Introduction
Gastric carcinoma (GC) is a common type of cancer
with an increasing incidence of malignancy in developing countries.
More cases are diagnosed in China each year compared with other
countries (1), and it is the second
most common type of cancer-associated mortality in China at present
(2). Different survival rates of
patients with the same tumor node metastasis (TNM) scores have been
observed in clinical observation. Therefore, the staging system of
the American Joint Committee on Cancer may not be sufficient to
predict clinical outcomes, as it does not consistently distinguish
which patients may have a poor prognosis within the same stage.
Increasing numbers of biomarkers have been reported that are
associated with different types of cancer (3). The discovery and understanding of
tumor-associated biomarkers may aid in improving the diagnosis of
GC and the efficacy of treatments. In addition, this information
may be used to select the most appropriate therapy, which is
particularly important for patients with early-stage GC.
T-box transcription factors (TBXs) are a conserved
gene family that are required for the embryonic development of the
heart and forelimbs. TBX5 is critical for forelimb development and
cardiogenesis (4,5) and is associated with Holt-Oram syndrome
(HOS) (6,7). Yu et al (8) reported that TBX5 may be a potential
tumor suppressor gene in colon cancer. However, Rosenbluh et
al (9) indicated that a
β-catenin/yes-associated protein 1 (YAP1)/TBX5 complex was required
for the survival of cancer cells, particularly for the initiation
and progression of colon cancer. However, the prognostic, clinical
and pathological significance of TBX5 in human GC has not yet been
identified. In the present study, the mRNA level of TBX5 was
evaluated by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) in 60 pairs of surgically resected GC and
healthy gastric tissues. Data from a large cohort of patients with
GC were used to evaluate the prognostic and clinicopathological
value of TBX5 expression by immunohistochemistry.
Materials and methods
Ethics statement
The study was officially approved by the Ethics
Committee of Sun Yat-sen University Cancer Center (Guangdong,
China). Written informed consent from the patients/patient's
families were obtained.
Patients
A total of 161 consecutive patients with
histologically diagnosed stage I and II GC that underwent surgery
between January 2003 and December 2006 were retrospectively
evaluated and the paraffin-embedded samples were obtained. A total
of 60 self-pairs fresh frozen tissue samples were obtained between
June 2011 and January 2012 from the tumor tissue bank for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis. Patients who possessed a second primary tumor, previous
malignant disease, died of postoperative complications or received
neoadjuvant/adjuvant treatments, were excluded. The surgical
procedures were performed by experienced surgeons using the
Japanese Gastric Cancer Association guidelines (10).
Tissue specimens
A total of 30 self-pairs each of early-stage (stage
I and II; collected between June 2011 and January 2012) and a total
of 30 self-pairs each of late-stage (stage III and IV; collected
between October 2011 and April 2012) gastric adenocarcinoma
specimens and adjacent non-cancerous tissues were snap-frozen and
stored at −80°C following surgery. The paraffin-embedded samples
for immunohistochemisrty (IHC) were obtained from a total of 161
consecutive patients with histologically diagnosed stage I and II
GC that underwent surgery between January 2003 and December 2006.
The patients were previously untreated with no distant metastasis
and had histologically proven GC of different stages.
Extraction of total RNA and
RT-qPCR
The total RNA was extracted using TRIzol solution
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions. DNA contamination was eliminated by
using RNAse-free DNAase. RT-qPCR was performed using the Maxima
First Strand cDNA Synthesis Kit for RT-qPCR (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). For the reverse transcription
(RT) reaction, 2 µg total RNA was used to synthesize first strand
cDNA. After that the cDNA was used as template for RT-qPCR
detection, which was performed using the SYBR Green PCR Master Mix
(Invitrogen Life Technologies, Carlsbad, CA, USA). For the
evaluation of the association between the GAPDH (internal control)
and TBX5, the primer sequences were as follows: TBX5, F
5′-TCCACCCAACCCATACCC-3′ and R 5′-GCTGTGCCGACTCTGTCCTGT-3′; GAPDH,
F 5′-CTCCTCCTGTTCGACAGTCAGC-3′ and R 5′-CCCAATACGACCAAATCCGTT-3′. A
RT-qPCR machine (ABI 7900HT; Applied Biosystems Life Technologies,
Foster City, CA, USA) that measured the binding of SYBR Green I to
double-stranded DNA was used to perform gene-specific
amplification. The cycling conditions were as follows: initial step
at 95°C for 10 min, then 45 cycles of 95°C for 30 sec and at last
60°C for 60 sec. The instrument's software (SDS 2.0; Applied
Biosystems Life Technologies) was used to calculate the amplicated
sample's relative quantity.
Immunohistochemistry
Paraffin-embedded sections (2-µm thick) were put
into the graded ethanol washes (through 100, 95, 90, 80 and 70%
ethanol) to deparaffinize and rehydrate the samples. Antigen
retrieval was then performed as follows: The slides were boiled in
EDTA (1 mM; pH 8.0) for 15 min in a microwave oven. The sections
were placed into 0.3% hydrogen peroxide solution for 10 min at room
temperature. Next, the sections were washed with PBS and incubated
overnight at 4°C with a 1:600 dilution of rabbit anti-human TBX5
polyclonal IgG antibody (LifeSpan Biosciences, Inc., Seattle, WA,
USA). Following 3 washes with PBS, the secondary antibody was
applied for 30 min at room temperature. Subsequently, the slides
were developed with 3-diaminobenzidine tetrahydrochloride (Tianjin
Fuyu Fine Chemical Co., Ltd., Tianjin, China). The sections were
counterstained with 20% hematoxylin (Shanghai Huntz Enterprises,
Inc., Shanghai, China) and then the slides were dehydrated and
cleared.
Semi-quantitative methods
For immunohistochemical analysis, TBX5 expression
was evaluated according to the percentage of positively stained
cells. The scores of staining intensity were defined as ‘3’
(strongly stained; strikingly positive at low magnification); ‘2’
(moderately stained; visible at low magnification); ‘1’ (weakly
stained; visible at high magnification); or ‘0’ (no staining). The
positive percentage score was as follows: ‘3’ (>50%, diffuse);
‘2’ (25–50%, focal); ‘1’ (5–25%, sporadic); or ‘0’ (<5%,
negative). Positive percentage score × staining intensity score =
total TBX5 score. A total score of ≥4 was defined as high
expression and <4 as low expression. Three investigators (Dr Yan
Zheng, Dr Dan-Dan Wang and Dr Wei Wang) who were blind to the
clinical outcomes independently evaluated TBX5 staining under a
light microscope (Nikon Ecli, PSE 80i; Nikon Corporation, Tokyo,
Japan). The results between the observers differed in ≤15% of the
examined slides.
Follow-up
The surveillance studies following pulmonary
resection included clinical and laboratory examinations every 3
months for the first 2 years, every 6 months for the next 2 years,
and every 12 months thereafter until the patients were lost in
follow-up (the patient could not be contacted) or patient
mortality. The overall survival (OS) was used as a measure of
prognosis, which was defined as the time from the surgery to
mortality or the final follow-up.
Statistical analysis
All statistical analyses were performed with SPSS
software, version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). A
Wilcoxon matched- pairs signed-rank test was used to compare the
TBX5 protein levels in the tumor tissue and the adjacent normal
tissue samples. The correlation between TBX5 and the
clinicopathological characteristics were assessed using the
χ2 test. Survival curves were plotted by the
Kaplan-Meier method with the log-rank test. P≤0.05 was considered
to indicate a statistically significant difference.
Results
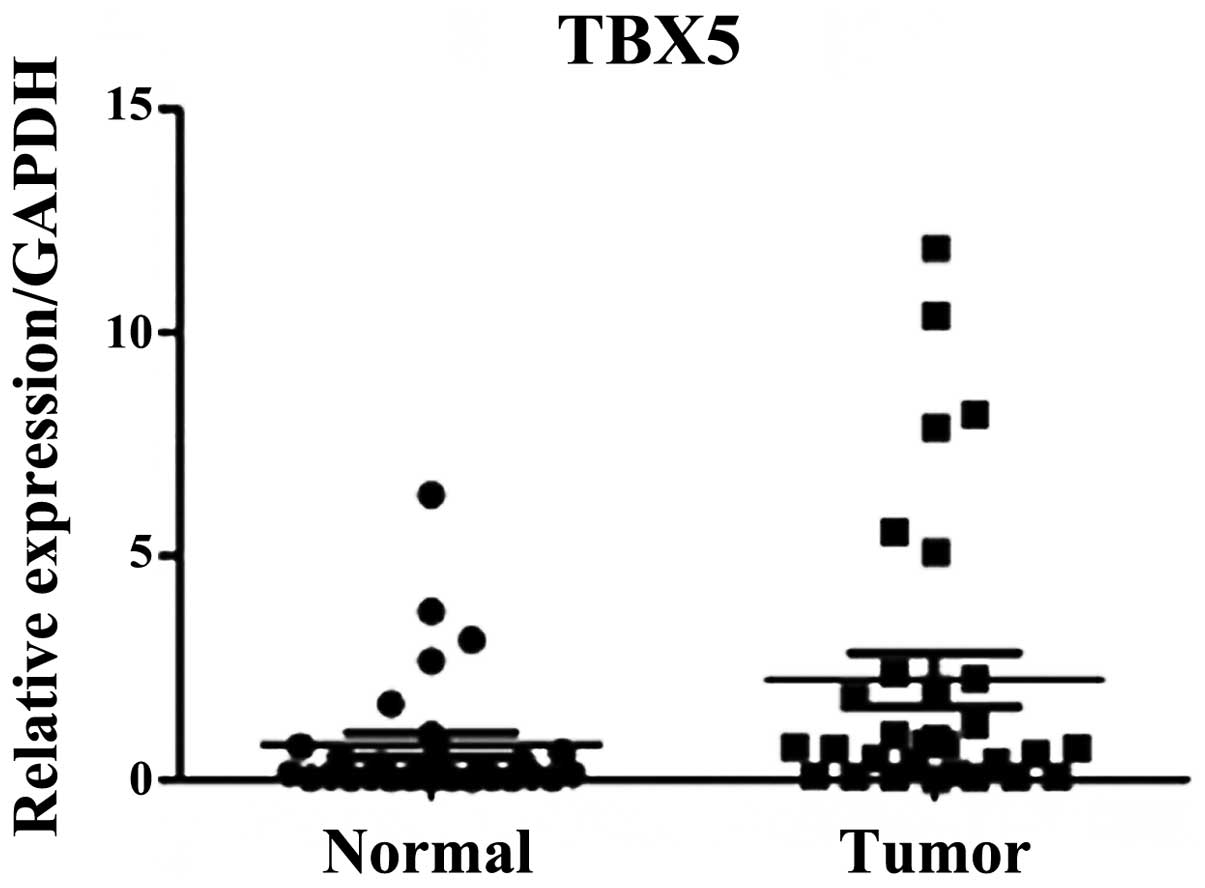
RT-qPCR analysis
RT-qPCR was performed on 60 pairs of surgical
specimens (tumor and adjacent non-tumor tissue samples) to examine
the mRNA expression levels of TBX5. A significant difference was
identified between the stage I and II tumor and paired non-tumor
tissue samples (P=0.01; Fig. 1).
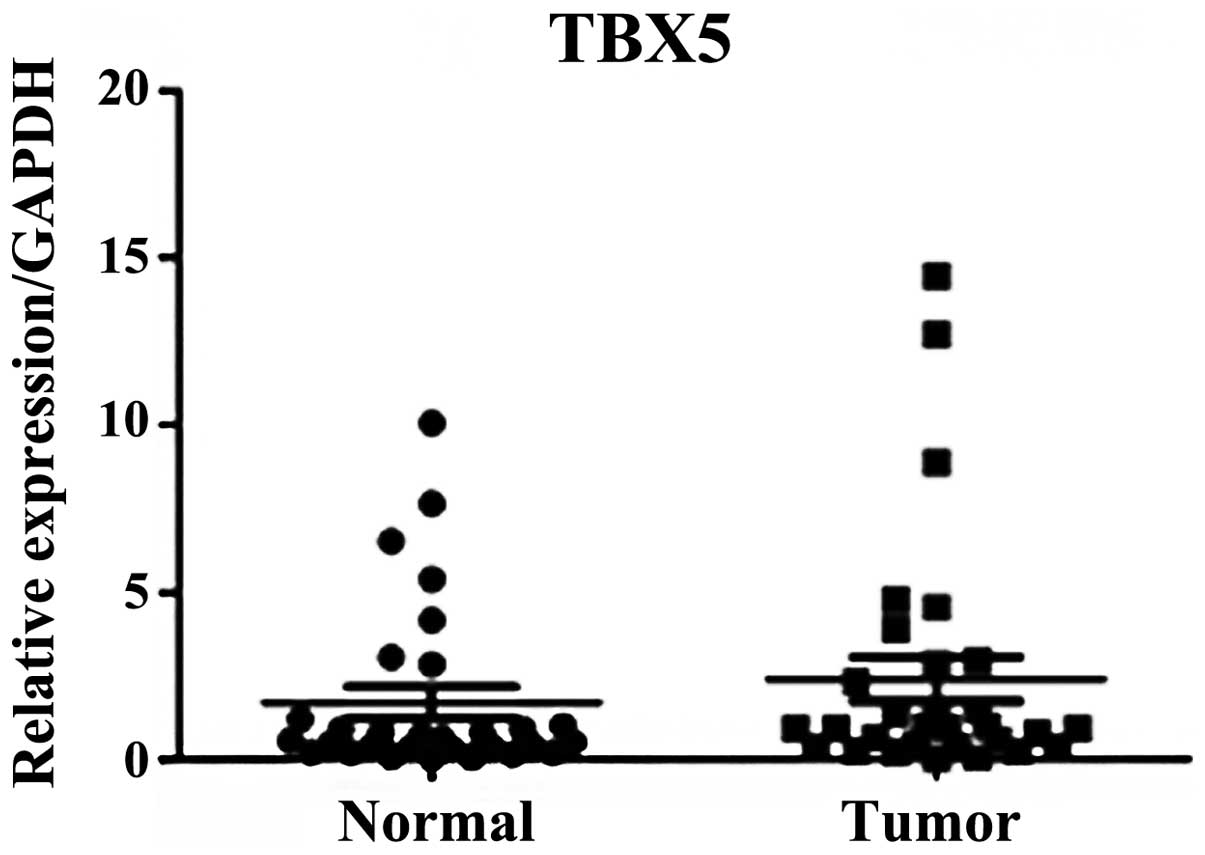
However, no significant difference was observed in TBX5 mRNA
expression levels in the stage III and IV GC samples compared with
the adjacent normal tissues (P=0.318; Fig. 2).
Immunohistochemical analysis and
clinicopathological characteristics
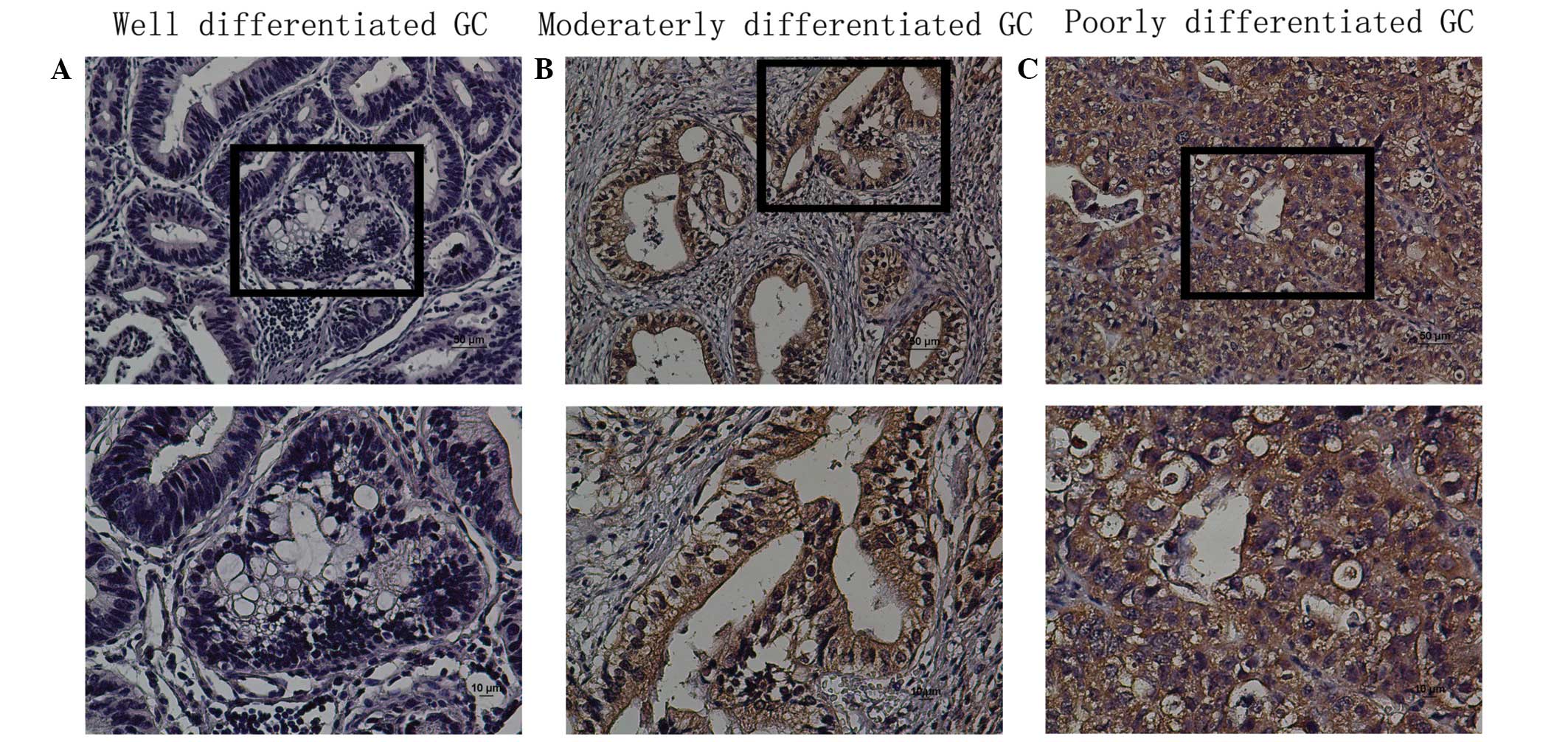
The protein expression levels of TBX5 in situ
were evaluated by immunohistochemical analysis of paraffin-embedded
GC tissue blocks (n=161). TBX5 was expressed in a nuclear and
cytoplasmic pattern in tissues, and TBX5 protein expression was
observed in the tumor tissue (Fig.
3). The expression of TBX5 was high in poor-differentiated
group and low in well-differentiated group. TBX5 expression was
‘low’ in 76/161 (47.2%) and ‘high’ in 85/161 (52.8%) as assessed
using the criteria mentioned above. No correlations between the
clinicopathological variables and TBX5 expression were observed
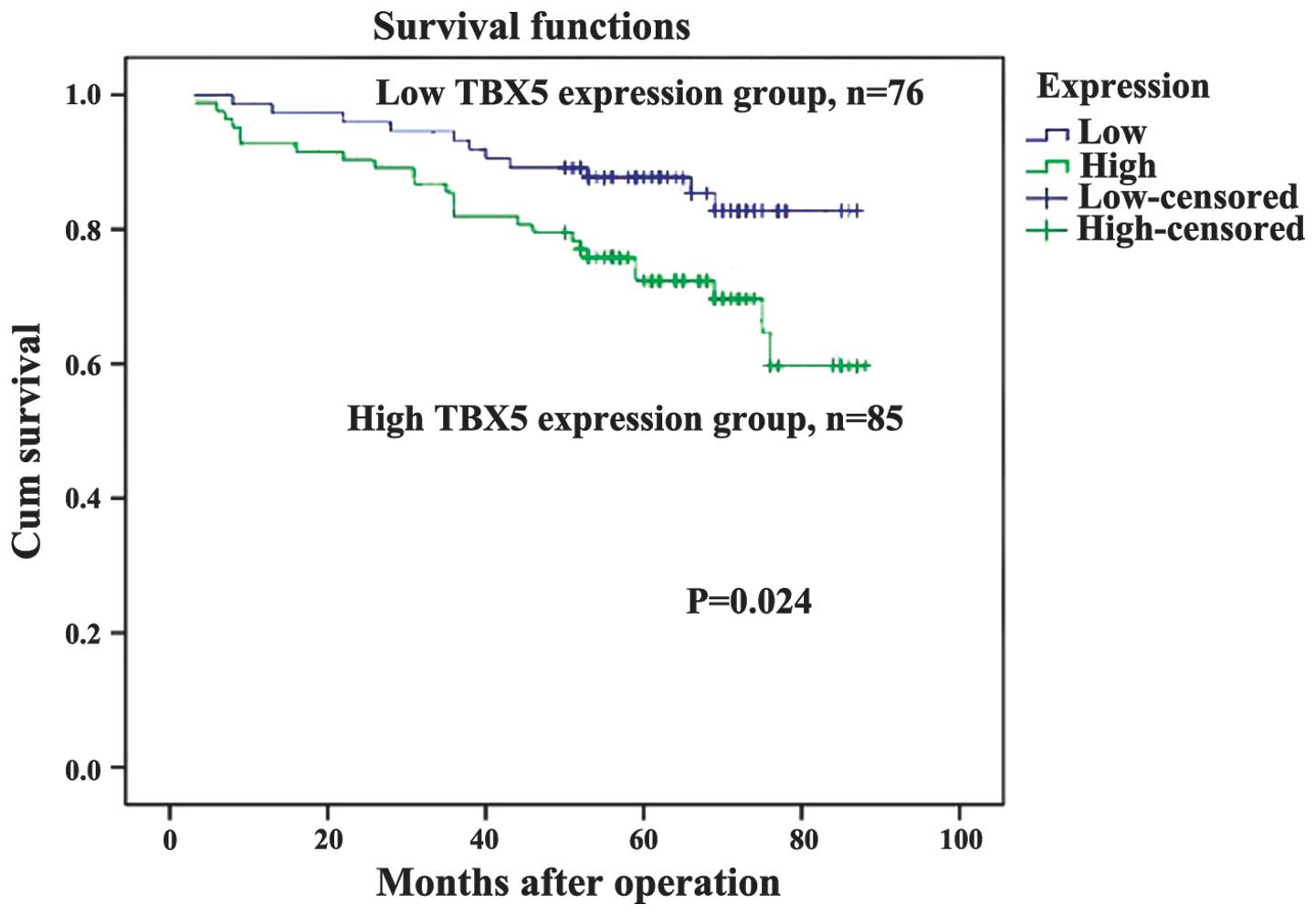
(Table I). As demonstrated in the
Kaplan-Meier survival curves, TBX5 expression may be used to
predict the OS of stage I and II GC (P=0.024, Fig. 4). The expression of TBX5 was
demonstrated to be a significant prognostic factor for patients
with GC following univariate analysis (P=0.028; Table II). In addition, TBX5 expression was
identified as an independent prognostic factor in the multivariate
Cox proportional hazards model analysis (P=0.017; Table II).
 | Table I.The expression of TBX5 and the
clinicopathologic characteristics of patients with gastric cancer
stage I and II. |
Table I.
The expression of TBX5 and the
clinicopathologic characteristics of patients with gastric cancer
stage I and II.
| Characteristics
(n) | Low TBX5 expression
(n) | High TBX5 expression
(n) | χ2 | P-value |
|---|
| Gender |
|
| 0.117 | 0.740 |
| Male
(108) | 52 | 56 |
|
|
| Female
(53) | 24 | 29 |
|
|
| Location |
|
| 1.565 | 0.687 |
| Fundus of
stomach (68) | 31 | 37 |
|
|
| Proximal
(25) | 14 | 11 |
|
|
| Distant
(65) | 29 | 36 |
|
|
| Total
(3) | 2 | 1 |
|
|
| Tumor invasion
(T) |
|
| 0.071 | 0.968 |
| T1
(33) | 15 | 18 |
|
|
| T2
(31) | 15 | 16 |
|
|
| T3
(46) | 21 | 25 |
|
|
| T4a
(51) | 25 | 26 |
|
|
| Nodal status (N) |
|
| 2.028 | 0.363 |
| N0
(124) | 55 | 69 |
|
|
| N1
(31) | 17 | 14 |
|
|
| N2
(6) | 4 | 2 |
|
|
| TNM staging,
7thed. |
|
| 0.15 | 0.698 |
| Stage I
(49) | 22 | 27 |
|
|
| Stage II
(112) | 54 | 58 |
|
|
 | Table II.Univariate and multivariate analyses
of overall survival in 161 patients with stage I and II gastric
cancer. |
Table II.
Univariate and multivariate analyses
of overall survival in 161 patients with stage I and II gastric
cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.016 | 0.986–1.046 | 0.295 |
|
|
|
| Gendera | 1.363 | 0.697–2.666 | 0.365 |
|
|
|
|
Locationb | 0.57 | 0.385–0.846 | 0.005e | 0.65 | 0.435–0.97 | 0.035e |
| TNMc | 5.623 | 1.724–18.339 | 0.004e | 4.699 | 1.417–15.585 | 0.011e |
| TBX5d | 2.213 | 1.088–4.501 | 0.028e | 2.378 | 1.168–4.844 | 0.017e |
Discussion
The aim of the present study was to observe the
expression of TBX5 in primary GC samples, in addition to
identifying its potential clinical relevance.
T-box (TBX) transcription factors belong to a
conserved gene family with critical roles in organogenesis and
embryogenesis (11). TBX5 is a member
of the T-box family and is essential for the embryonic development
of the forelimbs and heart (4,5). HOS is
caused by mutations in TBX5 (12). In
a previous study by Rosenbluh et al (9), it was demonstrated that TBX5 forms a
complex with β-catenin and YAP1, which is essential for the process
of tumorigenesis in colorectal cancer. Numerous previous studies
have reported that β-catenin may be associated with GC (13,14).
Therefore, additional studies are required to investigate the
potential association between TBX5 expression and
clinicopathological features and survival data in GC. The present
study evaluated the expression of TBX5 in GC patients who received
uniform treatment and determined its clinicopathological
significance by correlating this data with the characteristics of
the patients and long-term follow-up information. The findings of
the present study indicated that TBX5 may be a useful biomarker to
identify patients with stage I and II GC who may have unfavorable
survival rates.
In the present study, RT-qPCR analysis was used to
determine that the mRNA level of TBX5 was reduced in normal
paracancerous tissues compared with stage I and II GC tumor tissues
(P<0.01). However, no significant difference was demonstrated in
TBX5 mRNA expression levels in tissue samples from patients with
stage III and IV GC compared with normal tissues. These results
indicated that TBX5 expression may be involved in the progression
of stage I and II GC. Immunohistochemical analysis demonstrated
that high expression of TBX5 was detected in 52.8% (n=161) of the
GCs. The clinical and pathological significance of TBX5 expression
in GC was systematically evaluated; however, no significant
correlation was observed between disease characteristics and the
level of TBX5 expression. Since the present study was a single
institute retrospective analysis, further studies are required to
evaluate the potential association between TBX5 expression and
clinicopathological features in other populations.
Kaplan-Meier survival analysis demonstrated that the
TBX5 expression level was a significant and independent predictive
factor in cases of surgically resected stage I and II GC. High TBX5
expression was observed in patients with significantly shorter
median OS, compared with patients with low expression of TBX5.
Rosenbluh et al (9)
demonstrated that the YAP1/β-catenin/TBX5 complex is localized to
the Bcl-2-like protein 1 and baculoviral IAP repeat containing 5
promoters (15). This is in
accordance with another previous study that demonstrated that TBX5
forms a complex and induces transcription of atrial natriuretic
factor (16). The transcriptional
factors were observed to regulate developmental and
cancer-associated phenotypes (17).
Rosenbluh et al (9) also
demonstrated that TBX5 was a key transcription factor target of the
β-catenin/YAP1 complex, which regulated cancer phenotypes.
Therefore, TBX5 may be activated and overexpressed in stage I and
II GC. The mechanisms underlying the potential function of TBX5
were explained in the study by Rosenbluh et al (9). Wnt/β-catenin signaling has been
demonstrated to be involved in the pathogenesis of cancer and is
essential for cancer initiation and progression (18). YAP1, β-catenin and the transcription
factor TBX5 form a complex and move to the promoters of
anti-apoptotic genes, including BCL2L1 and BIRC5 through the
phosphorylation of YAP1 (9). This
hypothesis has been investigated in cell lines and animal models
(4–6,8).
Collectively, these data demonstrate that TBX5 may be a novel
biomarker that is potentially an independent predictor of the
survival rate of patients with stage I and II GC and that high
expression of TBX5 may aid in distinguishing which patients with
stage I and II GC may have unfavorable survival rates.
At present, TNM stage is widely accepted as a
powerful predictive parameter of survival rates (19). However, cases of the same TNM stage
are often observed to result in varied clinical outcomes, and TNM
alone may not be sufficient to predict clinical outcomes.
Therefore, it may be useful to determine those biomarkers that may
aid in the identification of patients with potentially poor
survival rates within the same TNM stage, so that they may be
selected for specific treatments. Having demonstrated the
clinicopathological significance of TBX5 expression in the
prognosis of OS in patients with GC, additional studies are
required to investigate the significance of TBX5 in patients with
stage I and II GC treated with chemotherapy, and the association
between TBX5 and β-catenin.
In conclusion, patients with stage I and II GC and
high expression of TBX5 resulted in unfavorable survival rates
compared with those with low expression of TBX5. The present study
demonstrates that the expression level of TBX5 in stage I and II GC
following surgery may be a potential prognostic biomarker of
survival rates in patients with GC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172080 and
81201773) and the Specialized Research Fund for the Doctoral
Program of Higher Education of China (grant no. 20100171110084 and
20120171120114).
References
|
1
|
Wang YC, Wei LJ, Liu JT, et al: Comparison
of cancer incidence between China and the USA. Cancer Biol Med.
9:128–132. 2012.PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zhang S, et al: The
incidences and mortalities of major cancers in China, 2009. Chin J
Cancer. 32:106–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanash SM, Pitteri SJ and Faca VM: Mining
the plasma proteome for cancer biomarkers. Nature. 452:571–579.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hiroi Y, Kudoh S, Monzen K, et al: Tbx5
associates with Nkx2-5 and synergistically promotes cardiomyocyte
differentiation. Nat Genet. 28:276–280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeuchi JK, Ohgi M, KoshibaTakeuchi K, et
al: Tbx5 specifies the left/right ventricles and ventricular septum
position during cardiogenesis. Development. 130:5953–5964. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Basson CT, Bachinsky DR, Lin RC, et al:
Mutations in human TBX5 [corrected] cause limb and cardiac
malformation in Holt-Oram syndrome. Nat Genet. 15:30–35. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Atik T, Dervisoglu H, Onay H, et al: A new
mutation in the TBX5 gene in Holt-Oram syndrome: Two cases in the
same family and prenatal diagnosis. J Trop Pediatr. 60:257–259.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu J, Ma X, Cheung KF, et al: Epigenetic
inactivation of T-box transcription factor 5, a novel tumor
suppressor gene, is associated with colon cancer. Oncogene.
29:6464–6474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosenbluh J, Nijhawan D, Cox AG, et al:
β-Catenin-driven cancers require a YAP1 transcriptional complex for
survival and tumorigenesis. Cell. 151:1457–1473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee MH, Choi D, Park MJ and Lee MW:
Gastric cancer: Imaging and staging with MDCT based on the 7th AJCC
guidelines. Abdom imaging. 37:531–540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Minguillon C and Logan M: The comparative
genomics of T-box genes. Brief Funct Genomics Proteomics.
2:224–233. 2003. View Article : Google Scholar
|
|
12
|
Lu J, Tsai T, Choo S, et al: Induction of
apoptosis and inhibition of cell growth by tbx5 knockdown
contribute to dysmorphogenesis in Zebrafish embryos. J Biomed Sci.
18:732011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong L, Deng J, Sun ZM, Pan AP, Xiang XJ,
Zhang L, Yu F, Chen J, Sun Z, Feng M and Xiong JP: Interference
with the β-catenin gene in gastric cancer induces changes to the
miRNA expression profile. Tumour Biol. Apr 10–2015.(Epub ahead of
print). View Article : Google Scholar
|
|
14
|
DiBartolomeo M, Pietrantonio F,
Pellegrinelli A, Martinetti A, Mariani L, Daidone MG, Bajetta E,
Pelosi G, de Braud F, Floriani I and Miceli R: Osteopontin,
E-cadherin, and beta-catenin expression as prognostic biomarkers in
patients with radically resected gastric cancer. Gastric cancer.
Apr 11–2015.(Epub ahead of print). View Article : Google Scholar
|
|
15
|
He A, Kong SW, Ma Q and Pu WT:
Co-occupancy by multiple cardiac transcription factors identifies
transcriptional enhancers active in heart. Proc Natl Acad Sci USA.
108:5632–5637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murakami M, Nakagawa M, Olson EN and
Nakagawa O: A WW domain protein TAZ is a critical coactivator for
TBX5, a transcription factor implicated in Holt-Oram syndrome. Proc
Natl Acad Sci USA. 102:18034–18039. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao B, Ye X, Yu J, et al: TEAD mediates
YAP-dependent gene induction and growth control. Genes Dev.
22:1962–1971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li D, Beisswenger C, Herr C, et al:
Myeloid cell RelA/p65 promotes lung cancer proliferation through
Wnt/β-catenin signaling in murine and human tumor cells. Oncogene.
33:1239–1248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu M, Pan H, Zhang F, et al:
Identification of TNM stage-specific genes in lung adenocarcinoma
by genome-wide expression profiling. Oncol Lett. 6:763–768.
2013.PubMed/NCBI
|