Introduction
Thyroid cancer is the most common malignant
endocrine tumor, with significant morbidity and mortality (1,2). A steady
increase in the incidence of this cancer type has been recorded
over the last three decades (3). It
is known that the female North American population has the highest
incidence of thyroid cancer, with an age-standardized rate of 15.1
per 100,000 females. In Western Europe, the age-standardized rate
is 5.8 per 100,000 females (4).
Thyroid cancer comprises tumors with different clinical and
histological features (5).
Specifically, papillary and follicular thyroid cancers are
slow-growing and well-differentiated. However, anaplastic thyroid
cancers are undifferentiated neoplasias with much more aggressive
activity, usually leading to patient mortality within one year of
diagnosis (6).
Antimicrobial peptides (AMPs) are naturally
occurring molecules found in microorganisms, plants and animals,
and are considered as an essential component in the innate immunity
(7,8).
Despite their structural conservation, AMPs are emerging as a novel
source of molecules and have a broad spectrum of activity, such as
antibacterial, antifungal and antiviral functions (9). More recently, their abilities against
tumor cells have been confirmed by selectively killing cancer
cells, whose membrane proteins are negatively charged through
glycosylation (10). AMPs exert
anticancer activity by a membranolytic mode of action or via
interaction with intracellular targets, or through use of a
combination of the two (11).
Cationic antitumor peptides represent potential antitumor therapy
agents, as they have a number of advantages compared with other
chemical agents, including a comparatively simple structure, a low
molecular mass, few adverse reactions, greater specific
cytotoxicity to tumor cells over healthy cells, ease of absorption
and a low risk for the induction of multi-drug resistance (12–14).
Bovine myeloid antimicrobial peptide 28 (BMAP-28) is
a cathelicidin, which is found in bovine neutrophils (15). It has been reported that BMAP-28 had a
wide range of antimicrobial activities and could confer protection
to bacterial infection and sepsis in animals (16,17).
Additionally, previous studies found that BMAP-28 exhibited
significantly reduced cytotoxicity against 3T3 cells and
erythrocytes (18). However, the
antitumor effect and clear mechanism of BMAP-28 on thyroid cancer
cells have not yet been investigated.
In the present study, the activity and mechanism of
BMAP-28 on human thyroid cancer TT cells were investigated in order
to highlight the therapeutic potential of BMAP-28 in thyroid
cancer.
Materials and methods
Regents
BMAP-28 (GGLRSLGRKILRAWKKYGPIIVPIIRIG) was
synthesized using solid-phase methodology at GL Biochemistry
Corporation (Shanghai, China). The resulting peptide was purified by
preparative reverse-phase high-performance liquid chromatography,
resulting in final products that were deemed >95% pure.
Synthetic antimicrobial peptide was reconstituted in
phosphate-buffered saline (PBS; pH 7.4) for subsequent experiments.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
sodium pyruvate and dimethyl sulfoxide (DMSO) were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Annexin V-fluorescein
isothiocyanate/propidium iodide (Annexin V-FITC/PI) were purchased
from Mbchemic (Shanghai, China). Colorimetric assay kits for
detection of capase-3 and -9 were obtained from BioVision (Mountain
View, CA, USA). Anti-human matrix metalloproteinase (MMP)-3
phycoerythrin mouse monoclonal antibody (MAb; 1:1,000) and
anti-human MMP-9 fluorescein rabbit MAb (1:1,000) were purchased
from R&D Systems Inc. (Minneapolis, MN, USA). The Easy Plus
Mini kit, iScript Select cDNA Synthesis kit, SyberGreen qPCR Primer
and iCycler iQ Multicolor Real-Time PCR Detection System were all
purchased from KeyGEN (Nanjing, China). The study was approved by
the ethics committee of the Department of Thyroid Surgery,
China-Japan Union Hospital of Jilin University (Changchun,
China).
Cell culture
Human thyroid cancer TT cells were obtained from the
American Type Culture Collection (Manassas, VA, USA) and maintained
as previously described (19).
Briefly, the TT cells were maintained in F-12K supplemented with
16% fetal bovine serum (Gibco Life Technologies, Carlsbad, CA,
USA), 100 U/ml penicillin and 100 U/ml streptomycin. The cells were
cultured at 37°C in a humidified incubator containing 5%
CO2.
MTT assay
An MTT assay was performed to evaluate the effect of
BMAP-28 on the TT cells. As previously described (20), the cells were seeded in 96-well plates
at a concentration of 5×103 cells/well with F-12K medium
containing 10% fetal calf serum and allowed to attach for 12 h.
Different concentrations of BMAP-28 (0, 0.5, 1, 2, 4, 8, 16 and 32
µM) were added to the cells and further incubated for 24, 48 or 72
h. Following incubation, the cells were incubated with MTT solution
(5 µg/ml) for 4 h at 37°C, then switched into 150 µl DMSO and
shaken for 5 min at room temperature prior to measuring absorbance
at 490 nm.
Cell apoptosis assay
Cell apoptosis assays (21) were conducted by double staining using
Annexin V and PI kits (eBioscience Inc., San Diego, CA, USA) to
investigate whether BMAP-28 induces apoptosis in TT cells. The TT
cells were seeded in 6-well plastic plates (5×105
cells/well) 24 h prior to BMAP-28 treatment. The medium supernatant
in the plates was then replaced, and various concentrations of
BMAP-28 (ranging from 0 to 4 µM), diluted in PBS, were added to the
plates. Subsequent to incubation at 37°C in a humidified atmosphere
with 5% CO2 for 48 h, the cells were harvested and
washed twice with PBS. For the Annexin V-FITC/PI assay, the cells
were treated according to the manufacturer's instructions. Briefly,
cells were suspended in 100 µl binding buffer (eBioscience, Inc.)
and 5 µl Annexin V-FITC. This mixture was incubated at room
temperature in the dark for 15 min. Next, cells were washed once
with binding buffer, suspended in 100 µl binding buffer and 5 µl PI
was added. Following this, the cells were analyzed using a BD FACS
Calibur flow cytometer (BD Biosciences, San Jose, CA, USA). Signals
of Annexin V-FITC were measured at 488 nm for excitation and 525 nm
for emission, and signals of PI were detected at 535 nm for
excitation and 615 nm for emission.
Detection of caspase-3, caspase-9,
MMP3 and MMP9 expression
Caspase-3, caspase-9, MMP3 and MMP9 expression was
assessed using colorimetric assay kits according to the
manufacturer's instructions (22).
The TT cells were treated with various concentrations of BMAP-28
(ranging from 0 to 4 µM) for 24 h. Next, cell pellets were washed
twice with PBS and scrapped with ice-cold lysis buffer. Active
caspase 3, caspase 9, MMP3 and MMP9 expression was measured
accordingly: The fluorescence intensity was measured at 405 nm with
a flow cytometer to detect the production of active molecules.
Quantitative polymerase chain reaction
(qPCR)
The effect of BMAP-28 on the caspase 3, caspase 9,
MMP3 and MMP9 RNA expression of the TT cells was examined by a
qPCR, as previously described (23).
Total RNA was extracted from the TT cells with or without BMAP-28
(ranging from 0 to 4 µM) treatment using TRIzol reagent and then
purified with an RNeasy Mini kit (Qiagen Sciences Inc., Germantown,
MD, USA). Next, qPCR was performed in an ABI PRISM 7300 sequence
detection system (Applied Biosystems Life Technologies, Foster
City, CA, USA), with 3 wells (10 µl/well) set for every reaction.
The reactions were performed under the following conditions: 95°C
for 10 min; 45 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C
for 30 sec to collect signals; followed by 72°C for 7 min to obtain
a solubility curve. Relative mRNA expression was calculated using
the comparative CT (2−ΔΔCt) method.
The sequences of the primers were as follows:
Caspase 3 forward, 5′-AGGAAGGTGGCAACG-3′ and reverse,
5′-CGCCAAATCTTGCTAAT-3′; caspase 9 forward,
5′-GGCTGTCTACGGCACAGATGGA-3′ and reverse,
5′-CTGGCTCGGGGTTACTGCCAG-3′; MMP-3 forward,
5′-CTCACAGACCTGACTCGGTT-3′ and reverse, 5′-CACGCCTGAAGGAAGAGATG-3′;
and MMP-9 forward, 5′-CGCAGACATCGTCATGT-3′ and reverse,
5′-GGATTGGCCTTGGAAGATGA-3′.
TT-xenograft mouse model and BMAP-28
administration in vivo
Nude mice purchased from the Academy of Military
Medical Science (Beijing, China) were housed in a rodent facility
at 22±1°C with a 12-h light-dark cycle and provided with continuous
standard rodent chow and water for acclimatization. The TT cells
(2×107 cells/ml; 0.1 ml) were inoculated intradermally
into the hind flank. When the diameter of the tumors reached 0.5
cm, the mice were randomly divided into four groups (n=6 per group)
consisting of a model control group administered with normal
saline, and three BMAP-28-treated groups administered at 0.25, 0.50
or 1 mg/kg body weight. These four groups were administered
intra-tumoral injections (50 µl) once every 5 days for 25 days. At
the indicated time-points, the mice were sacrificed by cervical
dislocation 24 h after the final administration. The tumor weights
of the mice from each group were measured. During the treatment,
the tumor volume (TV) of each mouse was measured.
The antitumor activity was expressed as the
inhibitory rate (%) and calculated as follows: (A − B) / A × 100,
where A and B were the average tumor weight of the model and
BMAP-28 groups, respectively.
The TV was calculated using the following formula:
TV = 1 / 2 × a × b2, where a and b are the long and
short diameters of the tumors in each mouse, respectively.
Statistical analysis
All experiments were performed in triplicate. The
data are presented as the mean ± standard deviation. Statistical
analysis was performed by a one-way analysis of variance, followed
by Dunnett's multiple comparison or Student's t-test, where
P<0.05 was considered to indicate a statistically significant
difference.
Results
BMAP-28 suppresses cell proliferation
of TT cells
To clarify the effects of BMAP-28 on thyroid cancer
cells in vitro, human thyroid cancer TT cells were treated
with BMAP-28 at different concentrations (ranging from 0 to 32 µM)
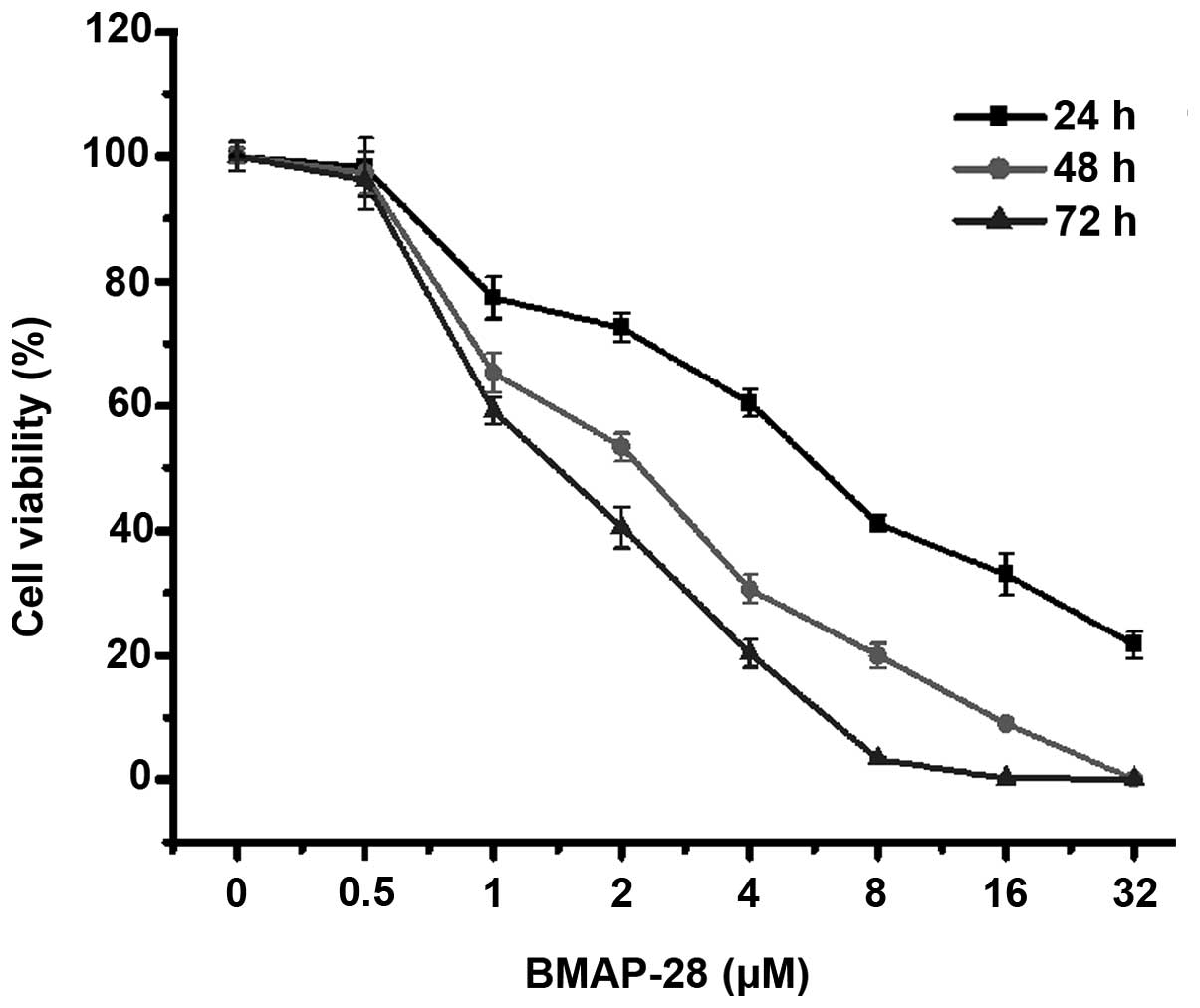
for 24, 48 and 72 h. As shown in Fig.
1, BMAP-28 time- and dose-dependently inhibited the cell
proliferation. Specifically, at 48 h, the cell viabilities were
65.2, 53.3, 30.7, and 19.9% at the dose of 1, 2, 4 and 8 µM
BMAP-28, respectively. The results illustrate that BMAP-28 exerted
a direct cytotoxic effect on the TT cells.
BMAP-28 induces cell apoptosis of TT
cells
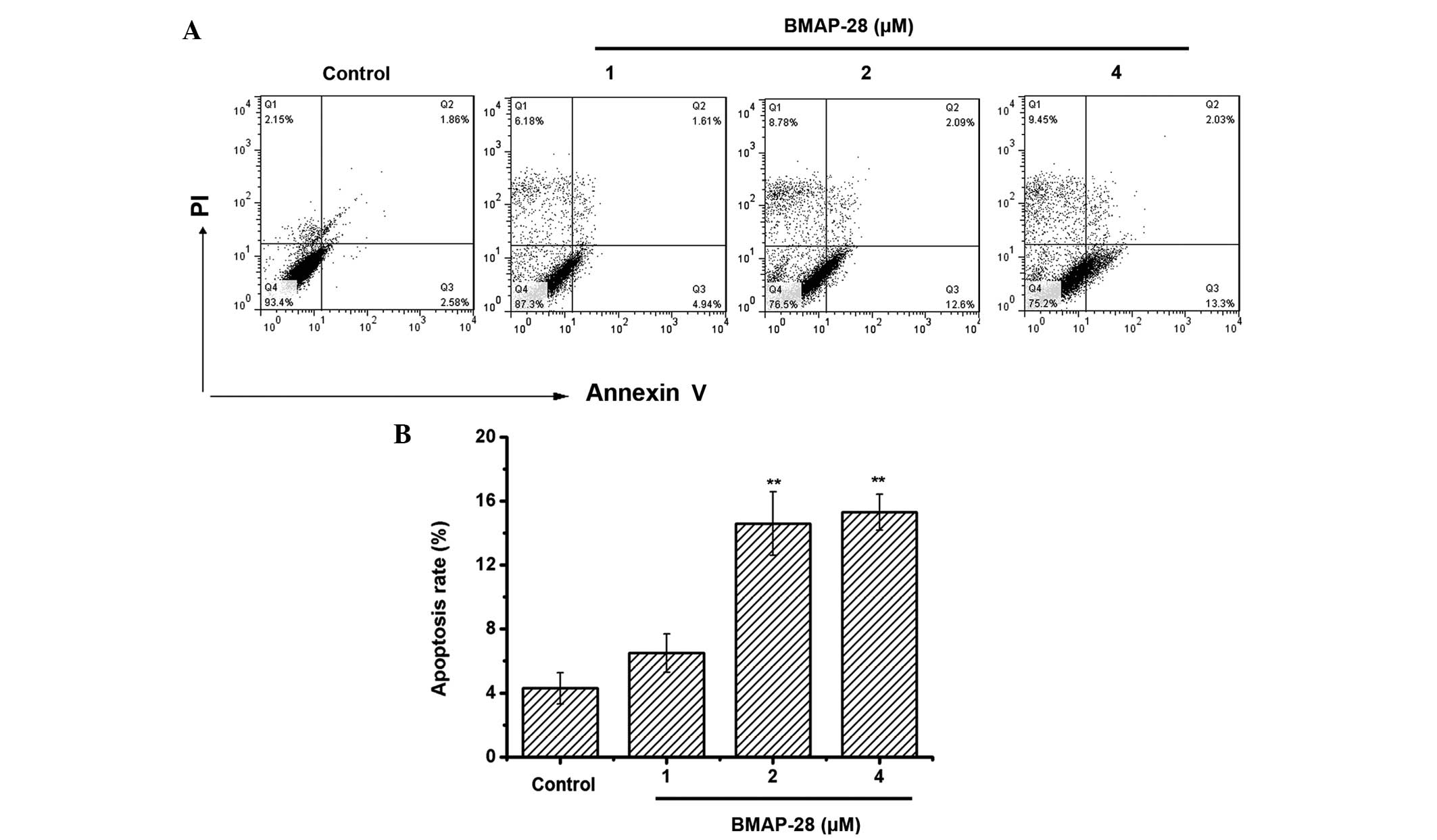
To test whether BMAP-28 induced cell death through
apoptosis, an Annexin V-FITC/PI assay was performed to measure the
percentage of apoptotic cells. Positive staining with Annexin
V-FITC correlated with the loss of membrane polarity, among which
the complete loss of membrane integrity lead to apoptosis. PI
entered the cells after the loss of membrane integrity. Therefore,
dual labeling with Annexin V and PI was adopted for discriminating
between unaffected and apoptotic cells. The results indicated that
BMAP-28 induced apoptotic effects on the TT cells (Fig. 2A). Specifically, the induced apoptotic
cell accumulation reached 6.5, 14.6 and 15.3% at the dose of 1, 2
and 4 µM BMAP-28, respectively (Fig.
2B). Therefore, induced apoptosis could be one mechanism for
BMAP-28 in preventing the proliferation of TT cells.
BMAP-28 activates caspase-3 and
caspase-9 in TT cells
Given that caspase-3 and caspase-9 are activated
during the early stage of apoptosis, they are treated as markers of
apoptotic cells (22). The present
results indicated that caspase-3 and caspase-9 were activated in
the BMAP-28-treated TT cells as the dose increased (Fig. 3A–D). Moreover, similar results were
obtained in the qPCR assay where the RNA levels of caspase-3 and
caspase-9 in the TT cells were increased after exposure to BMAP-28
(Fig. 3E and F).
BMAP-28 suppresses MMP3 and MMP9 in TT
cells
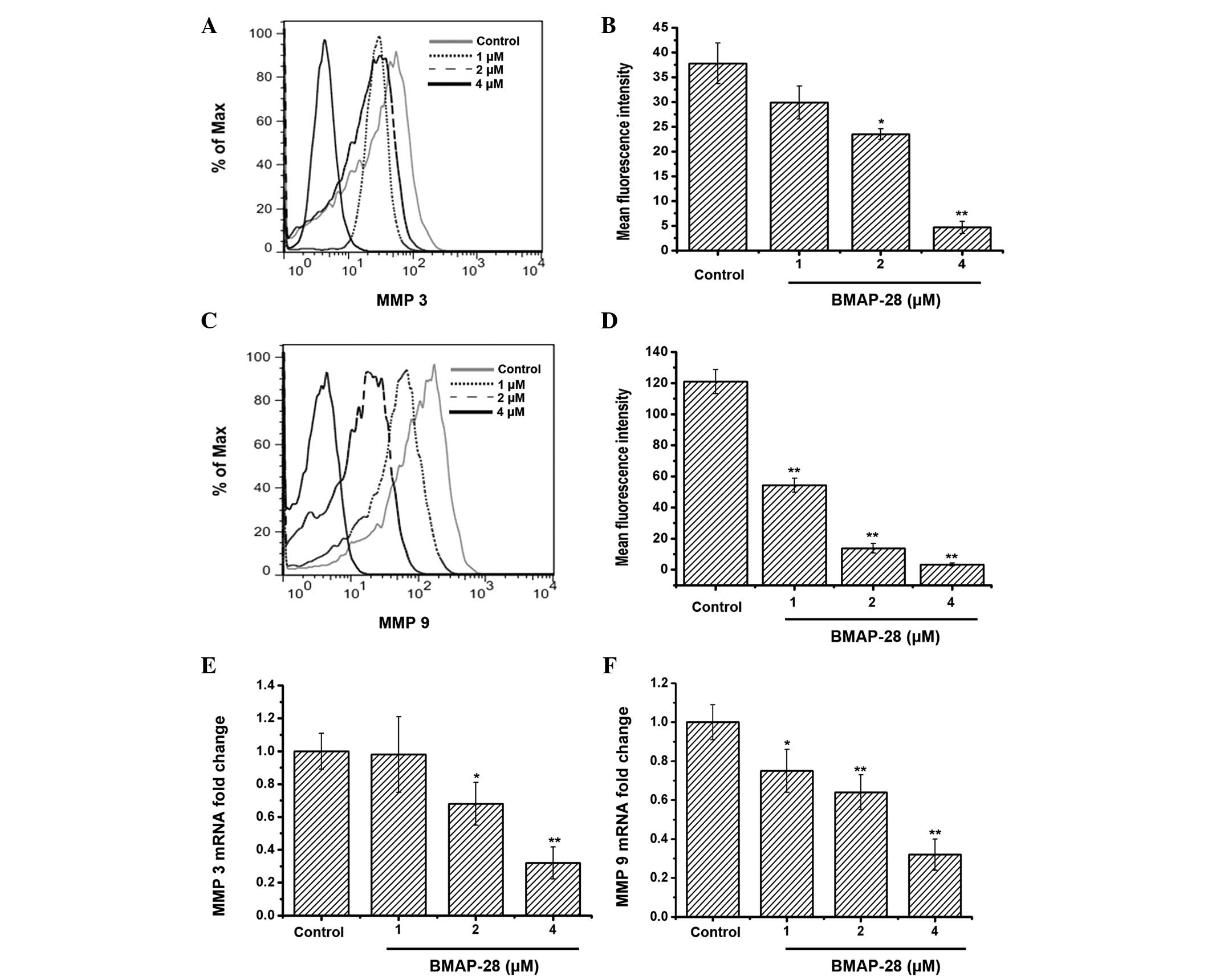
To investigate whether BMAP-28 could affect MMP3
and/or MMP9, further experiments were performed. The obtained data
indicated that the protein and mRNA expression of MMP3 and MMP9 in
the TT cells was downregulated in a dose-dependent manner following
exposure to BMAP-28 (Fig. 4).
BMAP-28 prevents tumor growth in the
TT-xenograft mouse model
In order to detect whether BMAP-28 could prevent the
TT growth in vivo, the TT-xenograft nude mouse model was
established. As shown in Fig. 5,
BMAP-28 significantly inhibited the TT cell growth as the dose
increased. Specifically, in the 1 mg/kg group, the tumor inhibitory
rate was up to 55.6%. These results suggested that BMAP-28
administration prevented the TT tumor growth in the mice.
Discussion
Thyroid cancers are the most commonly occurring
neoplasms of the endocrine system, with an excellent prognosis in
the initial stages (1). The cancer is
histopathologically classified into three groups: Differentiated
thyroid carcinoma (94%), medullary thyroid carcinoma (4%) and
anaplastic (undifferentiated) thyroid carcinoma (2%). However,
there are few available therapeutic options for advanced or
metastatic disease. Hence, it is necessary to develop novel drugs
with high efficiency and low toxicity.
AMPs, which are effective molecules of innate
immunity, have recently been shown to exhibit anticancer activity;
they have gained much attention as potential anticancer agents
(24). AMPs with cancer-selective
toxicity have become a potential source of alternative
chemotherapeutic agents that may overcome the limitations of
current drugs.
BMAP-28, a cathelicidin found in bovine neutrophils,
has been shown to possess broad-spectrum antimicrobial activity
(16). However, its anti-thyroid
cancer activity has not been elucidated. In the present study, the
anticancer activity and mechanisms of BMAP-28 on human TT cells
were investigated. It was found that BMAP-28 significantly
inhibited the proliferation of the TT cells in vitro
(Fig. 1), indicating that BMAP-28
possessed anti-TT cell activity. The mechanisms of BMAP-28 on the
TT cells were investigated in the further experiments.
Apoptosis, an active process leading to cell death,
is mediated by programmed signaling pathways that can be initiated
by a range of types of extracellular or intracellular stimulation.
In order to evaluate the efficacy of anticancer therapies, the
ability to induce apoptosis is essential (25). In the present study, the Annexin
V-FITC/PI double-staining assay suggested that BMAP-28 induced
apoptosis in the TT cells (Fig. 2).
Members of the caspase family have major roles in cell apoptosis
(26). The caspase cleavage cascade
is a well-known process that starts with initiator caspase
activation via the intrinsic or extrinsic pathways. Caspase-9 is
the key initiator caspase for the intrinsic pathway to induce cell
death, and activation of this caspase occurs with the release of
cytochrome c at the mitochondrial membrane. Caspase 3 is an
effector caspase that mediates the cleavage of multiple cellular
proteins in tandem with other effector caspases (27). The present study found that the
protein and mRNA expression of caspase-3 and caspase-9 increased
significantly in the BMAP-28-treated group, which indicated that
BMAP-28 could induce apoptosis in the TT cells partly via the
activation of caspase-9 and caspase-3 (Fig. 3). From these results, it may be
concluded that activated caspase-3 and -9 contribute to
BMAP-28-induced apoptosis.
MMPs family members are among the best characterized
proteases and consist of zinc endopeptidases sharing homologous
catalytic domains. The main characteristic of these enzymes is to
catalyze the cleavage of the extracellular matrix protein
components and to function in tissue remodeling during development,
wound healing, cancer invasion and metastasis, as well as other
physiological processes (28). A wide
variety of extracellular matrix components are known to be degraded
by the MMP-9 and MMP-3 enzymes, including collagen I, collagen IV
and the proteoglycans (29). MMP-9
has significant involvement in tumor metastasis due to its role in
basement membrane cleavage, which allows migratory phenotype cells
to be more motile and invasive (30).
The inhibition of MMP-9 and MMP-3 expression may therefore be
significant in the development of tumor metastasis treatments. As
shown in Fig. 4, in the present
study, BMAP-28 was able to inhibit the mRNA and protein of MMP-9
and MMP-3.
BAMP-28 exhibited a marked inhibitory effect on the
TT cells in vitro, which contributed to the induction of
apoptosis (caspase-3/9) and the inhibition of cancer invasion
(MMP-3/9). TT-xenograft tumor models were established in nude mice
(Fig. 5) to investigate whether
BAMP-28 also had an inhibitory effect in vivo. Compared with
the model control, BMAP-28 dose-dependently suppressed the
proliferation of the TT cells in the tumor-bearing mice. Hence,
BAMP-28 could inhibit the TT cell proliferation, not only in
vitro, but also in vivo.
In summary, BMAP-28 inhibited the proliferation of
human TT cells in vitro and in vivo through the
induction of the activation of caspase-3 and caspase-9 and the
inhibition of MMP-3 and MMP-9. These results suggested that BMAP-28
may be a potential candidate for the treatment of thyroid
cancer.
Acknowledgements
The present study was financially supported by a
grant from Jilin Provincial Finance Department (no.
SCZSY201504).
References
|
1
|
Kleiman DA, Buitrago D, Crowley MJ,
Beninato T, Veach AJ, Zanzonico PB, Jin M, Fahey TJ III and
Zarnegar R: Thyroid stimulating hormone increases iodine uptake by
thyroid cancer cells during BRAF silencing. J Surg Res. 182:85–93.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
HombachKlonisch S, Natarajan S,
Thanasupawat T, Medapati M, Pathak A, Ghavami S and Klonisch T:
Mechanisms of therapeutic resistance in cancer (stem) cells with
emphasis on thyroid cancer cells. Front Endocrinol (Lausanne).
25:5–37. 2014.
|
|
3
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: Update on epidemiology and risk factors. J Cancer
Epidemiol. 2013:9652122013.PubMed/NCBI
|
|
4
|
Stjepanovic N and Capdevila J: Multikinase
inhibitors in the treatment of thyroid cancer: Specific role of
lenvatinib. Biologics. 8:129–139. 2014.PubMed/NCBI
|
|
5
|
Massimino M, Vigneri P, Fallica M, Fidilio
A, Aloisi A, Frasca F and Manzella L: IRF5 promotes the
proliferation of human thyroid cancer cells. Mol Cancer. 16:11–21.
2012.
|
|
6
|
Fagin JA: Perspective: Lessons learned
from molecular genetic studies of thyroid cancer-insights into
pathogenesis and tumor-specific therapeutic targets. Endocrinology.
143:2025–2028. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hancock RE and Chapple DS: Peptide
antibiotics. Antimicrob Agents Chemother. 43:1317–1323.
1999.PubMed/NCBI
|
|
8
|
Hancock RE and Sahl HG: Antimicrobial and
host-defense peptides as new anti-infective therapeutic strategies.
Nat Biotechnol. 24:1551–1557. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mulder KC, Lima LA, Miranda VJ, Dias SC
and Franco OL: Current scenario of peptide-based drugs: The key
roles of cationic antitumor and antiviral peptides. Front
Microbiol. 4:3212013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mader JS and Hoskin DW: Cationic
antimicrobial peptides as novel cytotoxic agents for cancer
treatment. Expert Opin Investig Drugs. 15:933–946. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Camilio KA, Berge G, Ravuri CS, Rekdal O
and Sveinbjørnsson B: Complete regression and systemic protective
immune responses obtained in B16 melanomas after treatment with
LTX-315. Cancer Immunol Immunother. 63:601–613. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schweizer F: Cationic amphiphilic peptides
with cancer-selective toxicity. Eur J Pharmacol. 625:190–194. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riedl S, Zweytick D and Lohner K:
Membrane-active host defense peptides-challenges and perspectives
for the development of novel anticancer drugs. Chem Phys Lipids.
164:766–781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu S, Yang H, Wan L, Cheng J and Lu X:
Penetratin-mediated delivery enhances the antitumor activity of the
cationic antimicrobial peptide magainin II. Cancer Biother
Radiopharm. 28:289–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lynn MA, Kindrachuk J, Marr AK, Jenssen H,
Panté N, Elliott MR, Napper S, Hancock RE and McMaster WR: Effect
of BMAP-28 antimicrobial peptides on Leishmania major
promastigote and amastigote growth: Role of leishmanolysin in
Parasite Survival. PLoS Negl Trop Dis. 5:e11412011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benincasa M, Skerlavaj B, Gennaro R,
Pellegrini A and Zanetti M: In vitro and in vivo
antimicrobial activity of two alpha-helical cathelicidin peptides
and of their synthetic analogs. Peptides. 24:1723–1731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giacometti A, Cirioni O, Ghiselli R,
Bergnach C, Orlando F, D'Amato G, Mocchegiani F, Silvestri C, Del
Prete MS, Skerlavaj B, et al: The antimicrobial peptide BMAP-28
reduces lethality in mouse models of staphylococcal sepsis. Crit
Care Med. 32:2485–2490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahmad A, Asthana N, Azmi S, Srivastava RM,
Pandey BK, Yadav V and Ghosh JK: Structure-function study of
cathelicidin-derived bovine antimicrobial peptide BMAP-28: Design
of its cell-selective analogs by amino acid substitutions in the
heptad repeat sequences. Biochim Biophys Acta. 1788:2411–2420.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Starenki D and Park JI:
Mitochondria-targeted nitroxide, Mito-CP, suppresses medullary
thyroid carcinoma cell survival in vitro and in vivo.
J Clin Endocrinol Metab. 98:1529–1540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Ke M, Tian Y, Wang J, Li B, Wang
Y, Dou J and Zhou C: BF-30 selectively inhibits melanoma cell
proliferation via cytoplasmic membrane permeabilization and
DNA-binding in vitro and in B16F10-bearing mice. Eur J
Pharmacol. 707:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian Y, Wang H, Li B, Ke M, Wang J, Dou J
and Zhou C: The cathelicidin-BF Lys16 mutant Cbf-K16 selectively
inhibits non-small cell lung cancer proliferation in vitro.
Oncol Rep. 30:2502–2510. 2013.PubMed/NCBI
|
|
22
|
Cheng YX, Liu R, Wang Q, Li BS, Xu XX, Hu
M, Chen L, Fu Q, Pu DM and Hong L: Regular-induced apoptosis of
cervical cancer cell line siha via cytochrome c release and
caspase-3 and caspase-9 activation. Chin J Integr Med. 18:359–365.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu J, Yao YY, Dai QM, Ma GS, Zhang SF, Cao
L, Ren LQ and Liu NF: Erythropoietin attenuates cardiac dysfunction
by increasing myocardial angiogenesis and inhibiting interstitial
fibrosis in diabetic rats. Cardiovasc Diabetol. 11:1052012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han YY, Liu HY, Han DJ, Zong XC, Zhang SQ
and Chen YQ: Role of glycosylation in the anticancer activity of
antibacterial peptides against breast cancer cells. Biochem
Pharmacol. 86:1254–1262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tseng TH, Shen CH, Huang WS, Chen CN,
Liang WH, Lin TH and Kuo HC: Activation of
neutral-sphingomyelinase, MAPKs and p75 NTR-mediating caffeic acid
phenethyl ester-induced apoptosis in C6 glioma cells. J Biomed Sci.
21:612014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Floyd DH, Zhang Y, Dey BK, Kefas B, Breit
H, Marks K, Dutta A, HeroldMende C, Synowitz M, Glass R, et al:
Novel anti-apoptotic microRNAs 582-5p and 363 promote human
glioblastoma stem cell survival via direct inhibition of
caspase 3, caspase 9 and Bim. PLoS One. 9:e962392014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wyllie AH: ‘Where, O death, is thy sting?’
A brief review of apoptosis biology. Mol Neurobiol. 42:4–9. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim YS, Kim SH, Kang JG and Ko JH:
Expression level and glycan dynamics determine the net effects of
TIMP-1 on cancer progression. BMB Rep. 45:623–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim HR, Kim JM, Kim MS, Hwang JK, Park YJ,
Yang SH, Kim HJ, Ryu DG, Lee DS, Oh H, et al: Saussurea
lappa extract suppresses TPA-induced cell invasion via
inhibition of NF-κB-dependent MMP-9 expression in MCF-7 breast
cancer cells. BMC Complement Altern Med. 14:1702014. View Article : Google Scholar : PubMed/NCBI
|