a similar trend in
prognosis was noted between colorectal NETs and colorectal cancer
with lymph node metastasis (5). These
findings strongly suggest that colorectal NETs have malignant
potential, prompting a reconsideration of the term ‘carcinoid’
(benign carcinoma), which was specified by Oberndorfer more than a
century ago (6).
In 2010, NETs, including gastroenteropancreatic
NETs, were reclassified into two groups, NET grade G1 and NET grade
G2, by the World Health Organization (WHO) (7), with classical carcinoid tumors also
classified as NETs. Using this classification, the malignant
potential of tumors can be defined by simple evaluation of the
Ki-67 positivity index and mitotic count in NET cells. This
evaluation is also used for grading rectal NETs, and, to a certain
degree, for prognostication (8).
However, it remains difficult to predict the malignant potential of
tumors prior to treatments, such as surgery and chemotherapy, by
using small biopsy samples, as the classification is based on
non-specific histopathological markers of cell proliferation. In
this context, novel NET-specific markers, such as cancer stem cell
(CSC) markers, are required to identify tumor cell origin and
behavior.
Doublecortin-like kinase 1 (DCLK1) is a
microtubule-associated protein. The function of this protein has
been assessed mainly in nerve and neuroblastoma cells, but is not
yet fully understood (9–12). Recent accumulating evidence suggests
that DCLK1 serves as a putative marker for intestinal and
pancreatic stem cells or CSCs, attracting much attention from
oncologists and gastroenterologists (13–17).
The present study investigates the expression of
DCLK1 in rectal NET tissue, and discusses its relevance to the
origin of NET cells and their malignant potential from the point of
view of CSCs.
Patients and methods
Patients and tumor tissues
A total of 18 patients with rectal NETs, also known
as carcinoid tumors, were enrolled in the present study. Informed
consent to participate in the study was obtained from each patient,
in accordance with the principles stated in the Declaration of
Helsinki and the guidelines of the Ethical Committee of Kurume
University (study registration no. 13149). Between 2003 and 2012,
the tumors were resected via endoscopic mucosal resection (EMR) at
the Kurume University Hospital (Kurume, Japan). In total, 9
patients were male and 9 were female. The mean age of the patients
was 51 years old (range, 31–74 years old). The mean longest
diameter of the tumors was 5.2 mm (range, 2–8 mm). Histological
diagnosis was made by at least two pathologists independently,
according to the WHO guidelines for NETs (7). All the resected tumors were diagnosed as
G1 grade NETs (Table I). Prior to EMR
treatment, no distant metastasis or lymph node metastasis was
detected in any of the cases.
 | Table I.Characteristics of the patients and
tumors. |
Table I.
Characteristics of the patients and
tumors.
| Characteristics | Value |
|---|
| Gender |
|
| Male | 9 |
|
Female | 9 |
| Mean age (range),
years | 51 (31–74) |
| Tumor grade, n |
|
| G1 | 18 |
| G2 | 0 |
| Longest tumor
diameter (range), mm | 5.4 (2.0–8.0) |
Immunohistochemical analysis
Expression of DCLK1 and the commonly used
neuroendocrine markers, synaptophysin, chromogranin A and CD56, was
assessed via immunohistochemical analysis. The intensity of
staining was scored on a scale of 0–3 as follows: 0, negative
staining; 1, weakly-positive staining; 2, moderately-positive
staining; and 3, strongly-positive staining. The signal-positive
area was scored on a scale of 0–2 as follows: 0, positive staining
in 0–20% of cells; 1, positive staining in 21–60% of cells; and 2,
positive staining in 61–100% of cells. The sum of the scores was
calculated for each specimen. A total score of ≥2 was judged as
positive expression (Table II).
 | Table II.Evaluation criteria for
immunostaining. |
Table II.
Evaluation criteria for
immunostaining.
|
| Score |
|---|
|
|
|
|---|
| Criteria | 0 | 1 | 2 | 3 |
|---|
| Signal intensity | Negative | Mild | Moderate | Strong |
| Positive area, % | 0–10 | 11–60 | 61–100 | - |
Paraffin-embedded NET tissue samples were cut to
prepare 4-µm slices that were mounted on silane-coated glass
slides. Immunohistochemical analysis was performed using the
primary antibodies listed in Table
III. For Ki-67 staining, the BenchMark ULTRA autostainer
(Ventana Automated Systems, Inc., Tucson, AZ, USA) was used.
Briefly, each slide was heat-treated using CC1 retrieval solution
(Ventana Automated Systems, Inc.) for 60 min, and incubated with
the antibodies for 30 min. This automated system used the
streptavidin-biotin complex method with 3,3′-diaminobenzidine (DAB)
as a chromogen (Ventana UltraVIEW DAB detection kit; Ventana
Automated Systems, Inc.). Immunostaining for chromogranin A,
synaptophysin and CD56 was performed using the similar
fully-automated Bond-Max system (Leica Microsystems, Newcastle,
UK), with onboard heat-induced antigen retrieval for 10 min, and
the Bond Polymer Refine Detection kit (Leica Microsystems). For
staining of DCLK1 and NANOG, antigen retrieval was performed by
autoclaving tissue sections in 10 mM citrate buffer (pH 6.0) at
120°C for 5 min. The sections were pre-blocked using Protein Block
Serum-Free (DakoCytomation, Glostrup, Denmark) and incubated with
primary antibodies. Subsequent to being washed, the sections were
incubated with EnVision secondary antibodies labeled with
horseradish peroxidase-polymer complexes (DakoCytomation) and
visualized with DAB. The cell nuclei were counterstained with
hematoxylin. Specimens incubated with rabbit IgG alone were used as
negative controls.
 | Table III.Characteristics of antibodies used in
this study. |
Table III.
Characteristics of antibodies used in
this study.
| Antigen | Clone/type | Dilution | Antigen
retrieval | Source |
|---|
| DCLK1 | EPR6085 | 1:700 | H | Epitomics,
Burlingame, CA, USA |
| Synaptophysin | Z66 | 1:1 | H | Invitrogen,
Frederick, MD, USA |
| Chromogranin A | DAK-A3 | 1:400 | H | DakoCytomation,
Glostrup, Denmark |
| CD56 | 1B6 | 1:200 | H | Leica Microsystems,
Newcastle, UK |
| Ki-67 | MIB-1 | 1:200 | H | DakoCytomation,
Glostrup, Denmark |
Statistical analysis
Statistical significance was assessed using the
Mann-Whitney U test, using StatView 5.0 J software (SAS Institute
Inc., Cary, NC, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of DCLK1 and known
neuroendocrine markers
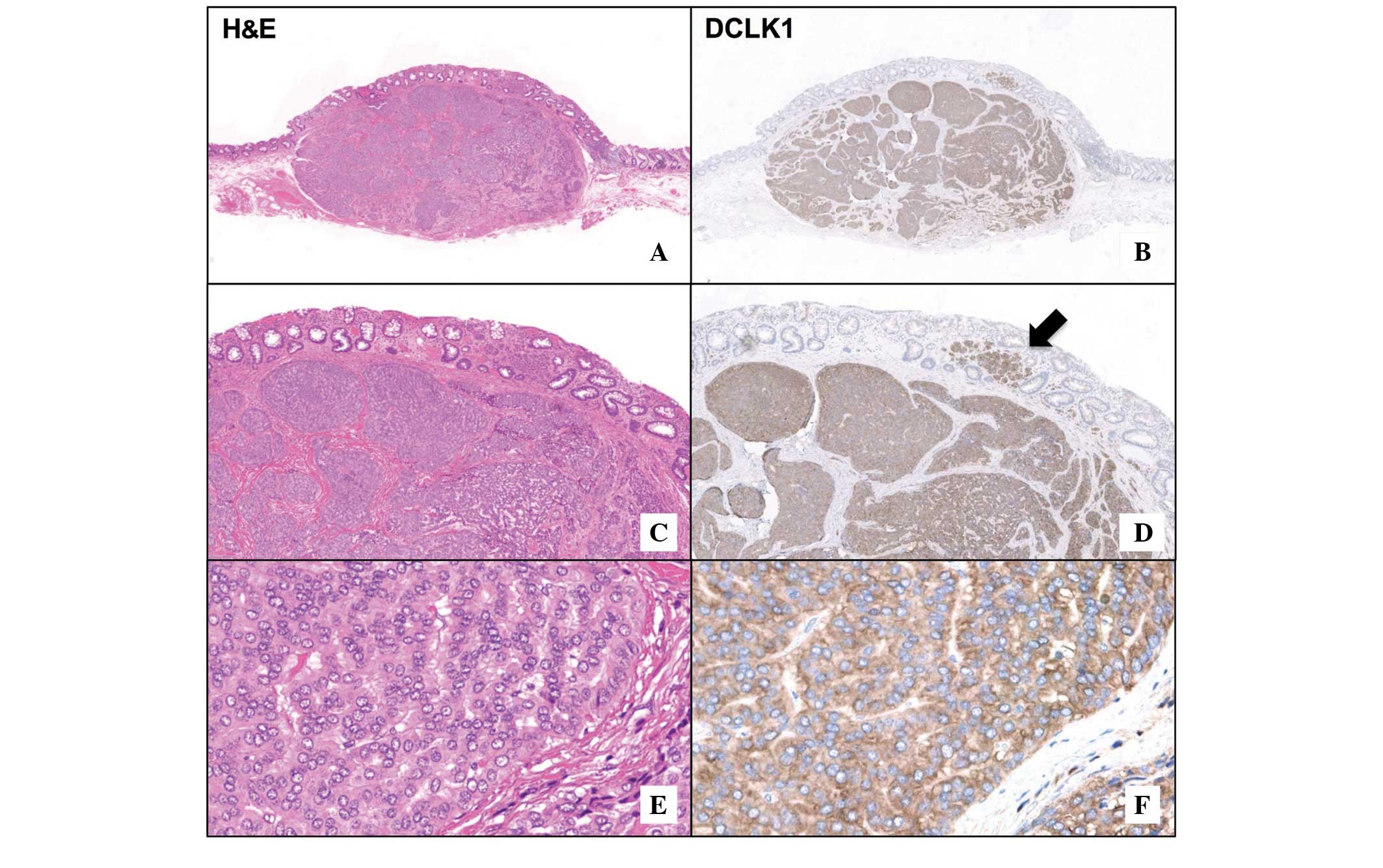
The expression of DCLK1 in rectal NET tissues is
demonstrated in Fig. 1. Diffuse
cytoplasmic expression of DCLK1 was observed in the tumor area
(Fig. 1B and F), while no positive
signal was visible in the surrounding non-tumorous tissue. The
invasive front of the tumor was clearly marked by the presence of
DCLK1-positive cells in the mucosal layer (Fig. 1D). Serial sections of NETs were
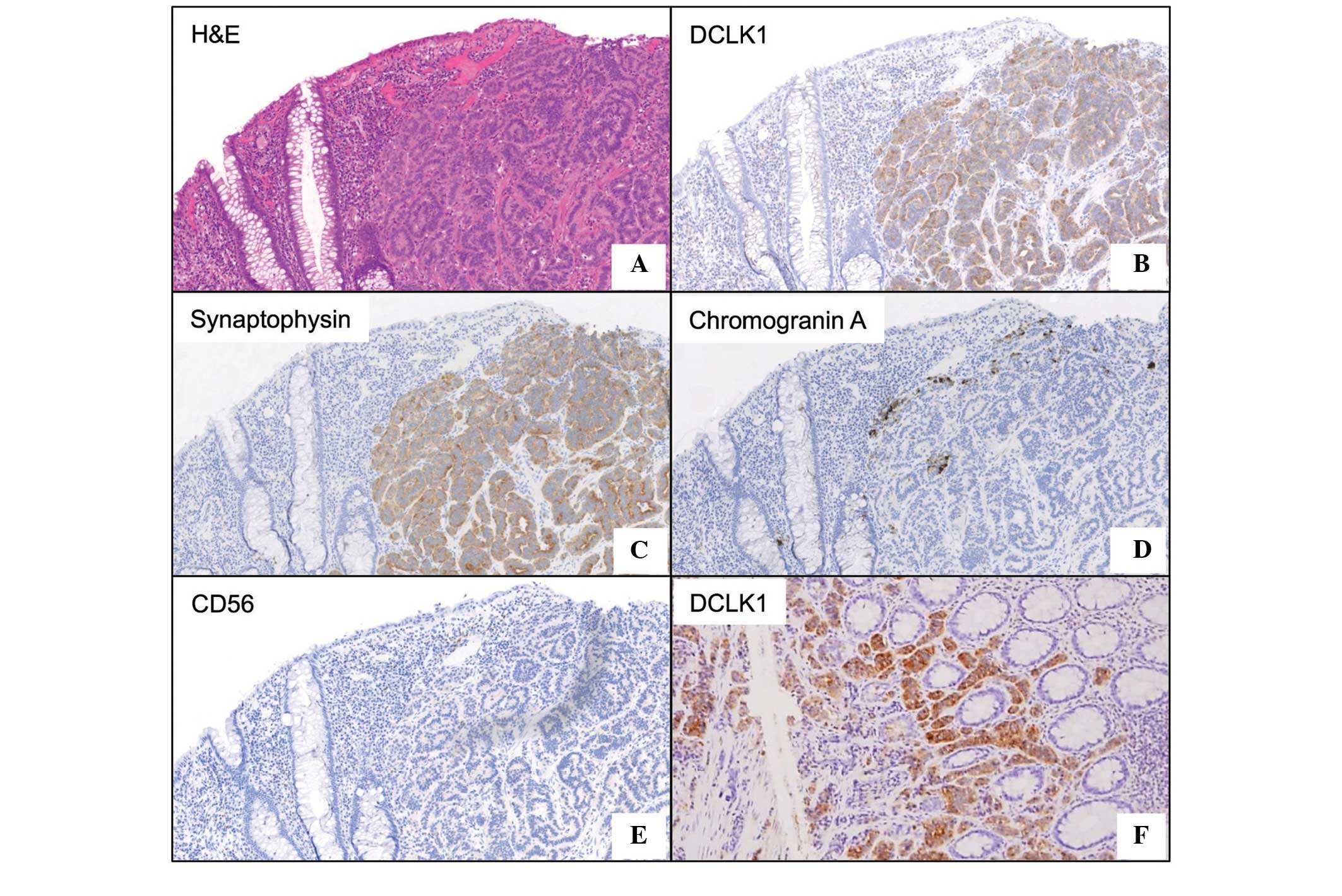
immunostained with the commonly used NET markers, synaptophysin,
chromogranin A and CD56 (Fig. 2).
Analysis of immunostaining scores
The immunostaining scores, including Ki-67 indices,
are summarized in Table IV. DCLK1
protein expression was observed in all the specimens, and the mean
± standard error of the total scores for DCLK1 was 4.83±0.12, which
was equivalent to that of synaptophysin (4.78±0.15). These two
scores were significantly higher than that of chromogranin A
(1.89±0.54; P<0.0001) and CD56 (3.33±0.42; P<0.01) (Fig. 3). There were no significant
differences in the scores between DCLK1 and synaptophysin, or
between chromogranin A and CD56.
 | Table IV.Immunoreactivity scores for DCLK1 and
known neuroendocrine markers. |
Table IV.
Immunoreactivity scores for DCLK1 and
known neuroendocrine markers.
| Case no. | Age, years | Gender | Tumor size, mm | Ki-67 index | DCLK1 | Synaptophysin | Chromogranin A | CD56 |
|---|
| 1 | 63 | F | 8×8 | <2% | 5 | 5 | 0 | 5 |
| 2 | 51 | M | 3×3 | <2% | 5 | 5 | 5 | 5 |
| 3 | 65 | F | 8×8 | <2% | 3 | 5 | 5 | 5 |
| 4 | 62 | F | 6×6 | <2% | 5 | 5 | 0 | 4 |
| 5 | 50 | F | 5×4 | <2% | 5 | 5 | 5 | 3 |
| 6 | 39 | F | 5×5 | <2% | 5 | 5 | 0 | 3 |
| 7 | 73 | M | 7×6 | <2% | 5 | 5 | 0 | 0 |
| 8 | 55 | F | 3×2 | <2% | 5 | 5 | 0 | 3 |
| 9 | 46 | F | 6×6 | <2% | 5 | 5 | 0 | 5 |
| 10 | 31 | F | 5×4 | <2% | 5 | 5 | 0 | 3 |
| 11 | 46 | M | 7×6 | <2% | 5 | 5 | 0 | 3 |
| 12 | 56 | M | 5×4 | <2% | 5 | 5 | 5 | 5 |
| 13 | 33 | M | 5×5 | <2% | 5 | 5 | 3 | 3 |
| 14 | 60 | M | 3×2 | <2% | 5 | 5 | 0 | 3 |
| 15 | 34 | F | 4×3 | <2% | 5 | 3 | 3 | 0 |
| 16 | 41 | M | 6×6 | <2% | 5 | 3 | 3 | 5 |
| 17 | 74 | M | 2×2 | <2% | 5 | 5 | 5 | 0 |
| 18 | 30 | M | 6×6 | <2% | 4 | 5 | 0 | 5 |
Expression of NANOG
The NANOG stem cell marker was widely expressed in
all NET tissues studied (Fig. 4).
Nuclear and cytoplasmic expression was observed (Fig. 4), and staining was particularly
predominant at the basal side of trabecular-like structures in the
tumor.
Discussion
In the present study, the strong expression of DCLK1
in rectal NETs was demonstrated. DCLK1 has two N-terminal
doublecortin domains that bind microtubules and regulate their
polymerization (18). The C-terminal
serine/threonine protein kinase domain, which has substantial
homology to the Ca2+/calmodulin-dependent protein
kinase, regulates a calcium-signaling pathway, thereby controlling
neurogenesis, neuronal migration and apoptosis in the developing
brain (9,10). This functional protein is also
abundantly expressed in neuronal neoplasms, including
neuroblastoma, and confers drug resistance to neoplastic cells
(19). On the basis of these
findings, DCLK1 was believed to play a fundamental role in tumor
cells, including NET cells, where neuroendocrine markers are
positive-for promoting cell migration, proliferation and
tumorigenesis. Several previous studies have underlined the crucial
involvement of DCLK1 in NET cell behavior. In animal models with
xenografted colon and pancreatic tumors, the silencing of the
Dclk1 gene resulted in a decrease in tumor size (15,17,20).
Although the precise tumor-promoting mechanism of DCLK1 has yet to
be fully elucidated, the DCLK1-mediated downregulation of the
expression of tumor-suppressing microRNAs (miR), such as miR-145,
miR-200 and let-7a, has been demonstrated to be a possible
mechanism (17,20). Another proposed mechanism involves the
DCLK1-dependent induction of vascular endothelial growth factor
receptor and epithelial-mesenchymal transition (EMT)-related
factors in tumor cells (17,20). These molecules, involved in the
inhibition of tumor suppressors, angiogenesis and EMT, may
contribute to the aggressive characteristics of not only cancer
cells, but also NET cells.
The present study also investigated the diagnostic
value of DCLK1 in NETs. NETs are conventionally diagnosed using
hematoxylin-eosin staining and immunostaining for chromogranin A,
synaptophysin and CD56. The results showed 100% positivity for
synaptophysin, and 83.3 and 44.4% positivity for CD56 and
chromogranin A, respectively. These findings are consistent with
the findings of a previous study of 114 cases of NETs, which
demonstrated 97.4% positivity for synaptophysin, 75.4% for CD56,
and 43.0% for chromogranin A (21).
Similar to the results for synaptophysin, DCLK1 positivity was also
observed in 100% of the tumors in the present study, suggesting its
diagnostic value in NETs. It was also noteworthy that DCLK1
staining could detect NET cells in a small rectal polypoid biopsy
sample with high sensitivity. The use of DCLK1 immunostaining may
extend diagnostic options prior to the treatment of NETs.
CSCs exhibit EMT, which is partly regulated by
DCLK1. Recent evidence suggested that the CSC-related stemness gene
products, including CDX2, OCT4 and SOX2, were expressed in NETs
(22,23). Due to this, the present study assessed
whether the expression of DCLK1 correlated with that of NANOG,
another marker of stem cells and CSCs. NANOG was found to be highly
expressed in the rectal NET tissues, and its distribution of
expression overlapped with that of DCLK1. This finding provided
insights into the CSC-like nature of NET cells, and it supports
previous findings that the knockdown of DCLK1 expression completely
blocked the expression of the stemness markers, NANOG, KLF4, OCT4
and SOX2, in human pancreatic cancer cells (20). Although further studies are required,
we speculate that the CSC marker, DCLK1, along with stemness gene
products, is also involved in the development of NETs, possibly
owing to interactions among them under specific conditions. From a
therapeutic point of view, targeting DCLK1 using emerging small
molecules may open novel avenues for the treatment of cancer,
including NETs, in various organs (24,25).
The histological grade of all resected NETs in the
present study was G1, possibly as the tumors were small enough to
be treatable via EMR. Despite the early developmental stage of the
tumors, DCLK1 and NANOG expression was strong and widely
distributed throughout. This point is important when considering
the origin and malignant potential of NETs. It is known that tuft
cells in intestinal crypts are DCLK1-positive and quiescent, and
have stem cell-like characteristics (26). Therefore, it is speculated that
DCLK1-positive tuft lineage cells are multipotent, or at least
bipotent, ectopically transforming into NET cells and
orthotopically into intestinal cancer cells, under specific
microenvironmental conditions. Recently, tuft cells have been shown
to become powerful colon cancer-initiating cells owing to
inflammatory insult (27). Therefore,
intestinal inflammation may affect the fate of tuft cell
transformation.
This study provides original findings and speculates
on novel concepts regarding the expression of the CSC and/or tuft
cell marker, DCLK1, in NETs. However, as the number of tumor
samples and the tumor grade were limited, further large-scale
studies are required to determine the role of DCLK1 in NETs more
precisely.
References
|
1
|
Pearse AG: The diffuse neuroendocrine
system and the apud concept: Related ‘endocrine’ peptides in brain,
intestine, pituitary, placenta, and anuran cutaneous glands. Med
Biol. 55:115–125. 1977.PubMed/NCBI
|
|
2
|
Ito T, Igarashi H, Nakamura K, Sasano H,
Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT, et
al: Epidemiological trends of pancreatic and gastrointestinal
neuroendocrine tumors in Japan: A nationwide survey analysis. J
Gastroenterol. 50:58–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsikitis VL, Wertheim BC and Guerrero MA:
Trends of incidence and survival of gastrointestinal neuroendocrine
tumors in the United States: A seer analysis. J Cancer. 3:292–302.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soga J: Carcinoids and their variant
endocrinomas. An analysis of 11842 reported cases. J Exp Clin
Cancer Res. 22:517–530. 2003.PubMed/NCBI
|
|
5
|
Konishi T, Watanabe T, Kishimoto J, Kotake
K, Muto T and Nagawa H: Japanese Society for Cancer of the Colon
and Rectum: Prognosis and risk factors of metastasis in colorectal
carcinoids: Results of a nationwide registry over 15-years. Gut.
56:863–868. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oberndorfer S: Karzinoide Tumoren des
Dünndarms. Frankf Z Pathol. 1:426–432. 1907.
|
|
7
|
Rindi G, Arnold R, Bosman FT, et al:
Nomenclature and classification of neuroendocrine neoplasms of
digestive systemWHO Classification of Tumours of the Digestive
System. Bosman FT, Carneiro F, Hruban R and Theise ND: 4th. IARC;
Lyon, France: pp. 13–14. 2010
|
|
8
|
Jann H, Roll S, Couvelard A, Hentic O,
Pavel M, Müller-Nordhorn J, Koch M, Röcken C, Rindi G, Ruszniewski
P, et al: Neuroendocrine tumors of midgut and hindgut origin:
Tumor-node-metastasis classification determines clinical outcome.
Cancer. 117:3332–3341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shu T, Tseng HC, Sapir T, Stern P, Zhou Y,
Sanada K, Fischer A, Coquelle FM, Reiner O and Tsai LH:
Doublecortin-like kinase controls neurogenesis by regulating
mitotic spindles and M phase progression. Neuron. 49:25–39. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koizumi H, Tanaka T and Gleeson JG:
Doublecortin-like kinase functions with doublecortin to mediate
fiber tract decussation and neuronal migration. Neuron. 49:55–66.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koizumi H, Higginbotham H, Poon T, Tanaka
T, Brinkman BC and Gleeson JG: Doublecortin maintains bipolar shape
and nuclear translocation during migration in the adult forebrain.
Nat Neurosci. 9:779–786. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verissimo CS, Molenaar JJ, Meerman J,
Puigvert JC, Lamers F, Koster J, Danen EH, van de Water B, Versteeg
R, Fitzsimons CP, et al: Silencing of the microtubule-associated
proteins doublecortin-like and doublecortin-like kinase-long
induces apoptosis in neuroblastoma cells. Endocr Relat Cancer.
17:399–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giannakis M, Stappenbeck TS, Mills JC,
Leip DG, Lovett M, Clifton SW, Ippolito JE, Glasscock JI, Arumugam
M, Brent MR, et al: Molecular properties of adult mouse gastric and
intestinal epithelial progenitors in their niches. J Biol Chem.
281:11292–11300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
May R, Sureban SM, Lightfoot SA, Hoskins
AB, Brackett DJ, Postier RG, Ramanujam R, Rao CV, Wyche JH, Anant
S, et al: Identification of a novel putative pancreatic
stem/progenitor cell marker DCAMKL-1 in normal mouse pancreas. Am J
Physiol Gastrointest Liver Physiol. 299:G303–G310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakanishi Y, Seno H, Fukuoka A, Ueo T,
Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, et
al: Dclk1 distinguishes between tumor and normal stem cells in the
intestine. Nat Genet. 45:98–103. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bailey JM, Alsina J, Rasheed ZA,
McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N,
Matsui W, et al: DCLK1 marks a morphologically distinct
subpopulation of cells with stem cell properties in preinvasive
pancreatic cancer. Gastroenterology. 146:245–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sureban SM, May R, Ramalingam S,
Subramaniam D, Natarajan G, Anant S and Houchen CW: Selective
blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a
MicroRNA-dependent mechanism. Gastroenterology. 137:649–659. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reiner O, Coquelle FM, Peter B, Levy T,
Kaplan A, Sapir T, Orr I, Barkai N, Eichele G and Bergmann S: The
evolving doublecortin (DCX) superfamily. BMC Genomics. 7:1882006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verissimo CS, Cheng S, Puigvert JC, Qin Y,
Vroon A, van Deutekom J, Price LS, Danen EH, van de Water B,
Fitzsimons CP, et al: Combining doublecortin-like kinase silencing
and vinca alkaloids results in a synergistic apoptotic effect in
neuroblastoma cells. J Pharmacol Exp Ther. 342:119–130. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sureban SM, May R, Qu D, Weygant N,
Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier
RG, et al: DCLK1 regulates pluripotency and angiogenic factors via
microRNA-dependent mechanisms in pancreatic cancer. PLoS One.
8:e739402013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao W, Liu SM, Lu HZ, Liang J, Yuan YL and
Liu XY: Analysis of clinicopathological features of intestinal
neuroendocrine neoplasms. Zhonghua Zhong Liu Za Zhi. 34:450–456.
2012.(In Chinese). PubMed/NCBI
|
|
22
|
Heverhagen AE, Geis C, Fendrich V,
Ramaswamy A, Montalbano R, Di Fazio P, Bartsch DK, Ocker M and
Quint K: Embryonic transcription factors CDX2 and Oct4 are
overexpressed in neuroendocrine tumors of the ileum: A pilot study.
Eur Surg Res. 51:14–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sholl LM, Long KB and Hornick JL: Sox2
expression in pulmonary non-small cell and neuroendocrine
carcinomas. Appl Immunohistochem Mol Morphol. 18:55–61. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weygant N, Qu D, Berry WL, May R,
Chandrakesan P, Owen DB, Sureban SM, Ali N, Janknecht R and Houchen
CW: Small molecule kinase inhibitor LRRK2-IN-1 demonstrates potent
activity against colorectal and pancreatic cancer through
inhibition of doublecortin-like kinase 1. Mol Cancer. 13:1032014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sureban SM, May R, Weygant N, Qu D,
Chandrakesan P, BannermanMenson E, Ali N, Pantazis P, Westphalen
CB, Wang TC, et al: XMD8-92 inhibits pancreatic tumor xenograft
growth via a DCLK1-dependent mechanism. Cancer Lett. 351:151–161.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
May R, Qu D, Weygant N, Chandrakesan P,
Ali N, Lightfoot SA, Li L, Sureban SM and Houchen CW: Brief report:
Dclk1 deletion in tuft cells results in impaired epithelial repair
after radiation injury. Stem Cells. 32:822–827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Westphalen CB, Asfaha S, Hayakawa Y,
Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H,
Muley A, et al: Long-lived intestinal tuft cells serve as colon
cancer-initiating cells. J Clin Invest. 124:1283–1295. 2014.
View Article : Google Scholar : PubMed/NCBI
|