Introduction
Thyroid cancer is the most common endocrine
carcinoma, with papillary thyroid carcinoma (PTC) accounting for
~80% of all thyroid cancer cases (1).
PTC is well-differentiated and prone to metastasizing to the
regional lymph nodes. The incidence and mortality of PTC are
increasing rapidly worldwide, as well as in China (2,3). Reports
have demonstrated the incidence and mortality rates of thyroid
cancer were raised at a rate of 14.51% and 1.42% respectively, each
year between 2003–2007 in China (4).
The majority of cancer-associated mortalities are caused by
metastasis, a process that involves changes in the cancer cells,
but also in the tumor microenvironment. Tumor cells and/or
tumor-associated leukocytes and platelets can produce inflammatory
cytokines that may contribute directly towards malignant
progression (5). A previous study
demonstrated that human thyroid samples express genes coding the
majority of inflammatory proteins that were identified in cell
cultures, including CXCR4, CD44, OPN, CXCL1, CXCL10 and SDF-1
(6). The oncogenes activated in
thyroid carcinomas, including RET/PTC, RAS and
BRAF, which trigger the MAPK cascade, can induce a
cell-autonomous proinflammatory transcriptional program in
thyrocytes, which includes expression of cytokines, chemokines and
their receptors (7). Proinflammatory
cytokines, such as tumor necrosis factor (TNF)-α and interferon
(IFN)-γ, are important inflammatory mediators of these processes
(8,9).
TNF-α and IFN-γ are pleiotropic cytokines expressed in various
types of tumor cells and cells in the tumor microenvironment,
exerting a variety of pro-tumoral activities in solid tumors
(10,11). Recent research into the inflammatory
microenvironment of malignant PTC tissues has supported the
hypothesis that a close association exists between inflammation and
tumor metastasis progression (12,13).
Despite attempts in the last few decades to elucidate the
inflammatory cytokines underlying invasion and metastasis in PTC
patients, the understanding about the role of TNF-α and IFN-γ in
PTC is limited.
Epithelial-mesenchymal transition (EMT) is a complex
process during which epithelial cells lose intercellular adhesion,
acquire fibroblast-like characteristics and increase migratory and
invasive properties. EMT was initially reported in embryonic
development; however, it also occurs during tumor metastasis
(14,15) and appears to be common in PTC invasion
(16–19). Transforming growth factor-β (TGF-β)
and epidermal growth factor (EGF) are inflammatory cytokines that
are able to stimulate EMT in thyroid cancer cells or thyroid cells
cultured ex vivo (20,21). Furthermore, chronic low levels of
TNF-α and IFN-γ have been demonstrated to induce invasion and
metastasis of cancer (11,12,22–24) via
mechanisms involving the SMAD, NF-κB, AKT/GSK-3β and JAK/STAT
signaling pathways. Thus, EMT represents a convergence point
between inflammation and the progression of cancer (25); however, the mechanisms through which
inflammation is involved in the different stages of tumor invasion,
intravasation and subsequent metastasis to the distant organ sites
remain poorly defined (26).
In the present study, the effects of TNF-α and IFN-γ
on the migration and invasion of various PTC cell lines were
investigated. In addition, the association of TNF-α and IFN-γ with
the expression levels of E-cadherin, N-cadherin and vimentin was
examined. The current study aimed to provide a basis for the
investigation of the chronic inflammatory microenvironment and EMT
in PTC tissues.
Materials and methods
Cell culture
The PTC cell line, BCPAP (harboring the BRAF
mutation), was purchased from Leibniz Institute DSMZ-German
Collection of Microorganisms and Cell Cultures GmbH (Braunschweig,
Germany). In addition, the PTC cell line, K1 (harboring the
BRAF mutation), was purchased from the Health Protection
Agency Culture Collections (Salisbury, UK). BCPAP and K1 cells were
cultured in RPMI 1640 medium. The PTC cell line, TPC-1 (harboring
the RET/PTC mutation), was acquired from Dr Bryan R.
Haugen of the Division of Endocrinology, Diabetes and Metabolism,
University of Colorado Denver (Aurora, CO, USA) and cultured in
high-glucose Dulbecco's modified Eagle's medium. All culture media
were supplemented with 10% fetal bovine serum and antibiotics (100
U/ml penicillin and 100 µg/ml streptomycin) and cells were cultured
in a humidified atmosphere containing 5% CO2 at 37°C.
All culture reagents were purchased from Life Technologies (Grand
Island, NY, USA).
Wound-healing assay
Cells (2×105/ml) were seeded in a 12-well
plate at 80% cell confluence, and stimulated with 20 ng/ml TNF-α
(Invitrogen Life Technologies, Grand Island, NY, USA) and 50 U/ml
IFN-γ (Roche Applied Sciences, New York, NY, USA) for 12 h, and
then the culture medium was replaced with fresh medium. Cells
treated only with medium were regarded as control groups. After 24
h, a scratch wound in the monolayer was created using a sterile 10
µl pipette tip. Phase contrast images were captured between 0 and
24 h using a DMi1 inverted microscope (Leica Microsystems, Wetzlar,
Germany). Data are presented as the percentages of the remaining
gap distance relative to the initial gap distance, and are
expressed as the mean ± standard deviation (SD) measurements from
three independent experiments.
Transwell-invasion assay
Costar Transwell® chambers (pore size, 8 μm;
Corning, Inc., Corning, NY, USA) were coated with 200 µl Matrigel
(BD Biosciences, Franklin Lakes, NJ, USA) at a 1:7 dilution and
incubated overnight. The cells were co-cultured with 20 mg/ml TNF-α
or 50 U/ml IFN-γ for 12 h, followed by incubation for 24 h in fresh
culture medium. Next, the cells were seeded in the top chamber and
medium containing 10% FBS was added to the lower chamber as a
chemoattractant. After 24 h, the cells were fixed in 4%
formaldehyde and stained with hematoxylin and eosin (Beyotime
Institute of Biology, Suzhou, China). Cells that invaded through
the pores to the lower surface of the filter were counted under a
microscope (DMi1; Leica Microsystems). Data are expressed as the
mean ± SD of triplicate measurements from three independent
experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA extraction, cDNA synthesis, and qPCR were
performed as previously described (27). Briefly, total RNA was extracted from
the cells using TRIzol reagent (Invitrogen Life Technologies)
according to the manufacturer's instructions. RNA integrity was
verified by 1.5% agarose gel electrophoresis, followed by staining
with ethidium bromide (Sigma-Aldrich, St. Louis, MO, USA). The
OD260/OD280 absorbance ratio (where OD is the
optical density at 260 and 280 nm, respectively) was between 1.9
and 2.0 in each RNA sample. Next, 1 mg total RNA was used to
prepare cDNA. A reverse transcriptase kit (PrimeScript™RT Reagent
kit; Takara Biotechnology Co., Ltd., Dalian, China) was used for
complementary DNA (cDNA) synthesis, at 37°C for 15 min, followed by
85°C for 5 sec, on an ABI 9700 GeneAmp® PCR system (Applied
Biosystems Life Technologies, Grand Island, NY, USA). The
transcripts were quantified using a Rotor-Gene 3000 Real-Time PCR
system (Qiagen, Manchester, UK) and SYBR® Premix Ex Taq™ II
(Takara Biotechnology Co., Ltd.), according to the manufacturer's
instructions. Reactions began with a 10-sec hot-start activation of
the Taq polymerase at 95°C, followed by 40–45 cycles of
amplification in three steps (denaturation at 95°C for 5 sec,
followed by 30-sec annealing at 60°C and 30-sec extension at 72°C).
The primers used in each reaction were as follows (28): E-cadherin forward,
5′TGCCCAGAAAATGAAAAAGG-3, and reverse, 5′-GTGTATGTGGCAATGCGTTC-3′;
N-cadherin forward, 5′-GAGAACTTTGCCGTTGAAGC-3′, and reverse,
5′-GTGTATGTGGCAATGCGTTC3′; vimentin forward,
5′-GAGAACTTTGCCGTTGAAGC-3′, and reverse,
5′-GCTTCCTGTAGGTGGCAATC-3′; GAPDH forward,
5′-ACCCAGAAGACTGTGGATGG-3′, and reverse,
5′-TCTAGACGGCAGGTCAGGTC-3′. Data are expressed as the mean ± SD of
three independent experiments.
Immunoblot analysis
Cells (1×105/ml) in 6-well plates were
cultured until 60% confluence and then incubated with TNF-α (10
ng/ml, 20 ng/ml, 40 ng/ml) or IFN-γ (25 U/ml, 50 U/ml, 100 U/ml)
for 36 h. Total cell extracts were prepared from the cell cultures
using radioimmunoprecipitation assay buffer (Sigma-Aldrich) on ice.
The extracts were centrifuged at 11,739 × g for 20 min, and the
supernatant was subjected to a bicinchoninic acid assay for protein
quantification. The samples were then boiled for 10 min at 100°C.
Proteins were resolved on a 10% gradient sodium dodecyl
sulfate-polyacrylamide gel and transferred to polyvinylidene
difluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Subsequently, the membranes were blocked with 5% bovine serum
albumin (BSA) in Tris-buffered saline/Tween 20 (TBST) for 2 h at
room temperature, and then incubated with primary antibodies
(dilution, 1:1,000 in 2.5% BSA/TBST). The following mouse
monoclonal antibodies were used: Anti-E-cadherin (cat no. 610812,
BD Biosciences); and anti-N-cadherin (cat no. sc-59987),
anti-vimentin (cat no. sc-66002) and anti-β-actin (cat no.
sc-47778) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Next, the samples were incubated with the appropriate
horseradish peroxidase-conjugated secondary antibody (dilution,
1:5,000 in TBST; Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China). The densitometry of the immunoblot analysis
results was measured using the ImageJ software (version 1.4w3u;
National Institutes of Health, Bethesda, MD, USA) and data from
three independent experiments.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 18.0; SPSS Inc., Chicago, IL, USA). The results
are represented as the mean ± SD. Differences between the groups
were determined using one-way ANOVA. P<0.05 was considered to
indicate a statistically significant difference.
Results
TNF-α and IFN-γ promote migration and
invasion of PTC cells
In order to explore whether TNF-α and IFN-γ can
contribute to the metastasis and invasion of PTC cells in
vitro, a wound-healing assay was used to assess cell migration
following stimulation with TNF-α and IFN-γ. Compared with the
control groups, incubation of the three thyroid carcinoma cell
lines (TPC-1, BCPAP and K1) with TNF-α (20 ng/ml) significantly
enhanced cell motility by ~2-fold (P<0.01; Fig. 1). The migration ability of the cells
under the stimulation of INF-γ (50 U/ml) was similar to this, the
motility of K1 and TPC-1 was increased by ~2-fold and the migration
ability of BCPAP was increased by ~1.5-fold compared with the
control (P<0.01; Fig. 1).
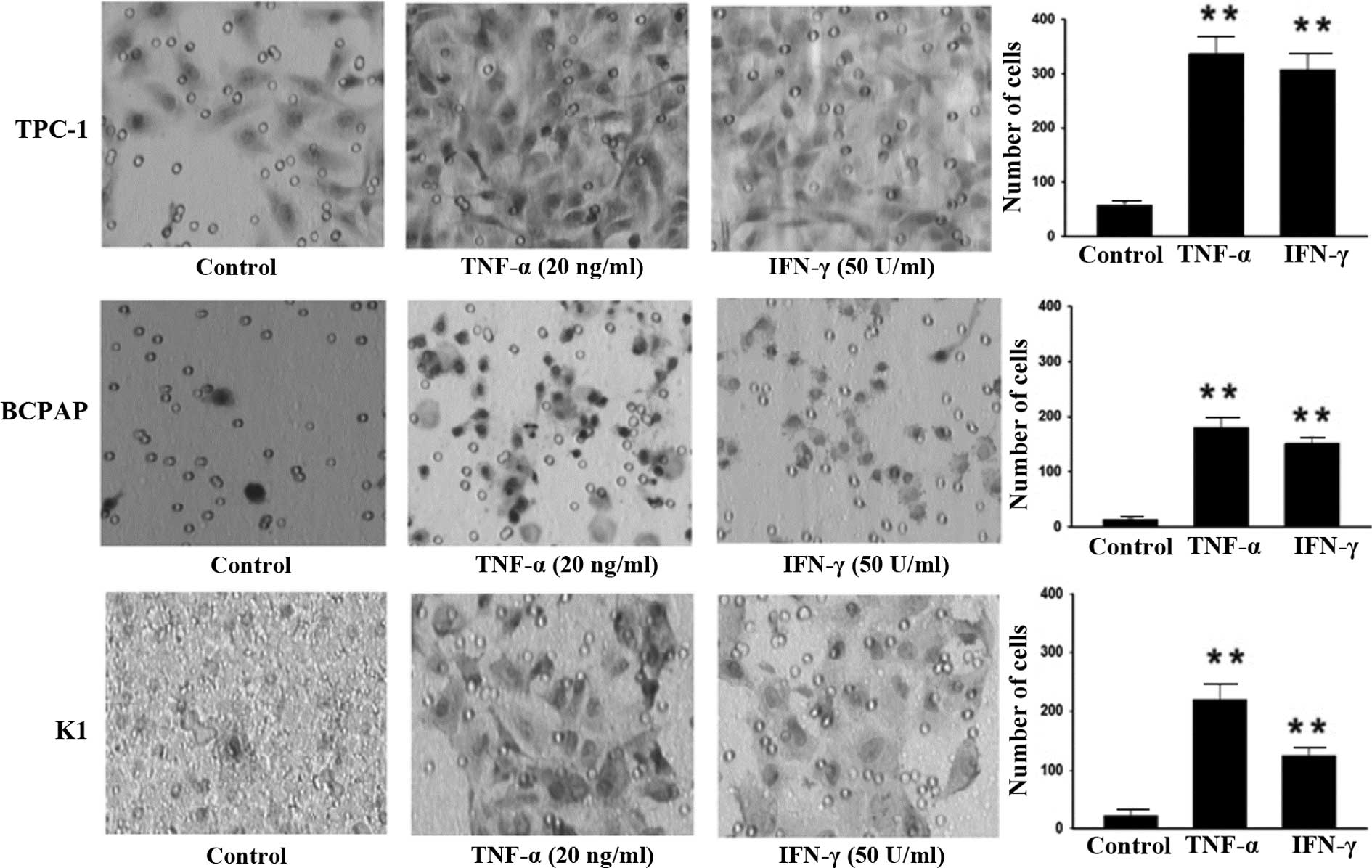
Similarly, significant changes in cell invasive ability were
observed compared with the control (P<0.01; Fig. 2). Following incubation with TNF-α and
IFN-γ, the number of invasive TPC-1 cells was increased by ~6-fold,
in comparison to cells incubated without any cytokines. In
addition, subsequent to incubation of BCPAP cells with TNF-α, the
fraction of invasive cells increased by 13-fold compared with the
control group, while it increased by 10-fold following incubation
with IFN-γ. After incubation of K1 cells with TNF-α, the fraction
of invasive cells increased by 10-fold compared with the control,
while it increased by 6-fold following incubation with IFN-γ. These
results suggest that TNF-α and IFN-γ are able to significantly
promote the invasiveness of PTC cells.
 | Figure 1.TNF-α and IFN-γ promote migration of
PTC cells. For the wound-healing assay, the human PTC cell lines,
(A) TPC-1, (B) BCPAP and (C) K1, were treated with 20 mg/ml TNF-α
and 50 U/ml IFN-γ. Images were captures using a light microscope 24
h after scratching a wound in the monolayer (magnification, ×40 for
TPC-1 and BCPAP cells, ×100 for K1 cells). The results from three
independent experiments are presented as graphs of the mean ±
standard deviation (*P<0.05 and **P<0.01, vs. control). Bars,
100 µm for TPC-1 and BCPAP cells, 200 µm for K1 cells. PTC,
papillary thyroid carcinoma; TNF, tumor necrosis factor; IFN,
interferon. |
EMT was induced by TNF-α and IFN-γ in
the PTC cell lines
TNF-α-induced EMT has been reported in numerous
tumor types; however, EMT induced by IFN-γ alone has rarely been
observed (11,22,23).
RT-qPCR was used to measure the expression levels of epithelial
cell marker, E-cadherin, and the mesenchymal cell markers,
N-cadherin and vimentin, in epithelial cell lines, and their
expression was used as a measure of EMT.
Epithelial cell lines treated with TNF-α and IFN-γ,
alone or in combination, expressed lower levels of E-cadherin mRNA
and higher levels of N-cadherin and vimentin mRNA compared with
untreated cells (Fig. 3). In
TNF-α-treated TPC-1 cells, RT-qPCR revealed that the mRNA
expression of E-cadherin was decreased by ~60–70% at 12 and 24 h,
while the mRNA expression of N-cadherin was increased by 2-fold at
12 h and 3-fold at 24 h; however, no evident increase was observed
in the mRNA expression of vimentin. By contrast, when treated with
IFN-γ, the mRNA expression of E-cadherin was decreased by ~20–40%
at 12 h and ~70% at 24 h, while that of vimentin was increased by
1.5-fold at 12 and 24 h; however, no evident increase was observed
in the mRNA expression of N-cadherin. In BCPAP cells, the
observations were similar.
Incubation with low concentrations of TNF-α and
IFN-γ was also found to exert a synergistic effect on E-cadherin in
TCP-1 and BCPAP cells. TPC-1 cells incubated with a combination of
10 ng/ml TNF-α and 25 U/ml IFN-γ for 12 h expressed 67% more
N-cadherin mRNA compared with cells incubated with 10 ng/ml TNF-α
alone and 108% more N-cadherin mRNA compared with cells incubated
with 25 U/ml IFN-γ alone (P<0.01). In addition, BCPAP cells
incubated with a combination of 10 ng/ml TNF-α and 25 U/ml IFN-γ
for 12 h presented a 6-fold increase in N-cadherin mRNA expression
compared with cells incubated with 10 ng/ml TNF-α alone, and a
5-fold increase compared with cells incubated with 25 U/ml IFN-γ
alone (P<0.01). Although TNF-α and IFN-γ were both able to
induce downregulation of E-cadherin mRNA expression, their effect
on N-cadherin and vimentin expression differed. TNF-α predominantly
upregulated N-cadherin expression, while IFN-γ predominantly
upregulated vimentin expression.
These results were further validated by immunoblot
analysis (Fig. 4), which indicated
that the expression of E-cadherin protein was decreased to varying
levels in all three cell lines, when compared with the control
groups. E-cadherin protein expression was reduced by 10–59% in
TPC-1 cells, by 23–78% in BCPAP cells and by 28–78% in K1 cells.
Consistent with the mRNA levels, TNF-α also predominantly induced
N-cadherin protein, while IFN-γ predominantly induced vimentin,
with detailed results shown in Fig.
4. However, when administered in higher concentrations (40
ng/ml TNF-α and 100 U/ml IFN-γ), the synergistic effect of these
cytokines on EMT progression was less pronounced. Upon measuring
the protein expression to evaluate the superposition effect of
these two cytokines (10 ng/ml TNF-α and 25 U/ml IFN-γ), the EMT
progression was unclear, potentially due to the excessively high
concentration of cytokine used for an extended period (36 h), which
may have induced apoptosis.
Discussion
In the present study, the influence of
proinflammatory cytokines TNF-α and IFN-γ on the malignant
progression of PTC cell lines in vitro was investigated.
TNF-α and IFN-γ were found to enhance the capacity of PTC cell
lines to migrate and invade, and this process coincided with the
downregulation of E-cadherin and upregulation of N-cadherin and
vimentin, which are hallmarks of EMT.
TNF-α and IFN-γ contributed to the metastasis and
invasion of three PTC cell lines, including the BCPAP and K1 cell
lines that harbor the BRAF mutation and the TPC-1 cell line
that harbors the RET/PTC mutation. The three cell
lines responded to TNF-α and IFN-γ treatment in a similar manner,
indicating that the oncogenic mutation did not influence the
response to these cytokines.
In order to investigate the mechanism of EMT
initiated by TNF-α and IFN-γ, the present study also examined the
expression of various EMT markers. The expression of E-cadherin in
untreated PTC cells was found to be high, which is consistent with
the findings of previous studies (29). Liu and Brown reported that E-cadherin
was expressed in well-differentiated thyroid carcinomas, including
papillary and follicular carcinomas, but not in anaplastic thyroid
carcinoma (29). E-cadherin was also
expressed at a high level in thyroid cells at all development
stages (30), indicating that
embryonic thyrocytes maintain original epithelial differentiation
and homotypic cell-cell adhesion is mediated by E-cadherin.
Following treatment with TNF-α and IFN-γ, the expression of
E-cadherin mRNA and protein was reduced, which indicated the first
observed functional consequence of the EMT process. Reduced
E-cadherin expression has also been observed in the WRO follicular
thyroid carcinoma cell line during TNF-α stimulation, and
E-cadherin expression was restored when TNF-α stimulation ceased
(31). Furthermore, in differentiated
thyroid carcinomas, reduced E-cadherin expression has been
associated with a poor outcome (32).
In the current study, the expression levels of
N-cadherin and the mesenchymal maker, vimentin, were also examined.
N-cadherin mRNA and protein levels were upregulated following
incubation with TNF-α, but no changes were observed in the level of
N-cadherin expression following incubation with IFN-γ. Loss of
E-cadherin and gain of N-cadherin is defined as ‘cadherin switch’
and indicates EMT in numerous solid tumors (10,11,13).
However, increased expression of N-cadherin is not observed in all
cells undergoing EMT. For instance, N-cadherin expression was not
significantly increased in the EMT induced by TGF-β treatment in
the BCPAP and TPC-1 cell lines, or as a result of endoplasmic
reticulum stress in PC Cl3 cells (33). The findings of previous studies
support the hypothesis that the process defined as EMT comprises a
wide spectrum of changes in epithelial plasticity, indicating that
different ‘subtypes’ of EMT exist, differing in the mechanism of
progression towards a mesenchymal phenotype (33,34).
Additionally, expression of vimentin, which was
increased following incubation with IFN-γ, was not found to be
altered by incubation with TNF-α. A number of studies have
demonstrated that vimentin is an important regulator of EMT
(35–37), and plays a key role in PTC (17). However, vimentin has to be localized
in the perinuclear region to be fully functional, and the present
study detected the total vimentin expression, which may not
accurately represent the concentration of perinuclear vimentin.
Therefore, this methodological limitation restricts the conclusions
that can be drawn about the influence of IFN-γ and TNF-α treatment
on vimentin activity in PTC cells. The present study was only
preliminary and thus the downstream pathway of TNF-α and IFN-γ
treatment, as well as any differences in TPC-1 and BCPAP cells
following exposure to these cytokines, should be further
analyzed.
In conclusion, the current study identified that
TNF-α and IFN-γ play an important role in regulating the adhesion
and migration of PTC cells. The results strongly implicated the
occurrence of EMT in the PTC metastases, which was induced by TNF-α
and IFN-γ treatment. These findings highlight the role of the tumor
microenvironment and EMT phenol-type in PTC metastases, and
recommend further investigation to assist the efforts to define
predictive diagnostic models and treatments for PTC with lymph node
metastases.
Acknowledgements
The authors would like to thank Dr Bryan R. Haugen
(Division of Endocrinology, Diabetes and Metabolism, University of
Colorado Denver, Denver, CO, USA) for providing the TPC-1 cell
line. This study was supported by a grant from the National Science
Foundation of China (no. 81102471).
References
|
1
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guan HX, Shan ZY, Mi XY, Wang E and Teng
W: Incidence of thyroid carcinoma before and after universal salt
iodization: An 11-year retrospective analysis of pathological
reports. J Chin Med Univ. 35:284–285. 2006.
|
|
4
|
Liu YQ, Zhang SQ, Chen WQ, Chen LL, Zhang
SW, Zhang XD and Zheng RS: Trend of incidence and mortality on
thyroid cancer in China during 2003–2007. Zhonghua Liu Xing Bing
Xue Za Zh. 33:1044–1048. 2012.(In Chinese).
|
|
5
|
Wu ST, Sun GH, Hsu CY, Huang CS, Wu YH,
Wang HH and Sun KH: Tumor necrosis factor-α induces
epithelial-mesenchymal transition of renal cell carcinoma cells via
a nuclear factor kappa B-independent mechanism. Exp Biol Med
(Maywood). 236:1022–1029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liotti F, Visciano C and Melillo RM:
Inflammation in thyroid oncogenesis. Am J Cancer Res. 2:286–297.
2012.PubMed/NCBI
|
|
7
|
Menicali E, Moretti S, Voce P, Romagnoli
S, Avenia N and Puxeddu E: Intracellular signal transduction and
modification of the tumor microenvironment induced by RET/PTCs in
papillary thyroid carcinoma. Front Endocrinol (Lausanne).
3:672012.PubMed/NCBI
|
|
8
|
Aust G, Heuer M, Laue S, Lehmann I,
Hofmann A, Heldin NE and Scherbaum WA: Expression of tumour
necrosis factor-alpha (TNF-α) mRNA and protein in pathological
thyroid tissue and carcinoma cell lines. Clin Exp Immunol.
105:148–154. 2006. View Article : Google Scholar
|
|
9
|
Bossowski A, Harasymczuk J, Moniuszko A,
Bossowska A, Hilczer M and Ratomski K: Cytometric evaluation of
intracellular IFN-γ and IL-4 levels in thyroid follicular cells
from patients with autoimmune thyroid diseases. Thyroid Res.
4:132011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jing Y, Han Z, Liu Y, Sun K, Zhang S,
Jiang G, Li R, Gao L, Zhao X, Wu D, et al: Mesenchymal stem cells
in inflammation microenvironment accelerates hepatocellular
carcinoma metastasis by inducing epithelial-mesenchymal transition.
PloS One. 7:e432722012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Wang HS, Zhou BH, Li CL, Zhang F,
Zhang G, Bu XZ, Cai SH and Du J: Epithelial-mesenchymal transition
(EMT) induced by TNF-a requires AKT/GSK-3b-mediated stabilization
of snail in colorectal cancer. PLoS One. 8:e566642013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Knauf JA, Sartor MA, Medvedovic M, et al:
Progression of BRAF-induced thyroid cancer is associated with
epithelial-mesenchymal transition requiring concomitant MAP kinase
and TGFβ signaling. Oncogene. 30:3153–3162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sancisi V, Gandolfi G, Ragazzi M, Nicoli
D, Tamagnini I, Piana S and Ciarrocchi A: Cadherin 6 is a new RUNX2
target in TGF-β signalling pathway. PloS One. 8:e754892013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Howard EW, Camm KD, Wong YC and Wang XH:
E-cadherin upregulation as a therapeutic goal in cancer treatment.
Mini Rev Med Chem. 8:496–518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bai Y, Kakudo K, Nakamura M, Ozaki T, Li
Y, Liu Z, Mori I, Miyauchi A and Zhou G: Loss of cellular
polarity/cohesiveness in the invasive front of papillary thyroid
carcinoma and periostin expression. Cancer Lett. 281:188–195. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vasko V, Espinosa AV, Scouten W, He H,
Auer H, Liyanarachchi S, Larin A, Savchenko V, Francis GL, de la
Chapelle A, et al: Gene expression and functional evidence of
epithelial-to-mesenchymal transition in papillary thyroid carcinoma
invasion. Proc Natl Acad Sci USA. 104:2803–2808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Z, Kakudo K, Bai Y, Li Y, Ozaki T,
Miyauchi A, Taniguchi E and Mori I: Loss of cellular
polarity/cohesiveness in the invasive front of papillary thyroid
carcinoma, a novel predictor for lymph node metastasis; possible
morphological indicator of epithelial mesenchymal transition. J
Clin Pathol. 64:325–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Puppin C, Fabbro D, Dima M, Di Loreto C,
Puxeddu E, Filetti S, Russo D and Damante G: High periostin
expression correlates with aggressiveness in papillary thyroid
carcinomas. J Endocrinol. 197:401–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Braun J, Hoang V, Dralle H and Hüttelmaier
S: Downregulation of microRNAs directs the EMT and invasive
potential of anaplastic thyroid carcinomas. Oncogene. 29:4237–4244.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grände M, Franzen Å, Karlsson JO, Ericson
LE, Heldin NE and Nilsson M: Transforming growth factor-β and
epidermal growth factor synergistically stimulate epithelial to
mesenchymal transition (EMT) through a MEK-dependent mechanism in
primary cultured pig thyrocytes. J Cell Sci. 115:4227–4236. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren J, Wang Y, Gao Y, Mehta SB and Lee CG:
FAT10 mediates the effect of TNF-α in inducing chromosomal
instability. J Cell Sci. 124:3665–3675. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shiozaki A, Bai X, Shen T, Moodley S,
Takeshita H, Fung SY, Wang Y, Keshavjee S and Liu M: Claudin 1
mediates TNFα-induced gene expression and cell migration in human
lung carcinoma cells. PLoS One. 7:e380492012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Borthwick LA, Gardner A, De Soyza A, Mann
DA and Fisher AJ: Transforming growth factor-β1 (TGF-β1) driven
epithelial to mesenchymal transition (EMT) is accentuated by tumour
necrosis factor α (TNFα) via crosstalk between the SMAD and NF-κB
pathways. Cancer Microenviron. 5:45–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
López-Novoa JM and Nieto MA: Inflammation
and EMT: An alliance towards organ fibrosis and cancer progression.
EMBO Mol Med. 1:303–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei
Y, Abbruzzese JL, Hortobagyi GN and Hung MC: Epidermal growth
factor receptor cooperates with signal transducer and activator of
transcription 3 to induce epithelial-mesenchymal transition in
cancer cells via up-regulation of TWIST gene expression. Cancer
Res. 67:9066–9076. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue H, Wang W, Li Y, Shan Z, Li Y, Teng X,
Gao Y, Fan C and Teng W: Selenium upregulates
CD4+CD25+ regulatory T cells in
iodine-induced autoimmune thyroiditis model of NOD H-2h4
mice. Endocr J. 57:595–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY and Weinberg RA: The epithelial-mesenchymal transition
generates cells with properties of stem cells. Cell. 133:704–715.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu J and Brown R: Immunohistochemical
detection of epithelial mesenchymal transition associated with
stemness phenotype in anaplastic thyroid carcinoma. Int J Clin Exp
Pathol. 3:755–762. 2010.PubMed/NCBI
|
|
30
|
Fagman H, Grande M, Edsbagge J, Semb H and
Nilsson M: Expression of classical cadherins in thyroid
development: Maintena-nce of an epithelial phenotype throughout
organogenesis. Endocrinology. 144:3618–3624. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jensen K, Patel A, Hoperia V, Larin A,
Bauer A and Vasko V: Dynamic changes in E-cadherin gene promoter
methylation during metastatic progression in papillary thyroid
cancer. Exp Ther Med. 1:457–462. 2010.PubMed/NCBI
|
|
32
|
Rocha A, Soares P, Seruca R, Máximo V,
MatiasGuiu X, CameselleTeijeiro J and Sobrinho-Simões M:
Abnormalities of the E-adherin/catenin adhesion complex in
classical papillary thyroid carcinoma and in its diffuse sclerosing
variant. J Pathol. 194:358–366. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ulianich L, Garbi C, Treglia A, Punzi D,
Miele C, Raciti GA, Beguinot F, Consiglio E and Di Jeso B: ER
stress is associated with dedifferentiation and an
epithelial-to-mesenchymal transition-like phenotype in PC Cl3
thyroid cells. J Cell Sci. 121:477–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vuoriluoto K, Haugen H, Kiviluoto S,
Mpindi J, Nevo J, Gjerdrum C, Tiron C, Lorens JB and Ivaska J:
Vimentin regulates EMT induction by Slug and oncogenic H-Ras and
migration by governing Axl expression in breast cancer. Oncogene.
30:1436–1448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mendez MG, Kojima S and Goldman RD:
Vimentin induces changes in cell shape, motility and adhesion
during the epithelial to mesenchymal transition. FASEB J.
24:1838–1851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kokkinos MI, Wafai R, Wong MK, Newgreen
DF, Thompson EW and Waltham M: Vimentin and epithelial-mesenchymal
transition in human breast cancer-observations in vitro and in
vivo. Cells Tissues Organs. 185:191–203. 2007. View Article : Google Scholar : PubMed/NCBI
|