Introduction
Human cytomegalovirus (HCMV) is a β-herpesvirus that
causes persistent infection in humans and may cause severe disease
in immunocompromized individuals. There is evidence that HCMV
infection and expression may be associated with human malignancies,
including malignant glioma, and colon and prostate cancer (1–5). HCMV may
increase the malignancy of infected cells by disrupting cellular
pathways involved in the cell cycle, apoptosis, angiogenesis, cell
invasion and host immune response (6). Glioma is the most common primary brain
tumor and arises from astrocytes and the precursors of astrocytes.
Although the treatment of glioma has markedly advanced, the
treatment efficacy is not so promising. It is of vital importance
for HCMV infection to affect the central nervous system, and glioma
is one of the most common primary diseases of the central nervous
system (1,7–9).
In 2002, Cobbs et al initially reported the
association between HCMV and malignant glioma (10). This study confirmed that HCMV nucleic
acids and proteins were present in a high proportion of high- and
low-grade gliomas, classified as World Health Organization (WHO)
grades II–IV. The expression of early and delayed gene products
occurred in these tumors (10). It is
well-known that the HCMV genome encodes >200 proteins with
expression that occurs in a program of three sequential stages.
Immediate-early (IE) proteins are necessary for replication and
activate early genes. Early proteins are essential for certain
functional enzymes and, therefore, for the virus. Late proteins
comprise the structural components of the virus. The HCMV
immediate-early-1 (IE-1) protein was detected in >90% of
gliomas. Almost 80% of newly diagnosed patients with glioblastoma
multiforme (GBM) contain HCMV DNA in the peripheral blood, which
indicates either systemic reactivation or viral shedding from tumor
cells to the periphery. The immediate-early 2 (IE86) protein of
human cytomegalovirus (HCMV) binds to the tumor suppressor p53 and
inactivates the function of this protein through unknown
mechanisms. IE86 is able to suppress the histone acetyltransferase
(HAT) activity of the p53 transcriptional coactivators P300 and
cyclic adenosine monophosphate response element-binding protein
(CREB) binding protein (CBP). IE86 deletion mutants lacking the
minimal N-terminal HAT inhibitory domain fail to repress the in
vivo DNA binding of p53 and local histone acetylation.
The activating transcription factor 5 (ATF5) is a
member of the ATF/CREB family of basic zipper proteins (11,12). It
has previously been confirmed that ATF5 is highly expressed in
malignant glioma and is essential for glioma cell survival
(13). It has also been demonstrated
that HCMV infection inhibits apoptosis by regulating the ATF5
signaling pathway in human malignant glioma cells (14,15). ATF5
expression is downregulated by trophic factors and this is required
for the promotion of neuroprogenitor cell cycle exit and
differentiation into other neurons, oligodendroglia or astrocytes.
Examination of the role of ATF5 in glioblastoma cells indicates
that interference with the expression or activity of ATF5 results
in the cells undergoing apoptotic death. By contrast, normal
astrocytes and neurons do not appear to require ATF5 for survival,
indicating that ATF5 may be a selective target for the treatment of
glioblastoma and other neural neoplasias (16,17).
Histone acetylation is closely associated with
certain biological effects, including gene transcription, and is
catalyzed by HATs. P300 is one of the numerous HATs that are able
to change the structure of the chromosome and then trigger the
process of transcription (18). In
the meantime, the HAT P300 acetylates the various cellular
proteins, and numerous studies indicate that the interaction
between P300 and certain viral proteins promote the replication of
the virus. It was revealed that IE86 binds to the HAT domain of the
p53 coactivators P300 and CBP, and blocks the acetyltransferase
activity of histones and p53 (19–22). HCMV
IE86 downregulates p53-dependent gene activation by inhibiting
P300/CBP-mediated local histone acetylation and that IE86 may have
oncogenic activity.
The acetylation histones affect the accessibility of
chromatin for DNA replication, repair and transcription.
Acetylation is the addition of an acetyl group from the co-factor
acetyl coenzyme A to the amino group of lysine residues on
histones, which is catalyzed by group enzymes termed HATs.
Targeting of cellular HATs by viral proteins is important in the
development of virus-associated diseases. Acetylation on histones,
in general, demonstrates two distinct functions (20). First, histone acetylation may loosen
the interactions between histones and DNA, as acetylation
neutralizes the positive charge on a lysine residue, and thereby
reduces electrostatic interactions between DNA and histones.
Second, acetylation on a particular lysine residue may also recruit
proteins to chromatin to perform specific functions. DNA is tightly
bound around the nucleosome core, which consists of histone
residues H2A, H2B, H3 and H4. It is known that histone
modifications regulate diverse cellular processes that are critical
for cancer prevention. In recent years, with the development of
molecular biology and the progression of studies investigating
glioma, it was found that the incidence and development of glioma
were associated with acetyl-histone H3K9 and acetyl-histone H3K14,
which were the most common histone acetylation forms. The
identification of histone acetylation profiles in human neoplasia
has been a major factor in constructing a novel paradigm, in which
acetylation histones contribute markedly to human disease (23).
Materials and methods
Tissue specimens and clinical
data
In total, 60 tissue samples were obtained from the
Department of Pathology of the Affiliated Hospital of Qingdao
University Medical College (Qingdao, Shandong, China) between March
and December 2012. No patients underwent radiation or chemotherapy
prior to surgical therapy. Ethical approval was obtained from the
Institutional Review Board of the Affiliated Hospital of Qingdao
University Medical College.
The pathological diagnosis and grading for each
glioma was assessed by neuropathologists, according to the 2007 WHO
Classification of Nervous System Tumors (24). In total, 60 patients with glioma were
analyzed, consisting of 25 patients with GBM, 16 patients with
anaplastic glioma and 19 patients with low-grade glioma. The
anaplastic tumors consisted of 9 anaplastic astrocytomas, 3
anaplastic ependymomas, 3 anaplastic oligodendrogliomas and 1
anaplastic oligoastrocytoma. The low-grade tumors consisted of 3
astrocytomas, 2 pilocytic astrocytomas, 2 ependymomas, 1 ganglioma,
10 oligodendrogliomas and 1 subependymoma. In addition, 9 tissue
specimens obtained from the normal cortex of patients without
glioma were used as the negative control. All tissue samples were
collected in accordance with Institutional Review Board-approved
protocols. The mean age of the patients at the time of diagnosis
was 55, 45, 43 and 42 years for GBM, anaplastic glioma, low-grade
glioma and normal cortex, respectively. Overall, 36% of all
patients were female. The formalin-fixed, paraffin-embedded tissue
blocks were archived in the Section of Neuropathology of the
Department of Pathology. The present study was approved by the
Institutional Review Board of the Affiliated Hospital of Qingdao
University Medical College and written informed consent was
obtained from all patients.
Immunohistochemical analysis
The paraffin-embedded sections were heated overnight
on slides in an oven at 60°C. The slides were removed from the oven
and deparaffinized by placing in xylene (BioGenex, San Ramon, CA,
USA). Subsequently, the slides were soaked in serial dilutions of
ethanol (100, 95, 75 and 50% ethanol) to deparaffinize the slides,
and then post-fixed in neutral-buffered saline. The sections were
blocked for endogenous peroxidase in 3% H2O2
for 12 min. Antigen retrieval was performed for 20 min in a
microwave oven on high power using citrate-buffered saline (pH
6.0). The slides were incubated in 5% bovine serum albumin to block
non-specific binding. The sections were then incubated with the
primary antibody overnight at 4°C, washed in phosphate-buffered
saline (PBS) and incubated with secondary antibody for 1 h at 37°C.
The sections were stained using 3,3-diaminobenzidine, which was
stored at room temperature without light for 10 min, and the
staining was finished using distilled water. The slides were then
counterstained using hematoxylin. An additional step of
hydrochloric acid alcohol differentiation was performed.
Subsequently, dehydration, clearing and mounting with neutral gums
were performed in turn.
Mouse anti-human HCMV IE2-86 monoclonal antibody
(cat no. 0841) in the present experiment was purchased from
Virostat (Westbrook, ME, USA) and used at a 1:30 dilution. Rabbit
anti-human P300 polyclonal antibodies (cat no. sc-585) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) and
used at a dilution of 1:200. Rabbit anti-human acetyl-histone H3K9
polyclonal antibodies (cat no. YK0006) were obtained from Immunoway
Biotechnology and used at a dilution of 1:200. Rabbit anti-human
acetyl-histone H3K14 monoclonal antibodies (cat no. ab52946) were
purchased from Abcam (Cambridge, UK) and used at a dilution of
1:200. Rabbit anti-human ATF5 polyclonal antibodies (cat no.
ab6012) were also purchased from Abcam and used at a dilution of
1:250.
The slides were examined independently by two
investigators blinded to the clinical and pathological data of the
patients. Protein expression was quantified using a visual grading
system based on the extent of staining, determined by the
percentage of positive tumor cells, which was graded on a scale of
0–4, as follows: 0, none; 1, 1–25%; 2, 26–50%; 3, 51–75%; and 4,
>75%. The staining intensity was graded on a scale of 0–3, as
follows: 0, no staining; 1, weak staining; 2, moderate staining;
and 3, strong staining. For additional analysis, the product of the
staining extent and intensity grades was used to define the cut-off
value for high expression of the proteins, and the protein
expression was classified into two categories, high (grades 12–4)
and low (grades 0–3).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) to detect ATF5, P300 and IE2
mRNA expression in glioma
The PCR primers were as follows: ATF5 forward,
5′-AAGTCGGCGGCTCTGAGGTA-3′ and reverse, 5′-TCC
GAACTCAAAGAAGGCCA-3′; P300 forward, 5′-GACCCT CAGCTTTTAGGAATCC-3′
and reverse, 5′-TGCCGTAGC AACACAGTGTCT-3′; IE2 forward,
5′-GCGCAATATCAT GAAAGATAAGAACA-3′ and reverse, 5′-GATTGGTGTTGC
GGAACATG-3′; and β-actin forward, 5′-TGGAACGGTGAA GGTGACAG-3′ and
reverse, 5′-GGCTTTTAGGATGGC AAGGG-3′. β-actin was used as an
internal control. The primers were synthetized by Shanghai Sangon
Biological Co., Ltd. (Shanghai, China). PCR was performed at 95°C
for 10 min, followed by 40 cycles of 95°C for 15 sec and 55°C for 1
min. Each reaction was performed in triplicate and analyzed
individually relative to β-actin, which was calculated using the
2−∆∆Ct method.
RNA extraction and RT-qPCR
RNA was extracted using TRIzol reagent (Takara Bio,
Inc., Otsu, Shiga, Japan), according to the manufacturer's
instructions. To obtain cDNA, 1 µg RNA was reverse-transcribed
using the PrimeScript RT reagent kit with gDNA Eraser (Takara Bio,
Inc.), according to the manufacturer's instructions. RT-qPCR was
performed using the GoTaq qPCR Master Mix (Promega, Madison, WI,
USA). The primers used were as follows: ATF5 forward,
5′-AGTGGGCTGGGATGGCTCGTAGAC-3′ and reverse,
5′-CTCGGGTGGTGGCAGGATGTGG-3′; IE2 forward,
5′-GCGCAATATCATGAAAGATAAGAACA-3′ and reverse,
5′-GATTGGTGTTGCGGAACATG-3′; and β-actin forward,
5′-TGGAACGGTGAAGGTGACAG-3′ and reverse, 5′-GGC
TTTTAGGATGGCAAGGG-3′.
Western blot analysis
For each tissue specimen, 50 mg of tissue was
transferred into a 1.5 ml microcentrifuge tube. A total of 500 µl
cell lysis buffer was added to the tube. The tissue was homogenized
on ice by 10–15 strokes (3–4 sec/stroke) of a mini-homogenizer and
a plastic pestle. The sample was centrifuged at 12,000 × g for 15
min at 4°C and the supernatant was then transferred to a fresh
tube. A total of 50 µg protein and an equal volume of 2X sample
buffer were heated at 94°C for 5 min. The proteins were separated
on an 8% sodium dodecyl sulfate-polyacrylamide gel and then
transblotted onto polyvinylidene difluoride membranes. The
membranes were blocked in PBS with Tween 20 (Tianjin Guangfu Fine
Chemical Research Institute, Tianjin, China) and 5% skimmed dried
milk at 37°C for 1 h. The membranes were incubated with anti-ATF5
(dilution, 1:2,000), anti-IE86 (dilution, 1:40), anti-P300
(dilution, 1:1,000), anti-acetyl-histone H3K9 (dilution, 1:1,000)
and anti-acetyl-histone H3K14 (dilution, 1:2,000) primary
antibodies at 4°C overnight. The membranes were then treated with
goat anti-rabbit (dilution, 1:2,000; cat. no. BA1054-1) and goat
anti-mouse (dilution, 1:2,000; cat. no. BA1050-0.5) IgG secondary
antibodies (BosterBio, Pleasanton, CA, USA). The western blot
membranes were developed using enhanced chemiluminescence reagents
and visualized using the BIO-PRINT ST4 gel imaging system (Vilber
Lourmat, Marne-la-Vallée, France).
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(IBM, Armonk, NY, USA). One-way analysis of variance and least
significant difference test were used to analyze the present data.
P<0.05 was considered to indicate a statistically significant
difference. The values are expressed as the mean ± standard
deviation.
Results
Expression of ATF5, IE86, P 300,
acetyl-histone H3K9 and acetyl-histone H3K14 in GBM, anaplastic
glioma, low-grade glioma and normal cortex tissues
Immunohistochemical analysis revealed that the
expression of the ATF5, IE86, P300, acetyl-histone H3K9 and
acetyl-histone H3K14 proteins in GBM and anaplastic glioma tissues
was significantly increased compared with the expression in the
normal cortex tissues. However, there was little difference between
the expression levels of the ATF5, IE86, P300, acetyl-histone H3K9
and acetyl-histone H3K14 proteins in the low-grade glioma tissues
and normal cortex tissues (Fig. 1).
The results indicated that the protein expression levels of ATF5,
IE86, P300, acetyl-histone H3K9 and acetyl-histone H3K14 may be
associated with the clinical development and classification of
gliomas.
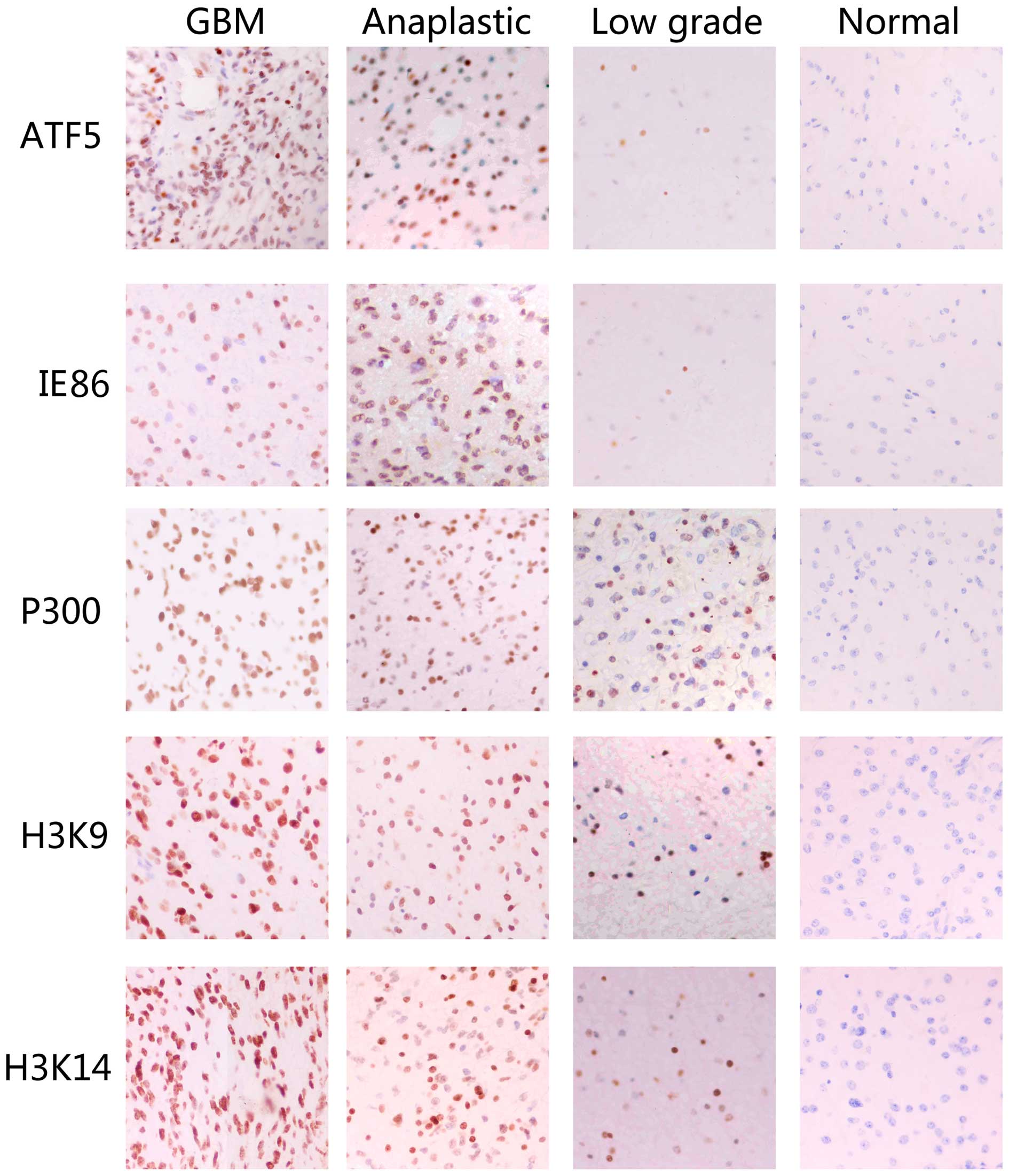 | Figure 1.IE86, ATF5, P300, acetyl-histone H3K9
and acetyl-histone H3K14 overexpression is associated with the
histological level of human glioma tissue specimens.
Immunohistochemical staining with the indicated specific antibodies
was performed on 25 glioblastoma, 16 anaplastic astrocytoma, 19
low-grade gloma and 9 normal cortex tissues samples. The
representative images of three tumor samples indicate the pattern
of IE86, ATF5, P300, acetyl-histone H3K9 and acetyl-histone H3K14
expression in different sections. IE86, immediate-early 2 protein;
ATF5, activating transcription factor 5; GBM, glioblastoma
multiforme. |
Analysis of protein expression in the tissue
specimens revealed that the expression level of ATF5 in GBM tissues
was evidently increased compared with the expression in anaplastic
glioma, low-grade glioma and normal cortex tissues (P<0.05). The
expression level of the ATF5 protein in anaplastic glioma tissues
was significantly increased compared with the expression in
low-grade glioma and normal cortex tissues (P<0.05). However,
the expression level of the ATF5 protein in low-grade glioma
tissues was slightly increased compared with the expression in the
normal cortex (P>0.05). It was concluded that patient age and
gender are not associated with the expression levels of ATF5 in
glioma tissues, but ATF5 expression is associated with the
classification of gliomas.
Immunohistochemical staining also revealed that IE
expression was markedly increased in GBM tissues compared with the
expression in low-grade glioma and normal cortex tissues
(P<0.05). However, the expression level of IE86 was not
evidently increased in GBM tissues compared with the expression in
anaplastic glioma tissues (P>0.05). In addition, the expression
level of IE86 in low-grade glioma tissues was not significantly
different from the expression in the normal cortex tissues
(P>0.05). However, the expression level of IE86 in anaplastic
glioma tissues was significantly increased compared with the
expression in low-grade glioma and normal cortex tissues
(P<0.05). The expression level of IE86 in low-grade glioma
tissues was also significantly increased (P<0.05) compared with
the expression in the normal cortex tissues. Therefore, the
expression level of IE86 in glioma was associated with the
classification of gliomas, which was independent of patient gender
and age.
The expression level of P300 in GBM tissues was
significantly increased (P<0.05) compared with the expression in
anaplastic glioma, low-grade glioma and normal cortex tissues. The
expression level of P300 in GBM tissues was significantly increased
(P<0.05) compared with the expression in low-grade glioma and
normal cortex tissues. However, there was no difference in the
expression levels of P300 between the low-grade glioma and normal
cortex (P>0.05). It was concluded that the expression level of
P300 is associated with the classification of glioma, but is not
associated with patient gender or age.
There are marked variations in the expression levels
of acetyl-histone H3K9 in GBM tissues compared with the expression
levels in anaplastic glioma, low-grade glioma and normal cortex
tissues (P<0.05). The expression level of acetyl-histone H3K9 in
anaplastic glioma tissues is extremely different from the
expression level in low-grade glioma and normal cortex tissues
(P<0.05). However, there is little difference between the
expression levels of acetyl-histone H3K9 in low-grade glioma
tissues and normal cortex tissues (P>0.05). The expression level
of acetyl-histone H3K9 was associated with the classification of
gliomas, but was not associated with age or gender.
The expression level of acetyl-histone H3K14 in GBM
tissues was markedly different from the expression in anaplastic
glioma, low-grade glioma and normal cortex tissues (P<0.05). The
expression level of acetyl-histone H3K14 in anaplastic glioma was
significantly different from the expression levels in low-grade
glioma and normal cortex tissues (P<0.05). There was no
difference between the expression levels of acetyl-histone H3K14 in
low-grade glioma and that in the normal cortex (P>0.05). The
expression levels of acetyl-histone H3K14 demonstrated no
association with age or gender.
Overall, the present results indicated that as the
malignancy degree of glioma increased, the increased expression of
ATF5, IE86 and P300 demonstrated a notable significance in
evaluating the malignancy degree of human glioma. The level of
histone acetylation, indicated by H3K9 and H3K14, was positively
associated with the malignant grade of brain glioma (Tables I–V).
 | Table I.Expression of ATF5 in GBM, anaplastic
glioma, low-grade glioma and the normal cortex. |
Table I.
Expression of ATF5 in GBM, anaplastic
glioma, low-grade glioma and the normal cortex.
|
| Expression of
ATF5 |
|
|---|
|
|
|
|
|---|
| Clinical
characteristics | High, n | Low, n | P-value |
|---|
| Age, years |
|
|
|
|
Median | 61 | 58 | 0.261 |
|
Range | 42–80 | 41–75 |
|
| Gender |
|
|
|
| Male | 18 | 14 | 0.171 |
|
Female | 21 | 16 |
|
| Grade |
|
|
|
| GBM | 24 | 1 |
|
|
Anaplastic glioma | 12 | 4 | 0.045a |
| Low-grade
glioma | 2 | 17 | 0.045a |
|
|
|
| 0.000b |
| Normal
cortex | 1 | 8 | 0.000a |
|
|
|
| 0.002b |
|
|
|
| 0.963c |
 | Table V.Expression of Acetyl-Histone H3K14 in
GBM, anaplastic glioma, low-grade glioma and the normal cortex. |
Table V.
Expression of Acetyl-Histone H3K14 in
GBM, anaplastic glioma, low-grade glioma and the normal cortex.
|
| Expression of
acetyl-histone H3K14 |
|
|---|
|
|
|
|
|---|
| Clinical
characteristics | High, n | Low, n | P-value |
|---|
| Age, year |
|
|
|
|
Median | 60 | 58 | 0.277 |
|
Range | 50–70 | 49–67 |
|
| Gender |
|
|
|
|
Male | 18 | 15 | 0.168 |
|
Female | 22 | 14 |
|
| Grade |
|
|
|
|
GBM | 25 | 0 |
|
|
Anaplastic glioma | 12 | 4 | 0.008a |
|
Low-grade glioma | 3 | 16 | 0.000a |
|
|
|
| 0.000b |
| Normal
cortex | 0 | 9 | 0.000a |
|
|
|
| 0.000b |
|
|
|
| 0.207c |
mRNA expression of ATF5 and P300
increased with IE2 as the malignancy of glioma increased
RT-qPCR and western blot analysis were performed to
investigate the expression of ATF5, IE2, P300, H3K9 and H3K14 mRNA
in human glioma and to elucidate the clinical significance of this
mRNA expression. As shown in Fig. 2,
the expression levels of ATF5, IE2 and P300 mRNA demonstrated a
sustainable increase between the normal cortex tissues and GBM
tissues. The highest levels of ATF5, IE2 and P300 mRNA expression
were identified in the samples obtained from patients with GBM. The
expression levels of ATF5, IE2 and P300 mRNA were by far the lowest
percentage in the LGG and normal cortex groups. GBM and anaplastic
gliomas were considered to be high-grade gliomas. As shown in
Fig. 3, western blot analysis
revealed that the expression of ATF5, IE2, P300, acetyl-histone
H3K9 and acetyl-histone H3K14 mRNA was significantly increased in
the high-grade glioma tissue specimens compared with the normal
cortex tissues. However, this increase in expression was not
evident in low-grade glioma tissues.
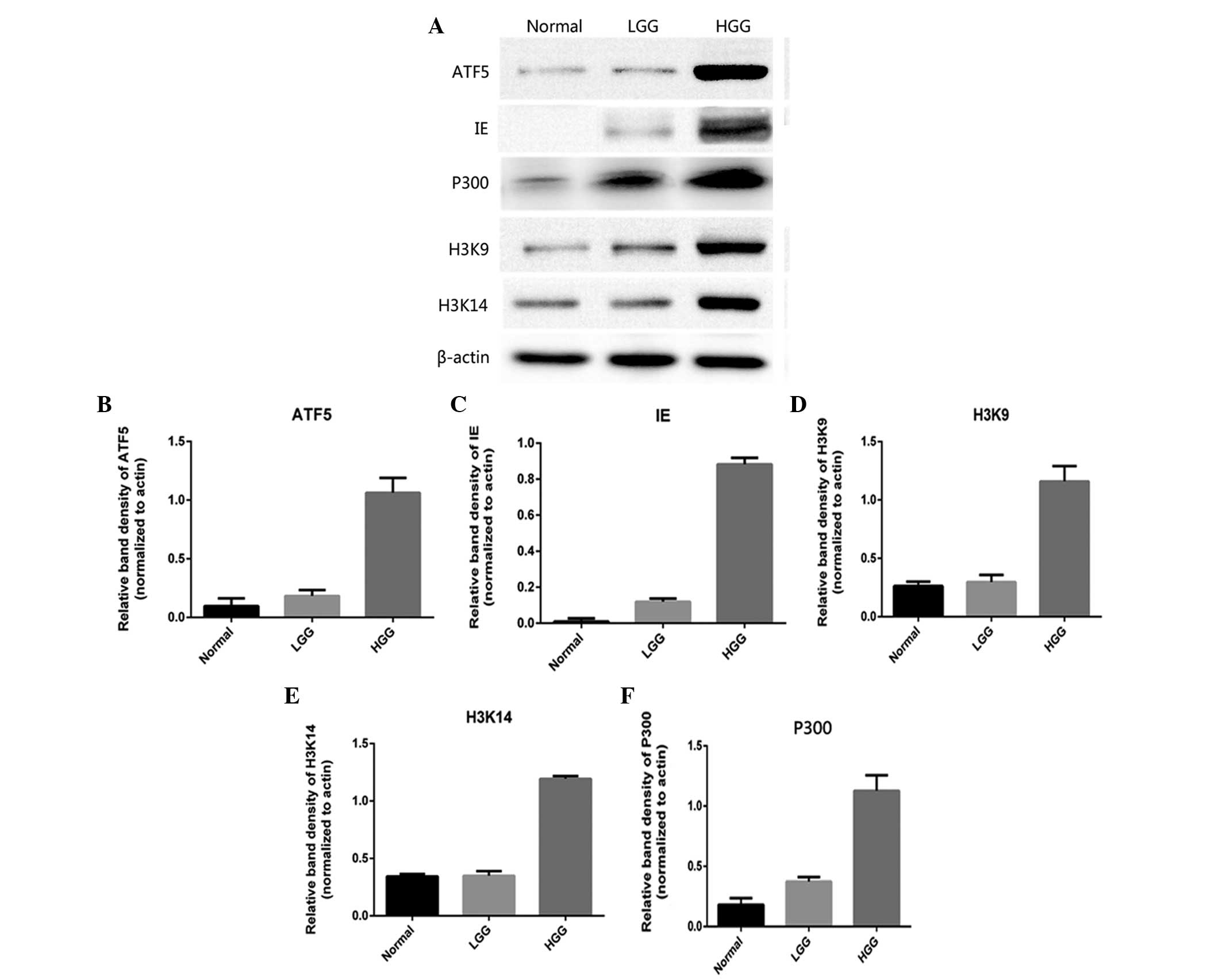 | Figure 3.(A) Western blot analysis of the
expression level of IE86, ATF5, P300, acetyl-histone H3K9 and
acetyl-histone H3K14 acetylation in various types of glioma.
Western blot analysis performed on various grades of human glioma
specimens confirmed the immunohistochemical data. The data
indicated that the expression of (B) ATF5, (C) IE86, (D)
acetyl-histone H3K9, (E) acetyl-histone H3K14 and (F) P300 was
highest in HGG, consisting of GBM and anaplastic glioma, followed
by LGG and then the normal cortex tissues. IE86, immediate-early 2
protein; ATF5, activating transcription factor 5; GBM, glioblastoma
multiforme; LGG, low-grade glioma; HGG, high-grade glioma. |
Discussion
Based on the findings and results of previous
studies that have investigated the activity of HCMV proteins in
glioma tissues and glioma cell lines, there is considered to be
sufficient evidence to conclude that HCMV sequences and viral gene
expression exist in the majority of malignant gliomas (3–5,8). However, previous studies have not
focused on determining the association between the host cell
proteins and high expression of HCMV, or on the role of HCMV as a
glioma-initiating event. In the present study, the expression of
ATF5, a basic leucine zipper transcription factor, and the cell
acetylation level were revealed to be positively associated with
the expression of the HCMV immediate-early regulator protein
IE86.
In general, there is considered to be sufficient
evidence to support the hypothesis that HCMV can alter the
malignant phenotype in glioma via modulation of the proliferation,
apoptosis and differentiation of glioma cells . It is becoming
clear that the post-translational modification of histone and
non-histone proteins by acetylation is a component of an important
cellular signaling process that controls tumor progression.
Previous studies have identified this signaling pathway as one of
the primary targets of viral proteins subsequent to HCMV infection.
IE proteins are the proteins that are initially expressed following
HCMV infection. There is also an association between high levels of
IE86 expression and the malignant progression of glioma (4,10,25). A previous study has reported that IE86
interacts directly with chromatin acetylation factor
P300/CBP-associated factor during infection. The interaction of the
IE86 protein with P300 has been revealed to lead to the
dissociation of the myoblast determination protein (MyoD)-P300
complex and to the downregulation of MyoD transcriptional activity
(20,26). The acetylation of histone H3 at K9
(H3K9) or K14 (H3K14) is increased during the initial phases of
infection at the immediate-early promoters in human MRC-5 embryonic
lung cells (27). Overall, these
results indicate that high levels of IE86 expression are associated
with the malignant progression of glioma and may affect the
acetylation of glioma cells. In the present study, the expression
of acetyl transferase P300 and the acetylation level of H3K9 and
H3K14 were detected. The high expression of P300, H3K9 and H3K14 in
high-grade glioma tissues and the association with the malignant
progression of glioma suggested that the presence of HCMV IE86 in
glioma cells may regulate the cell signaling pathway by acetylating
histone and non-histone proteins.
IE86 has been revealed to bind to a number of
cellular factors, indicating that IE86 may also act as a bridge
between transcription factors and increase the recruitment of
general transcription factors to the pre-initiation complex
(21,22,28–30).
Previous studies have reported that IE2-86 forms complexes with
P300 and CBP, which in turn bind to CREB and may act as adaptor
proteins for the function of CREB (22). As a member of the ATF/CREB family,
ATF5 contains associated basic leucine zipper domains. The basic
region is enriched with lysine and arginine residues and is
involved in DNA binding, whereas the leucine zipper motif mediates
protein-protein interactions (12,31).
Notably, it has previously been indicated that ATF5 may also form a
complex with P300 and enhance ATF5 acetylation (32). Therefore, it was hypothesized that
ATF5 is positively associated with the increased expression of
IE86. In the present study, immunohistochemical analysis revealed
that IE86 expression is associated with ATF5 expression and the
clinical development and classification of gliomas, but the
expression of IE86 is independent of patient gender and age. GBM
and anaplastic glioma were classified as high-grade gliomas, and
the present study identified no significant difference between the
expression levels of ATF5, IE86 and histone acetylation protein in
these two tissue types. RT-qPCR and western blot analysis were then
performed to investigate the expression of ATF5, IE86, P300,
acetyl-histone H3K9 and acetyl-histone H3K14 in the high-grade and
low-grade glioma tissues. The expression of all proteins was
significantly increased in the high-grade glioma tissues compared
with low-grade glioma tissues and tissues from the normal
cortex.
The exact role of HCMV in glioma remains under
investigation. There is no conclusive evidence of the
transformation of normal human cells subsequent to HCMV infection
(33). Therefore, HCMV may modulate
the malignant properties of the tumor cells through mechanisms that
affect the cell cycle, survival, invasive potential, chromosomal
stability, immunodetection and angiogenic properties of the cells.
Glioma cells provide a genetic environment, including the high
level of ATF5 and histone acetylation, that is characterized by
disturbances in intracellular signaling pathways, transcriptional
control and tumor suppressor proteins, which enables HCMV to exert
oncomodulatory effects on tumor cells, but not in normal cells
(12). Thus, the excessive expression
of the HCMV IE86 and ATF5 proteins is a predictor of the malignancy
level in glioma patients and may be a novel target of molecular
therapies for glioma. However, the exact role of HCMV in regulating
glioma malignancy through ATF5 should be investigated in future
studies.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81471958) and the Natural
Science Foundation of Shandong Province (grant no. J 122211).
Glossary
Abbreviations
Abbreviations:
|
ATF5
|
activating transcription factor 5
|
|
HCMV
|
human cytomegalovirus
|
|
IE
|
immediate early genes
|
|
WHO
|
World Health Organization
|
|
CREB
|
cyclic adenosine monophosphate
response element-binding protein
|
|
CBP
|
CREB-binding protein
|
|
GBM
|
glioblastoma
|
|
HGG
|
high-grade glioma, consisting of GBM
and anaplastic glioma
|
|
HAT
|
histone acetyltransferase
|
References
|
1
|
Ranganathan P, Clark PA, Kuo JS, Salamat
MS and Kalejta RF: Significant association of multiple human
cytomegalovirus genomic loci with glioblastoma multiforme samples.
J Virol. 86:854–864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dziurzynski K, Wei J, Qiao W, Hatiboglu
MA, Kong LY, Wu A, Wang Y, Cahill D, Levine N, Prabhu S, et al:
Glioma-associated cytomegalovirus mediates subversion of the
monocyte lineage to a tumor propagating phenotype. Clin Cancer Res.
17:4642–4649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soroceanu L and Cobbs CS: Is HCMV a tumor
promoter? Virus Res. 157:193–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barami K: Oncomodulatory mechanisms of
human cytomegalovirus in gliomas. J Clin Neurosci. 17:819–823.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Michaelis M, Doerr HW and Cinatl J: The
story of human cytomegalovirus and cancer: Increasing evidence and
open questions. Neoplasia. 11:1–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cinatl J, Scholz M, Kotchetkov R, Vogel JU
and Doerr HW: Molecular mechanisms of the modulatory effects of
HCMV infection in tumor cell biology. Trends Mol Med. 10:19–23.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duan YL, Ye HQ, Zavala AG, Yang CQ, Miao
LF, Fu BS, Seo KS, Davrinche C, Luo MH and Fortunato EA:
Maintenance of large numbers of virus genomes in human
cytomegalovirus-infected T98 G glioblastoma cells. J Virol.
88:3861–3873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dziurzynski K, Chang SM, Heimberger AB,
Kalejta RF, Dallas SR McGregor, Smit M, Soroceanu L and Cobbs CS:
HCMV and Gliomas Symposium: Consensus on the role of human
cytomegalovirus in glioblastoma. Neuro Oncol. 14:246–255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo MH and Fortunato EA: Long-term
infection and shedding of human cytomegalovirus in T98 G
glioblastoma cells. J Virol. 81:10424–10436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cobbs CS, Harkins L, Samanta M, Gillespie
GY, Bharara S, King PH, Nabors LB, Cobbs CG and Britt WJ: Human
cytomegalovirus infection and expression in human malignant glioma.
Cancer Res. 62:3347–3350. 2002.PubMed/NCBI
|
|
11
|
Liu X, Liu D, Qian D, Dai J, An Y, Jiang
S, Stanley B, Yang J, Wang B, Liu X and Liu DX: Nucleophosmin
(NPM1/B23) interacts with activating transcription factor 5 (ATF5)
protein and promotes proteasome- and caspase-dependent ATF5
degradation in hepatocellular carcinoma cells. J Biol Chem.
287:19599–19609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheng Z, Evans SK and Green MR: An
activating transcription factor 5-mediated survival pathway as a
target for cancer therapy? Oncotarget. 1:457–460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Angelastro JM, Canoll PD, Kuo J, Weicker
M, Costa A, Bruce JN and Greene LA: Selective destruction of
glioblastoma cells by interference with the activity or expression
of ATF5. Oncogene. 25:907–916. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu DX, Qian D, Wang B, Yang JM and Lu Z:
P300-Dependent ATF5 acetylation is essential for Egr-1 gene
activation and cell proliferation and survival. Mol Cell Biol.
31:3906–3916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang T, Qian D, Hu M, Li L, Zhang L, Chen
H, Yang R and Wang B: Human cytomegalovirus inhibits apoptosis by
regulating the activating transcription factor 5 signaling pathway
in human malignant glioma cells. Oncol Lett. 8:1051–1057.
2014.PubMed/NCBI
|
|
16
|
Zhao Y, Zhang YD, Zhang YY, Qian SW, Zhang
ZC, Li SF, Guo L, Liu Y, Wen B, Lei QY, et al: P300-dependent
acetylation of activating transcription factor 5 enhances C/EBPβ
transactivation of C/EBPα During 3T3-L1 differentiation. Mol Cell
Biol. 34:315–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu L, Zhang X, Che Y, Zhang Y, Tang S,
Liao Y, Na R, Xiong X, Liu L and Li Q: A cellular response protein
induced during HSV-1 infection inhibits viral replication by
interacting with ATF5. Sci China Life Sci. 56:1124–1133. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nevels M, Paulus C and Shenk T: Human
cytomegalovirus immediate-early 1 protein facilitates viral
replication by antagonizing histone deacetylation. Proc Natl Acad
Sci USA. 101:17234–17239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang ES, Zhang Z, Cai H, Huang DY, Huong
SM, Cha CY and Huang ES: Human cytomegalovirus IE1-72 protein
interacts with p53 and inhibits p53-dependent transactivation by a
mechanism different from that of IE2-86 protein. J Virol.
83:12388–12398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bryant LA, Mixon P, Davidson M, Bannister
AJ, Kouzarides T and Sinclair JH: The human cytomegalovirus
86-kilodalton major immediate-early protein interacts physically
and functionally with histone acetyltransferase P/CAF. J Virol.
74:7230–7237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodems SM, Clark CL and Spector DH:
Separate DNA elements containing ATF/CREB and IE86 binding sites
differentially regulate the human cytomegalovirus UL112-113
promoter at early and late times in the infection. J Virol.
72:2697–2707. 1998.PubMed/NCBI
|
|
22
|
Schwartz R, Helmich B and Spector DH: CREB
and CREB-binding proteins play an important role in the IE2
86-kilodalton protein-mediated transactivation of the human
cytomegalovirus 2.2-kilobase RNA promoter. J Virol. 70:6955–6966.
1996.PubMed/NCBI
|
|
23
|
Zhou Y, Xu Y, Wang H, Niu J, Hou H and
Jiang Y: Histone deacetylase inhibitor, valproic acid,
radiosensitizes the C6 glioma cell line in vitro. Oncol
Lett. 7:203–208. 2014.PubMed/NCBI
|
|
24
|
Lois DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: World
Organization Classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007.PubMed/NCBI
|
|
25
|
Scheurer ME, Bondy ML, Aldape KD, Albrecht
T and El-Zein R: Detection of human cytomegalovirus in different
histological types of gliomas. Acta Neuropathol. 116:79–86. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Caron C, Col E and Khochbin S: The viral
control of cellular acetylation signaling. Bioessays. 25:58–65.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cuevas-Bennett C and Shenk T: Dynamic
Histone H3 acetylation and methylation at human cytomegalovirus
promoters during replication in fibroblasts. J Virol. 82:9525–9536.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stinski MF and Petrik DT: Functional roles
of the human cytomegalovirus essential IE86 protein. Curr Top
Microbiol Immunol. 325:133–152. 2008.PubMed/NCBI
|
|
29
|
Taylor RT and Bresnahan WA: Human
cytomegalovirus IE86 attenuates virus- and tumor necrosis factor
alpha-induced NFkappaB-dependent gene expression. J Virol.
80:10763–10771. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoo YD, Chiou CJ, Choi KS, Yi Y, Michelson
S, Kim S, Hayward GS and Kim SJ: The IE2 regulatory protein of
human cytomegalovirus induces expression of the human transforming
growth factor beta1 gene through an Egr-1 binding site. J Virol.
70:7062–7070. 1996.PubMed/NCBI
|
|
31
|
Sheng Z, Li L, Zhu LJ, Smith TW, Demers A,
Ross AH, Moser RP and Green MR: A genome-wide RNA interference
screen reveals an essential CREB3L2-ATF5-MCL1 survival pathway in
malignant glioma with therapeutic implications. Nat Med.
16:671–677. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu DX, Qian D, Wang B, Yang JM and Lu Z:
p300-Dependent ATF5 acetylation is essential for Egr-1 gene
activation and cell proliferation and survival. Mol Cell Biol.
31:3906–3916. 2010. View Article : Google Scholar
|
|
33
|
Liao XH, Dong X, Wu C, Wang T, Liu F, Zhou
J and Zhang TC: Human cytomegalovirus immediate early protein 2
enhances myocardin-mediated survival of rat aortic smooth muscle
cells. Virus Res. 192:85–91. 2014. View Article : Google Scholar : PubMed/NCBI
|

















