Introduction
Lung cancer is responsible for the majority of
mortalities from cancer worldwide, and is classified into
small-cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC)
(1–3).
NSCLC is observed in ≤85% of cases of lung cancer, and its overall
5-year survival rate is 17.1% (1–3). The
radiotherapy treatment for NSCLC via conventional low-linear energy
transfer (LET) X-rays and γ-rays (typically used to treat malignant
cancer) presents disadvantages, including radiation-induced lung
injury and radioresistance of lung cancer cells (4,5). By
contrast, high-LET carbon ions possess several advantages on cancer
treatment, including higher relative biological effectiveness,
lower oxygen enhancement ratio and more uniform depth dose
distribution than low-LET rays (6).
However, the detailed molecular mechanisms underlying heavy ion
radiotherapy and its ability to reduce cellular radioresistance
remain largely unclear.
Intrinsic radioresistance, cell proliferation and
hypoxia are the 3 major radioresistance mechanisms in cancer cells
(7,8).
The problems associated with cell proliferation and hypoxia may be
eliminated by accelerated radiotherapy with hypoxia modification.
However, intrinsic radioresistance is a complex problem difficult
to eliminate, due to the multiple signaling pathways involved
(7,8).
Previous studies have demonstrated that the phosphoinositide-3
kinase (PI3K)/protein kinase B (Akt) and PI3K/mammalian target of
rapamycin (mTOR) signaling pathways may be frequently activated in
certain cancer cells, regulating cellular processes such as
proliferation, apoptosis and survival (8–10). The
PI3K family includes DNA-dependent protein kinase catalytic subunit
(PKcs), ataxia telangiectasia mutated (ATM) and ataxia
telangiectasia and Rad3-related (ATR), which participate in the DNA
damage response pathway (11).
DNA-PKcs and ATM are involved in non-homologous end joining (NHEJ)
and homologous recombination (HR) repair of DNA double-strand
breaks (DSBs) (12), while ATR is
involved in the DNA damage response pathway to repair interrupted
replication forks and extensive lesions in single-strand breaks
(SSBs) (13). Previous studies on the
signaling differences between the DNA damage response to γ-rays and
carbon ion irradiation observed an increased number of ATM and ATR
foci following carbon ion irradiation, and suggested that the
differences in DNA damage response to low- or high-LET may be due
to distinct macromolecular complexes (14). Similarly, Okayasu et al
(15) noticed that radiation with
iron ions at 2 Gy dose induced complex DNA damage, which was not
repaired by the NHEJ pathway. Since members of the PI3K family
participate in maintaining the genomic integrity and chromosome
stability, it has been hypothesized that these physiological
processes may be associated with the radiosensitivity of NSCLC
cells.
In the present study, the DNA-PKcs-inhibitor NU7026
and the ATM and ATR-inhibitor CGK733 were used to disrupt the NHEJ
repair pathway, in order to investigate the potential alterations
in the transcription and translation levels of the ATM, ATR,
DNA-PKcs genes, and to determine the radiosensitivity of lung
cancer A549 cells exposed to ionizing radiation. The results
suggested that the upregulation of ATR/ATM potentially enhanced
cellular radiosensitivity in A549 cells treated with the
DNA-PKcs-inhibitor, since part of the DNA damage-sensing apparatus
was inhibited following carbon ion irradiation. Therefore, high-LET
carbon ions instead of low-LET X-rays may be used in the future to
treat patients with lung cancer in the clinic. Further studies are
required to investigate the potential use of DNA-PKcs, ATM and ATR
in specific gene-radiotherapy approaches for the treatment of lung
cancer.
Materials and methods
Cell culture and irradiation
treatment
Normal lung fibroblast MRC-5 and lung cancer A549
cells were purchased from the American Type Culture Collection
(Manassas, USA), and cultured in minimum essential medium and
Dulbecco's modified eagle medium (Gibco Life Technologies,
Carlsbad, USA) supplemented with 10% fetal bovine serum (HyClone,
GE Healthcare Life Sciences, Logan, USA), respectively. The cells
were incubated in humidified atmosphere at 37°C in the presence of
5% CO2 to maintain exponential cell growth.
A549 cells were irradiated at room temperature with
6 MV X-rays delivered by a PRIMUS linear accelerator (Siemens AG,
Berlin, Germany) located in the Gansu Province Tumor Hospital
(Lanzhou, China), at a dose rate of 200 cGy/min and source skin
distance of 100 cm; or with 300 MeV carbon ion
(12C6+) beams, provided at a dose rate of 1
Gy/min and LET of 49 KeV/µm, at the Heavy Ion Research Facility in
Lanzhou (Institute of Modern Physics, Chinese Academy of Sciences,
Lanzhou, China). The cells were exposed to 2 Gy, and radiation
doses were determined based on previous pilot studies (11,13,14).
Non-irradiated A549 cells were handled in parallel with the
irradiated cells.
MTT assay
MRC-5 and A549 cells were plated into 96-well dishes
at a density of 5×104 cells/well. NU7026 and CGK733
(Abcam, Cambridge, UK) were added to each well at a final
concentration of 5–50 µM, and incubated for 48 h. Thereafter, MTT
(final concentration, 5 mg/ml) was added to each well. The medium
was then removed, and the formazan crystals were dissolved by
adding 150 µl dimethyl sulfoxide. The absorbance at 490 nm was
subsequently measured in a microplate reader (Infinite M200; Tecan
Group Ltd., Männedorf, Switzerland) (16,17).
Colony formation assay
A549 cells (2,000 cells) were seeded in a culture
dish of 100 µm in diameter, and treated with 10 µM NU7026 or CGK733
for 30 min, prior to be exposed to 2 Gy X-ray and carbon ion
irradiation. Following the addition of fresh medium, cell
incubation continued under standard culture conditions (37°C and 5%
CO2). The cells were washed with phosphate-buffered
saline (PBS), fixed with ethanol and stained with Giemsa 10 days
later. The number of colonies was calculated using a Multi Image™
Light Cabinet and the AlphaEase™ software (Alpha Innotech
Corporation, San Leandro, CA, USA).
Cell cycle analysis
Cells were harvested and fixed in 70% ice-cold
ethanol for >48 h at −20°C. Subsequently, the cells were washed
twice with PBS, resuspended in propidium iodide (PI) staining
solution (5 µg/ml PI, 10 kU/ml RNaseA and 0.005% Triton X-100 in
PBS) at a concentration of 1×106 cells/ml, and incubated
in the dark at room temperature for 30 min. The cell cycle
distribution was analyzed using a FACScan flow cytometer (BD
Biosciences, Franklin Lakes, USA) and FlowJo version 7.6 software
(FlowJo, LLC, Ashland, USA).
γH2AX foci immunofluorescence
Cells were seeded in a 6-well plate at a density of
1×105 cells/well, and covered with a coverslip for 24 h
to allow the cells to attach. Next, the cells were treated with
NU7026 for 30 min, prior to being subjected to 2 Gy X-rays and
carbon ion irradiation. At 0.5 h and 24 h post-irradiation, the
cells were fixed with 4% paraformaldehyde for 15 min, and then
treated with 0.1% Triton X-100 for 30 min and 5% bovine serum
albumin (BSA) for 1 h. Next, the cells were incubated overnight at
4°C with primary monoclonal antibody anti-γH2AX (1:500; Bioworld
Technology, Inc., St. Louis, MO, USA) in the presence of 1% BSA.
Cells were washed 3 times with PBST (0.05% Tween20) for 10 min, and
incubated at room temperature for 1 h with IgG-fluorescein
isothiocyanate in the presence of 1% BSA (1:5000; Cell Signaling
Technology, Inc.). Following the addition of 1.5 µg/ml
4′,6-diamidino-2-phenylindole (DAPI) to counterstain the nuclei.
The coverslip containing the cells was then mounted with
VECTASHIELD® Antifade Mounting Medium (Vector Laboratories, Inc.,
Burlingame, CA, USA). γH2AX foci were detected with a LSM 700 laser
scanning confocal microscope (Zeiss, Oberkochen, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from A549 cells with TRIzol
(Invitrogen Life Technologies, Carlsbad, USA). cDNA was synthesized
with a PrimeScript™ RT reagent kit (Takara Biotechnology Co., Ltd.,
Dalian, China) in a total volume of 20 µl. Then, multiplex qPCR was
performed in a total volume of 25 µl, using SYBR Green Master Mix
(Takara Biotechnology Co., Ltd.), 50 ng DNA and 10 µM of each of
the following primers from Takara Biotechnology Co., Ltd.: DNA-PKcs
(forward, 5′-AGG GAA GAA GAG TCT CTG GTGG-3; reverse, 5′-ATT AGG
GGA TCT GTT GCC TGGC-3) (18); ATM
(forward, 5′-ACTATCCCAATACACTGCTGGAGA-3; reverse,
5′-TTTGAGCAACTGACTGGCAAAC-3); ATR (forward, 5′-CCA AAG CGC CAC TGA
ATGAA-3; reverse, 5′-ACC TTG TAG TCG CTG CTC AAT GTC-3); and GAPDH
(forward, 5′-GAA GGT GAA GGT CGG AGTC-3; reverse, 5′-GAA GAT GGT
GAT GGG ATTTC-3).
The reaction was conducted on an FTC-3000 qPCR
system (Shanghai Funglyn Biotech Co., Ltd., Shanghai, China), with
the following cycling conditions: 30 sec at 95°C, 40 cycles of 5
sec at 95°C and 30 sec at 59°C. Each PCR was repeated 3 times. The
relative gene quantification approach (2−ΔΔCt) was used
according to the method previously described (19).
Western blotting
A549 cells were lysed in lysis buffer supplemented
with 1 mM phenylmethylsulfonyl fluoride, and the protein
concentration in the cell lysate was determined using a BCA assay
kit (Pierce Biotechnology Inc., Rockford, USA). 20 µg of proteins
from the whole-cell lysate was mixed with SDS buffer, separated on
SDS-PAGE at 80 V for 2 h and transferred to polyvinylidene
difluoride membranes (Bio-Rad Laboratories, Inc.) for 2 h. The
membranes were then blocked for 1 h with PBS containing 5% BSA
(Sigma-Aldrich), and incubated with the corresponding primary
monoclonal rabbit antibody IgG anti-ATM, ATR, DNA-PKcs or β-actin
(Cell Signaling Technology, Inc., Danvers, MA, USA) (1:1,000) at
4°C overnight. Next, the membranes were washed with PBST for 30
min, and incubated with a goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:5,000, Cell Signaling
Technology, Inc.) for 1 h at room temperature. Following 3 washes
with PBST for 10 min, a chemiluminescence kit (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) was used to detect the
reactive proteins. The data were represented as values relative to
the protein levels of β-actin.
Statistical analysis
The data, presented as the mean ± standard deviation
from ≥3 independent experiments, were evaluated for statistical
significance with the Students t-test, using Microsoft Excel
software (Microsoft Corporation, Redmond, USA). P﹤0.05 was
considered to indicate a statistically significant difference.
Results
The cytotoxicity of NU7026 and CGK733
was evaluated in A549 and MRC-5 cells
The effect of NU7026 or CGK733 on cell proliferation
was determined by MTT assay following 48-h exposure of A549 and
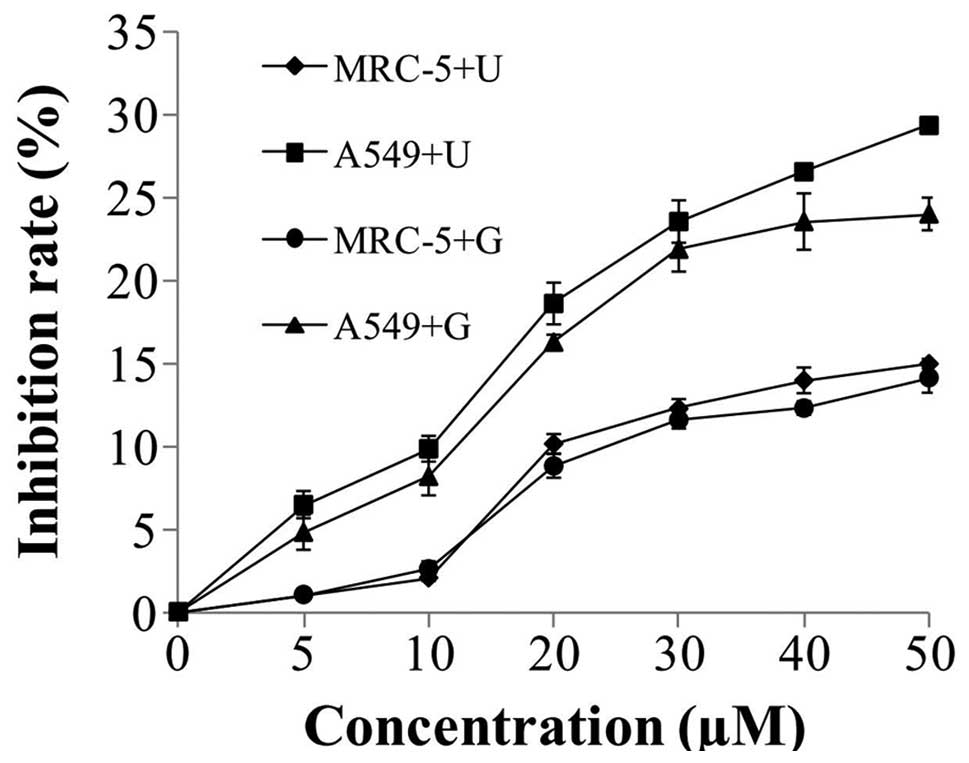
MRC-5 cells to the above inhibitors. At concentrations of NU7026
and CGK733 <10 µM, MRC-5 and A549 cells exhibited 2 and 10%
inhibition rate, respectively, whereas at concentrations of NU7026
and CGK733 >20 µM, the inhibition rate of MRC-5 and A549 cells
increased to >8 and 16%, respectively (Fig. 1). Compared with A549 cells treated
with 5–50 µM NU7026 or CGK733, no obvious cytotoxicity was observed
in MRC-5 cells, following treatment with NU7026 or CGK733 for 48 h
(Fig. 1). Consequently, 10 µM NU7026
and CGK733 were selected to examine the radiosensitivity of A549
cells in subsequent experiments.
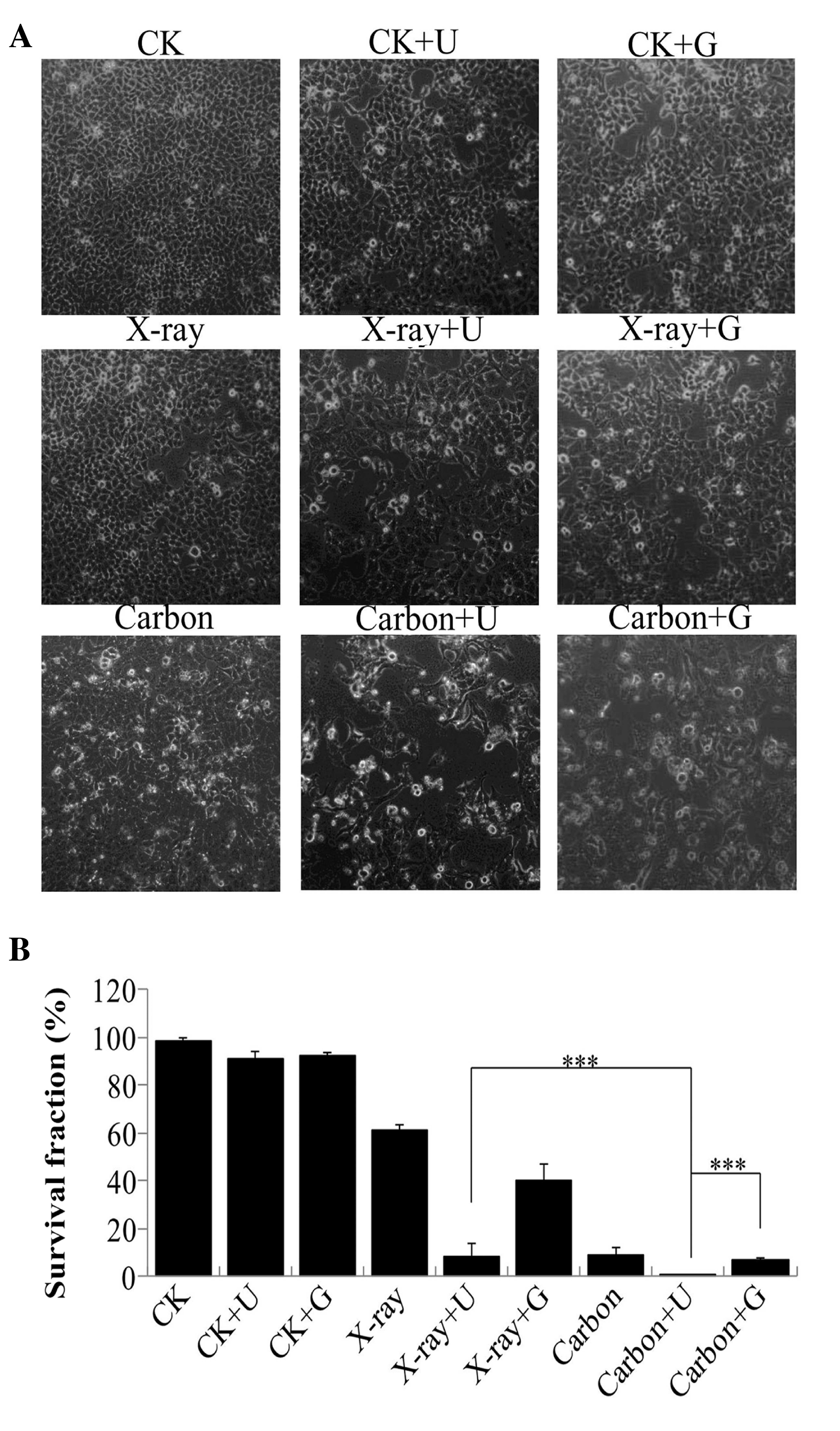
NU7026-treatment and irradiation
enhance cellular radiosensitivity
A549 cells were pretreated with NU7026 or CGK733 for
30 min, prior to be irradiated. To identify cellular alterations
upon inhibitors-treatment and/or irradiation, the cells were
analyzed under a microscope. A reduction in the number of cells and
alterations in cell morphology were observed (Fig. 2A). Following 10 days, the survival
fraction of cells exposed to carbon ion irradiation that had been
pretreated with NU7026 reduced to 0.67%, compared with the control
group (CK 98.75%, CK+U 90.82% and CK+G 92.63%) and the
NU7026-pretreated cells exposed to 2 Gy X-ray irradiation (8.75%).
The survival fraction of cells exposed to ionizing radiation
following CGK733-treatment was higher than that of cells exposed to
ionizing radiation following NU7026-treatment (Fig. 2B). These results implied there was a
significant increase in the radiosensitivity of A549 cells
following NU7026-treatment and carbon ion irradiation.
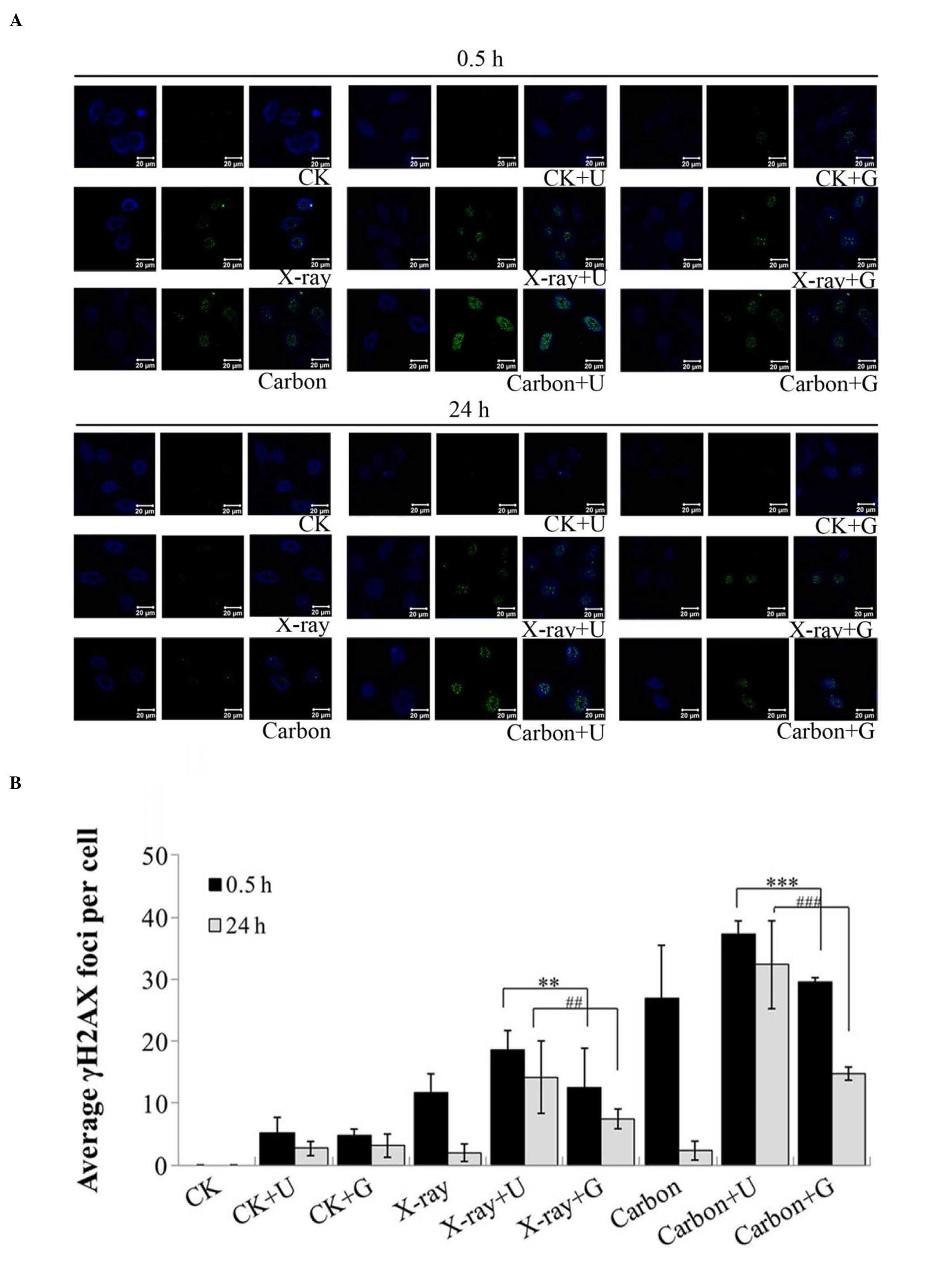
NU7026-treatment and irradiation
reduce DNA damage repair
γH2AX is a marker of DSBs and also an important
factor that recruits downstream proteins in response to DNA damage
(20). γH2AX foci were detected in
A549 cells at 0.5 h and 24 h post-irradiation, regardless of prior
NU7026 or CGK733-treatment (Fig. 3).
The X-ray+NU7026 and carbon+NU7026 groups exhibited a significant
increase in γH2AX foci, compared with the groups treated with
irradiation or CGK733 at 0.5 h (P<0.001). However, these γH2AX
foci remained unaltered from 0.5 to 24 h post-irradiation, and did
not dephosphorylate to repair DNA damage, indicating that the
process of DNA repair had been completed (Fig. 3). Therefore, the X-ray+NU7026 and
carbon+NU7026 groups appeared to display a poorer ability to repair
DNA damage from 0.5 to 24 h post-irradiation, compared with the
other groups (Fig. 3B). These results
indicated that the NU7026-treatment led to a significant reduction
in DNA damage repair, compared with the CGK733-treatment, in A459
cells exposed to ionizing radiation.
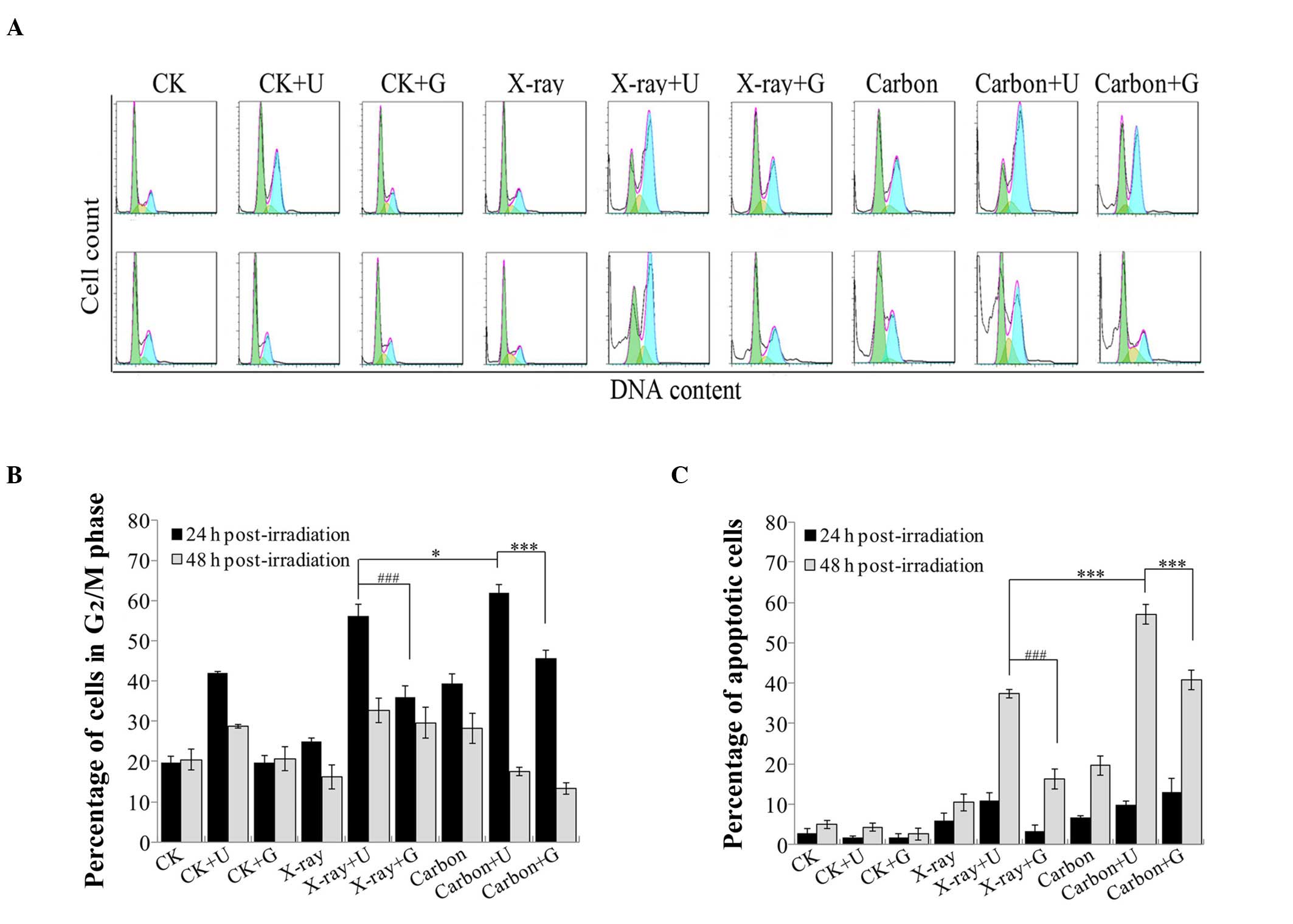
Exacerbated cell cycle G2/M
phase arrest increases cell apoptosis
Cell cycle arrest was examined by flow cytometry,
and represented as a blue G2/M peak in a DNA histogram
(Fig. 4A). In contrast to 2 Gy X-rays
irradiation with NU7026-treatment or 2 Gy carbon ion irradiation
with CGK733-treatment, a clear increase in the percentage of cells
arrested at the G2/M phase was observed in
NU7026-pretreated A549 cells exposed to 2 Gy carbon ion irradiation
at 24 h post-irradiation (P<0.05 and P<0.001; Fig. 4B). However, at 48 h post-irradiation,
the number of A549 cells arrested at the G2/M phase
significantly reduced following 2 Gy carbon ion irradiation,
regardless of the pretreatment with NU7026 or CGK733. Therefore, as
the time post-irradiation increased, the fraction of cells in the
G2/M phase reduced. In contrast, the number of apoptotic
cells rapidly increased (Fig. 4C),
and the percentage of cells treated with NU7026 or CGK733 at 48 h
post-irradiation was higher than at 24 h post-irradiation. These
results indicated that a pronounced G2/M arrest may
contribute to cell apoptosis.
DNA-PKcs-inhibition enhances the
transcription and translation of ATM and ATR
When DNA-PKcs is inhibited, ATM and ATR regulate
cell cycle arrest and apoptosis (21). Therefore, RT-qPCR was performed at 24
h post-irradiation to quantify the relative expression levels of
ATM and ATR in A549 cells that had been exposed to 2 Gy
irradiation. Compared with the CK group (non-irradiated A549
cells), the gene levels of ATM and ATR were markedly upregulated in
A459 cells following irradiation, and appeared to decline in A549
cells exposed to carbon ion irradiation plus NU7026-treatment
versus NU7026-treatment alone (P<0.001; Fig. 5A). In addition, the gene expression
levels of ATM and ATR gradually increased in A549 cells, following
X-rays and carbon ion irradiation alone, compared with the gradual
reduction observed in NU7026-treated cells (Fig. 5A). The ability of carbon ion
irradiation to regulate the intracellular levels of ATM and ATR was
opposite to the effects observed with X-rays irradiation. The
results of western blotting for ATM and ATR were in agreement with
those from RT-qPCR, and indicated high expression levels of ATR and
ATM in A459 cells following carbon ion irradiation alone and carbon
irradiation with NU7026-pretreatment, compared with control cells
(Fig. 5B).
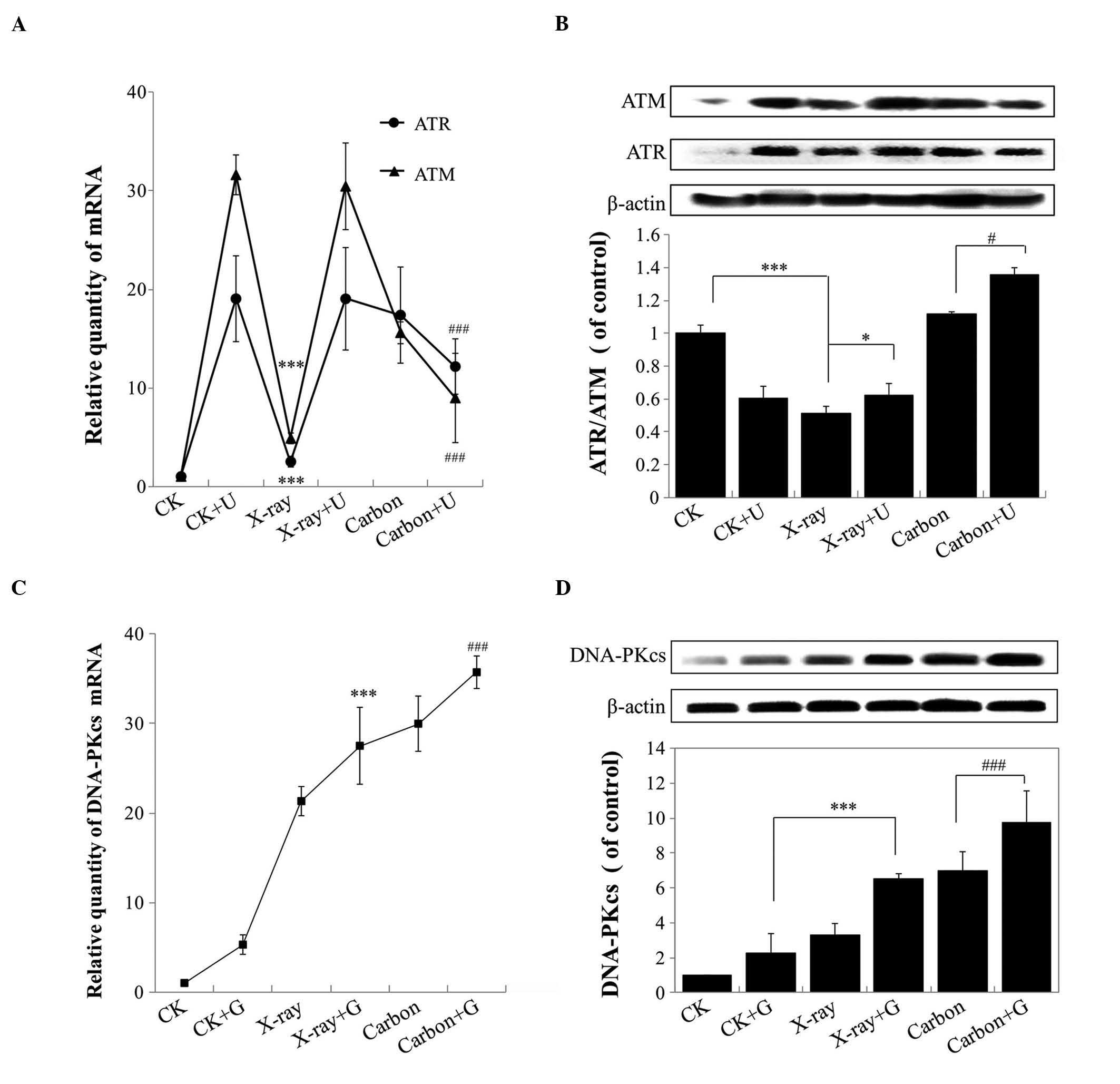 | Figure 5.(A) Products derived from the
transcription of the ATM and ATR genes were detected by RT-qPCR at
24 h post-irradiation in A459 cells subjected to different LET
and/or NU7026-pretreatment. (B) The protein levels of ATM and ATR
were determined by western blotting at 24 h post-irradiation in
A549 cells, following exposure to ionizing radiation of different
LET and/or NU7026-pretreatment. (C) Products derived from the
transcription of DNA-PKcs were detected by RT-qPCR at 24 h
post-irradiation in A459 cells subjected to different LET and/or
CGK733-pretreatment. (D) The protein expression levels of DNA-PKcs
were detected by western blotting at 24 h post-irradiation in A459
cells subjected to different LET and/or CGK733-pretreatment. In all
cases, A549 cells were incubated with 10 µM NU7026 or CGK733 for 30
min prior to being irradiated. ATM, ataxia telangiectasia mutated;
ATR, ataxia telangiectasia and Rad3-related; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; LET, linear
energy transfer; DNA-PKcs, DNA-dependent protein kinase catalytic
subunit; CK, non-irradiated A549 cells; U, NU7026; G,
CGK733.***P<0.001; ###P<0.001;*P<0.05;
#P<0.05. |
To investigate the mechanisms that led to the
observed reduced survival rate, increased percentage of cells in
the G2/M phase, and increased apoptosis rate, following
X-rays and carbon ion irradiation with or without NU7026-treatment
in A459 cells, the expression levels of DNA-PKcs were examined in
A549 cells treated with the ATM and ATR-inhibitor CGK733 and
ionizing radiation. The gene and protein expression levels of
DNA-PKcs were observed to be upregulated in A549 cells exposed to
different LET rays and CGK733-pretreatment (Fig. 5C and D). In particular, the
pretreatment with CGK733 and carbon ion irradiation significantly
enhanced the levels of DNA-PKcs in A549 cells, compared with the
other groups (P<0.001). These findings indicated that the
inhibition of DNA-PKcs regulated cell apoptosis and cell cycle
arrest following carbon ion irradiation through a mechanism
upstream of ATM and ATR.
Discussion
Previous studies have demonstrated that high-LET
radiation may produce high-clustered DNA damage, including DSBs,
SSBs, oxidized bases, and apurinic and apyrmidinic sites (22). Thus, the complexity of DNA damage
produced by high-LET radiation is higher than that from low-LET
radiation (22). DNA-PKcs and ATM
exhibit a critical sensory role in DSB repair through the NHEJ
pathway in mammalian cells, while ATR is activated by SSBs to
alleviate cellular genotoxic stress (21,23–25). DSBs
can activate checkpoint signaling, and are usually repaired through
the NHEJ and HR pathways (26). HR
occurs in the S and G2 phases of the cell cycle, whereas
NHEJ exists during the whole cell cycle (26). Therefore, the additional inhibitory
effect on NHEJ may retard the repair of carbon ion
irradiation-induced DNA damage. In the present study, the
inhibition of the NHEJ pathway led to the repair of carbon
ion-induced DNA damage to become dependent on the slow HR DNA
repair pathway. H2AX can be rapidly phosphorylated at Ser139, which
is dependent on effector signals upstream of ATM, ATR and DNA-PKcs
(13,27,28).
Previous studies have indicated that γH2AX foci may be a sensitive
indicator of DSB formation and repair (29). The results of the present study
revealed that γH2AX foci of A549 cells were also increased by
mechanisms upstream of ATM and ATR when the cells were treated with
NU7026 or CGK733 and radiation for 0.5 h. The number of green foci
observed in the irradiated A459 cells markedly reduced with time
post-irradiation when the cells were subjected to X-rays and carbon
ion irradiation alone, but not following X-rays and carbon ion
irradiation with NU7026 or CGK733-pretreatment (Fig. 3). These results indicated that the
A459 cells that had been subjected to X-rays and carbon ion
irradiation alone were experiencing DNA damage repair, as indicated
by the marked reduction in the number of green foci, whereas the
DNA damage repair was limited in those cells subjected to X-rays
and carbon ion irradiation with NU7026-pretreatment, as indicated
by their persistent green foci. The DNA damage repair induced by
NU7026-treatment was limited, compared with the CGK733-treatment
plus radiation, suggesting that the DNA damage repair ability of
DNA-PKcs was stronger than that of ATM and ATR. In addition, a
larger number of γH2AX foci were induced by high-LET particles,
compared with low-LET X-rays. However, the downstream repair
factors recruited by the formation of γH2AX foci did not function,
leading to the continuous localization of γH2AX at unrepaired
damage sites. These data also suggested that numerous damaged cells
arrested in the G2/M phase were not capable of being
repaired via the slow HR repair pathway alone. Previous studies
have demonstrated that the formation of γH2AX foci depends on the
presence of ATM, ATR and DNA-PKcs, following exposure to ionizing
radiation (30,31). Furthermore, γH2AX triggered the
G2/M checkpoint arrest by mediating the concentration of
p53 binding protein 1 at the DSBs induced by ionizing radiation.
Otherwise, the sole presence of these DSBs would be insufficient to
prevent the damaged cells from entering mitosis (32).
Previous studies have demonstrated that the
principal biological task of the cell cycle is to create time for
DNA damage repair. If the repair is successful, the cell cycle
continues operating. If the DNA damage is too serious to be
repaired, cells will undergo apoptosis (33,34). In
the present study, the number of A549 cells arrested at the
G2/M phase increased at 24 h post-irradiation when the
cells were pretreated with NU7026 or CGK733 (Fig. 4). Previous studies have suggested that
cells accumulated in the G2/M phase were potentially
subjected to apoptosis and sensitive to ionizing radiation
(35,36). In the present study, the number of
cells arrested at the G2/M phase markedly reduced with
time post-irradiation, while the number of apoptotic cells
increased from 24 to 48 h post-irradiation. This increase in
G2/M phase arrest and apoptosis was induced by high-LET
particles, compared with low-LET X-rays radiation. Furthermore, the
effects exhibited by the DNA-PKcs-inhibitor NU7026 were more
pronounced than those displayed by the ATM and ATR-inhibitor
CGK733. Therefore, the increase in G2/M arrest appeared
to enhance the radiosensitivity of A459 cells to carbon ion
irradiation via apoptosis-associated cell death.
Previous studies have demonstrated that effectors
located upstream of ATM, ATR and DNA-PKcs were able to initiate the
G2/M cell cycle arrest and DNA damage repair pathway
through other kinases (21,37,38). In
the present study, upregulation of ATM, ATR and DNA-PKcs in A459
cells was also evident following carbon ion irradiation with NU7026
or CGK733-pretreatment, which indicated that ATR, ATM and DNA-PKcs
were involved in DNA damage repair, possibly through regulation of
cell cycle arrest and apoptosis, rather than through the slow HR
pathway. The upregulated ATR/ATM rate in ionized A459 cells
observed in the present study was consistent with the results of
previous studies (13,14). In addition, the effect of the
DNA-PKcs-inhibitor on enhancing the radiosensitivity of A549 cells
was stronger than the effect of the ATM and ATR-inhibitor, since
the potent repair ability of DNA-PKcs was inhibited following
carbon ion irradiation.
In conclusion, in the present study, the treatment
with NU7026 increased the cellular radiosensitivity of lung cancer
A549 cells to high-LET carbon ion irradiation, which may be due to
the inhibition of DNA damage repair and the additional activation
of ATM and ATR. Therefore, the results of the present study
highlight the potential use of ATM and ATR in clinical radiotherapy
for the treatment of NSCLC, since the exposure of lung cancer cells
to high-LET rays jointly with NU7026, improved their cellular
sensitivity to radiotherapy, compared with irradiation alone.
Furthermore, the findings of the present study indicate the use of
NU7026 in animal experiments and as a novel agent in
gene-radiotherapy, since the compound did not produce any obvious
toxic effects on normal lung fibroblasts. Future in vitro
and in vivo studies on the combination of DNA-PKcs, ATM and
ATR-inhibitors (39) are required in
order to assess the beneficial effects of these drugs on the
treatment of NSCLC in the clinic.
Acknowledgements
The authors would like to thank the National
Laboratory of Heavy Ion Accelerator, the Gansu Province Tumor
Hospital and the Central Laboratory of the School of Life Sciences
of Lanzhou University (Lanzhou, China) for their support. The
authors would also like to thank the international science editors
who provided assistance with the use of the English language during
the elaboration of the present manuscript. The present study was
supported by the National Natural Science Foundation of China
(Beijing, China) (grant no. 81160283).
References
|
1
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang HJ, Kim N, Seong KM, Youn H and Youn
B: Investigation of radiation-induced transcriptome profile of
radioresistant non-small cell lung cancer A549 cells using RNA-seq.
PLoS One. 8:e593192013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang W, Wang J, Tang M, Pan J, Bai P, Lin
D, Qian F, Lin F, Yang X and Zhang S: Quantitative study of lung
perfusion SPECT scanning and pulmonary function testing for early
radiation-induced lung injury in patients with locally advanced
non-small cell lung cancer. Exp Ther Med. 3:631–635.
2012.PubMed/NCBI
|
|
6
|
Hamada N, Imaoka T, Masunaga S, Ogata T,
Okayasu R, Takahashi A, Kato TA, Kobayashi Y, Ohnishi T, Ono K, et
al: Recent advances in the biology of heavy-ion cancer therapy. J
Radiat Res (Tokyo). 51:365–383. 2010. View Article : Google Scholar
|
|
7
|
Janssens GO, Rademakers SE, Terhaard CH,
Doornaert PA, Bijl HP, van den Ende P, Chin A, Marres HA, de Bree
R, van der Kogel AJ, et al: Accelerated radiotherapy with carbogen
and nicotinamide for laryngeal cancer: Results of a phase III
randomized trial. J Clin Oncol. 30:1777–1783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bussink J, van der Kogel AJ and Kaanders
JHAM: Activation of the PI3K/Akt pathway and implications for
radioresistance mechanisms in head and neck cancer. Lancet Oncol.
9:288–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagelkerke A, Bussink J, van der Kogel AJ,
Sweep FCGJ and Span PN: The PERK/ATF4/LAMP3-arm of the unfolded
protein response affects radioresistance by interfering with the
DNA damage response. Radiother Oncol. 108:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Serra V, Markman B, Scaltriti M, Eichhorn
PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S,
et al: NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K
signaling and inhibits the growth of cancer cells with activating
PI3K mutations. Cancer Res. 68:8022–8030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yajima H, Lee K-J, Zhang S, Kobayashi J
and Chen BPC: DNA double-strand break formation upon UV-induced
replication stress activates ATM and DNA-PKcs kinases. J Mol Biol.
385:800–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhuang W, Li B, Long L, Chen L, Huang Q
and Liang Z: Induction of autophagy promotes differentiation of
glioma-initiating cells and their radiosensitivity. Int J Cancer.
129:2720–2731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stiff T, ODriscoll M, Rief N, Iwabuchi K,
Löbrich M and Jeggo PA: ATM and DNA-PK function redundantly to
phosphorylate H2AX after exposure to ionizing radiation. Cancer
Res. 64:2390–2396. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghosh S, Narang H, Sarma A and Krishna M:
DNA damage response signaling in lung adenocarcinoma A549 cells
following γ and carbon beam irradiation. Mutat Res. 716:10–19.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okayasu R, Okada M, Okabe A, et al: Repair
of DNA damage induced by accelerated heavy ions in mammalian cells
proficient and deficient in the non-homologous end-joining pathway.
Radiat Res. 165:59–67. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vaucher RA, da Motta AS and Brandelli A:
Evaluation of the in vitro cytotoxicity of the antimicrobial
peptide P34. Cell Biol Int. 34:317–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Song J, Yang P, Zou R, Fan X and
Zhao Z: Establishment of a three-dimensional culture and mechanical
loading system for skeletal myoblasts. Cell Biol Int. 33:192–198.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen H, Schultz M, Kruh GD and Tew KD:
Increased expression of DNA-dependent protein kinase confers
resistance to adriamycin. Biochim Biophys Acta. 1381:131–138. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kantor AF, Li FP, Janov AJ, Tarbell NJ and
Sallan SE: Hypertension in long-term survivors of childhood renal
cancers. J Clin Oncol. 7:912–915. 1989.PubMed/NCBI
|
|
20
|
Vandersickel V, Depuydt J, Van Bockstaele
B, Perletti G, Philippe J, Thierens H and Vral A: Early increase of
radiation-induced γH2AX foci in a human Ku70/80 knockdown cell line
characterized by an enhanced radiosensitivity. J Radiat Res
(Tokyo). 51:633–641. 2010. View Article : Google Scholar
|
|
21
|
Yang J, Xu ZP, Huang Y, Hamrick HE,
Duerksen-Hughes PJ and Yu YN: ATM and ATR: Sensing DNA damage.
World J Gastroenterol. 10:155–160. 2004.PubMed/NCBI
|
|
22
|
Hada M and Georgakilas AG: Formation of
clustered DNA damage after high-LET irradiation: A review. J Radiat
Res (Tokyo). 49:203–210. 2008. View Article : Google Scholar
|
|
23
|
Wang C and Lees-Miller SP: Detection and
repair of ionizing radiation-induced DNA double strand breaks: New
developments in nonhomologous end joining. Int J Radiat Oncol Biol
Phys. 86:440–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mukherjee B, Kessinger C, Kobayashi J, et
al: DNA-PK phosphorylates histone H2AX during apoptotic DNA
fragmentation in mammalian cells. DNA Repair (Amst). 5:575–590.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Durocher D and Jackson SP: DNA-PK, ATM and
ATR as sensors of DNA damage: Variations on a theme? Curr Opin Cell
Biol. 13:225–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JS, Krasieva TB, Kurumizaka H, et al:
Independent and sequential recruitment of NHEJ and HR factors to
DNA damage sites in mammalian cells. J Cell Biol. 170:341–347.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang H, Wang M, Wang H, Böcker W and
Iliakis G: Complex H2AX phosphorylation patterns by multiple
kinases including ATM and DNA-PK in human cells exposed to ionizing
radiation and treated with kinase inhibitors. J Cell Physiol.
202:492–502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang J, Yu Y, Hamrick HE and
Duerksen-Hughes PJ: ATM, ATR and DNA-PK: Initiators of the cellular
genotoxic stress responses. Carcinogenesis. 24:1571–1580. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Löbrich M, Shibata A, Beucher A, et al:
γH2AX foci analysis for monitoring DNA double-strand break repair:
Strengths, limitations and optimization. Cell Cycle. 9:662–669.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Falck J, Coates J and Jackson SP:
Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of
DNA damage. Nature. 434:605–611. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takahashi A and Ohnishi T: Does γH2AX foci
formation depend on the presence of DNA double strand breaks?
Cancer Lett. 229:171–179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fernandez-Capetillo O, Chen HT, Celeste A,
Ward I, Romanienko PJ, Morales JC, Naka K, Xia Z, Camerini-Otero
RD, Motoyama N, et al: DNA damage-induced G2-M checkpoint
activation by histone H2AX and 53BP1. Nat Cell Biol. 4:993–997.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stucki M, Stagljar I, Jónsson ZO and
Hübscher U: A coordinated interplay: Proteins with multiple
functions in DNA replication, DNA repair, cell cycle/checkpoint
control, and transcription. Prog Nucleic Acid Res Mol Biol.
65:261–298. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ferguson DO, Sekiguchi JM, Frank KM, Gao
Y, Sharpless NE, Gu Y, Manis J, DePinho RA and Alt FW: The
interplay between nonhomologous end-joining and cell cycle
checkpoint factors in development, genomic stability, and
tumorigenesis. Cold Spring Harb Symp Quant Biol. 65:395–403. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong J, Zhang Z, Lv W, Zhang M, Chen C,
Yang S, Li S, Zhang L, Han D and Zhang W: Icaritin synergistically
enhances the radiosensitivity of 4T1 breast cancer cells. PLoS One.
8:e713472013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang CJ, Wang CS, Hung JY, Huang HW, Chia
YC, Wang PH, Weng CF and Huang MS: Pyrogallol induces
G2-M arrest in human lung cancer cells and inhibits
tumor growth in an animal model. Lung Cancer. 66:162–168. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun Y, Sun D, Li F, Tian L, Li C, Li L,
Lin R and Wang S: Downregulation of Sirt1 by antisense
oligonucleotides induces apoptosis and enhances radiation
sensitization in A549 lung cancer cells. Lung Cancer. 58:21–29.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zangemeister-Wittke U and
Hopkins-Donaldson S: Apoptosis regulation and drug resistance in
malignant pleural mesothelioma. Lung Cancer. 49(Suppl 1):
S105–S108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Williams TM, Nyati S, Ross BD and
Rehemtulla A: Molecular imaging of the ATM kinase activity. Int J
Radiat Oncol Biol Phys. 86:969–977. 2013. View Article : Google Scholar : PubMed/NCBI
|