Introduction
Breast cancer is a common malignant tumor and
accounts for the majority of cancer mortality in females (1). Breast cancer has an estimated incidence
of 1,676,633 and had an estimated mortality rate of 521,817 in 2012
worldwide (2). In China, there has
been an increasing incidence of breast cancer in recent decades
(3). In 2012, it was estimated that
~48,000 females would succumb to breast cancer. To date,
chemotherapy has been the mainstay for the treatment of breast
cancer. However, the side effects and drug resistance associated
with chemotherapy have limited its effectiveness for the treatment
of breast cancer (4,5). A number of natural extractions have
demonstrated a wide range of biological activity and low toxicity
in animal models, and have been considered as an alternative method
of breast cancer treatment (6,7).
Emodin (1,3,8-trihydroxy-6-methylanthraquinone) is a
biologically active anthraquinone identified in the roots and bark
of a number of plants. Emodin is also observed naturally in a
number of widely used Chinese medicinal herbs, including Rheum
officinale (8) and Polygonam
cuspidatummedicine (9). It has
been widely studied for its antibacterial, diuretic,
immunosuppressive, anti-inflammatory and vasorelaxant effects. It
has been reported that emodin induces apoptosis of several types of
human cancer cells (10–13) by modulating various signaling
pathways. However, the biological mechanisms by which emodin
induces cytotoxicity remain largely unknown. Furthermore, the
biological effects of emodin on breast cancer cells remain to be
determined.
In this study, the effects of emodin on the
proliferation and apoptosis of breast cancer cells were assessed.
In addition, the mechanism by which emodin mediates these
biological effects was elucidated. We demonstrated that emodin
inhibited the proliferation and induced apoptosis of Bcap-37 and
ZR-75-30 breast cancer cells. These observations suggest that
emodin may be a potential agent in the treatment of breast
cancer.
Materials and methods
Drugs and antibodies
Emodin was purchased from Sigma-Aldrich (St. Louis,
MO, USA). A stock solution of emodin (10 mg/ml) was prepared in
dimethyl sulfoxide (DMSO) and further diluted in culture medium.
DMSO (0.05%) was used as vehicle control in all experiments. The
antibodies used for western blot analysis were as follows:
anti-cleaved caspase-3 (#9661), anti-Bcl-2 (#2876), anti-Bax
(#2772), anti-PARP (#9532), anti-p53 (#2527) and anti-β-actin
(#4970). All antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA).
Cell culture
Human breast cancer cells Bcap-37 and ZR-75-30 were
obtained from the Cell Resource Center of Shanghai Life Science
Research Institute, Chinese Academy of Sciences and cultured in
RPMI-1640 medium (Invitrogen Corporation, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (FBS; Thermo Scientific
Hyclone, Logan, UT, USA), 100 µg/ml streptomycin and 100 U/ml
penicillin at 37°C in a humidified incubator in an atmosphere of 5%
CO2.
Cell viability assay
Cells were trypsinized and seeded into 96-well
plates (Corning, Tewksbury, MA, USA) at a density of
1×104 cells per well and incubated overnight. The cells
were replenished with fresh medium containing various
concentrations (0, 10, 20, 40 and 80 µM) of emodin for 24, 48 and
72 h. At each time point, 10 ml of 5 mg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich) solution was added into each well and incubated for
an additional 4 h at 37°C. The culture medium was then removed, and
100 µl DMSO was added into each well. The absorbance was recorded
at 490 nm on a microplate reader (BioTek, Winooski, VT, USA). The
results represent the average values of three experiments. Each
experiment was performed at least three times.
Apoptosis analysis
Apoptosis was evaluated by Annexin V-fluorescein
isothiocyanate (Annexin V-FITC; KeyGEN Biotech, Nanjing, China).
Cells were treated with emodin (0, 10 and 40 µM) for 24 h. Then,
5×105 cells were collected, resuspended in 500 ml
binding buffer containing an additional 5 µl Annexin V-FITC and 5
µl propidium iodide, and incubated for 15 mins at room temperature
without direct sunlight. Samples were analyzed by flow cytometry
(BD Pharmingen, San Diego, CA, USA). Experiments were performed at
least three times.
Quantitative polymerase chain reaction
(qPCR)
Breast cancer cells were treated with emodin (0, 10
and 40 µM) for 24 h. Total RNA was extracted by the RNAiso Plus kit
(Takara, Dalian, China). First-strand complementary DNA (cDNA) was
synthesized using the PrimeScript RT reagent kit (Takara) according
to the instructions. Amplification of the cDNA for PCR was
performed in a reaction volume of 20 µl which included 2 µl cDNA.
The primer sequences used were as follows: p53 (forward,
ATCCTCACCATCATCACACTGG; reverse, ACAAACACGCACCTCAAAGC); Bcl-2
(forward, CAAATGCTGGACTGAAAAATTGTA; reverse,
TATTTTCTAAGGACGGCATGATCT); Bax (forward, GACACCTGAGCTGACCTTGG;
reverse: AGGAAGTCCAGTGTCCAGC); β-actin (forward,
CTGGGACGACATGGAGAAAA, reverse: AAGGAAGGCTGGAAGAGTGC). The PCR
cycling conditions were as follows: 95°C for 30 sec followed by 40
cycles at 95°C for 5 sec, and annealing at 60°C for 30 sec. Each
experiment was repeated three times. β-actin was used as an
internal reference gene to normalize gene expression. Relative gene
expression was analyzed by the comparative threshold cycle (Ct)
method. The value was used to plot the expression of apoptotic
genes by 2−∆∆Ct.
Matrigel invasion assay
Transwell chambers (Corning) were used to assess the
invasiveness of breast cancer cells. Two chambers were separated by
Matrigel-coated polycarbonate membrane (6.5-mm diameter inserts
with an 8-µm pore size). Cells were treated with FBS-free medium
for 24 h, and 5×105 cells/ml with various concentrations
of emodin (0, 10 and 40 µM) in a total volume of 200 µl were placed
in the upper chamber. A total of 500 µl RPMI-1640 medium and 10%
FBS were added to the lower chamber. After 24 h, the cells
remaining on the upper surface of the filter were removed with a
cotton swab. The membrane was fixed with methanol and then stained
with crystal violet. Cell migration was measured by a cell staining
count in five separate areas using a light microscope (Olympus
BX41; Olympus Inc., Center Valley, PA, USA). Each experiment was
performed at least three times.
Western blot analysis
Western blot analysis was used to detect the levels
of apoptotic protein. Breast cancer cells were treated with various
concentrations of emodin (0, 10 and 40 µM) for 24 h. Cells were
harvested and lysed in lysis buffer (Beyotime Biotech, Shanghai,
China). The total protein concentration of the cell was determined
by the bicinchoninic acid assay (KeyGEN Biotech). Forty micrograms
of protein were separated by electrophoresis on 10% sodium dodecyl
sulphate-polyacrylamide gel. The gel was then transferred to
polyvinylidene fluoride membranes, and then blocked for 2 h with
Tris-buffered saline and Tween-20 containing 5% non-fat milk. Next,
the membranes were incubated with the primary antibodies at 4°C
overnight. This was followed by incubation at room temperature with
the appropriate horseradish peroxidase-conjugated secondary
antibodies for 2 h. The results were detected using an
electrochemiluminesence kit (Pierce, Rockford, IL, USA).
Statistical analysis
All assays were performed independently in
triplicate. All values were expressed as mean ± standard deviation
and analyzed by Student's t-test using SPSS 19.0 software (IBM
SPSS, Armonk, MY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of emodin on the viability of
breast cancer cells
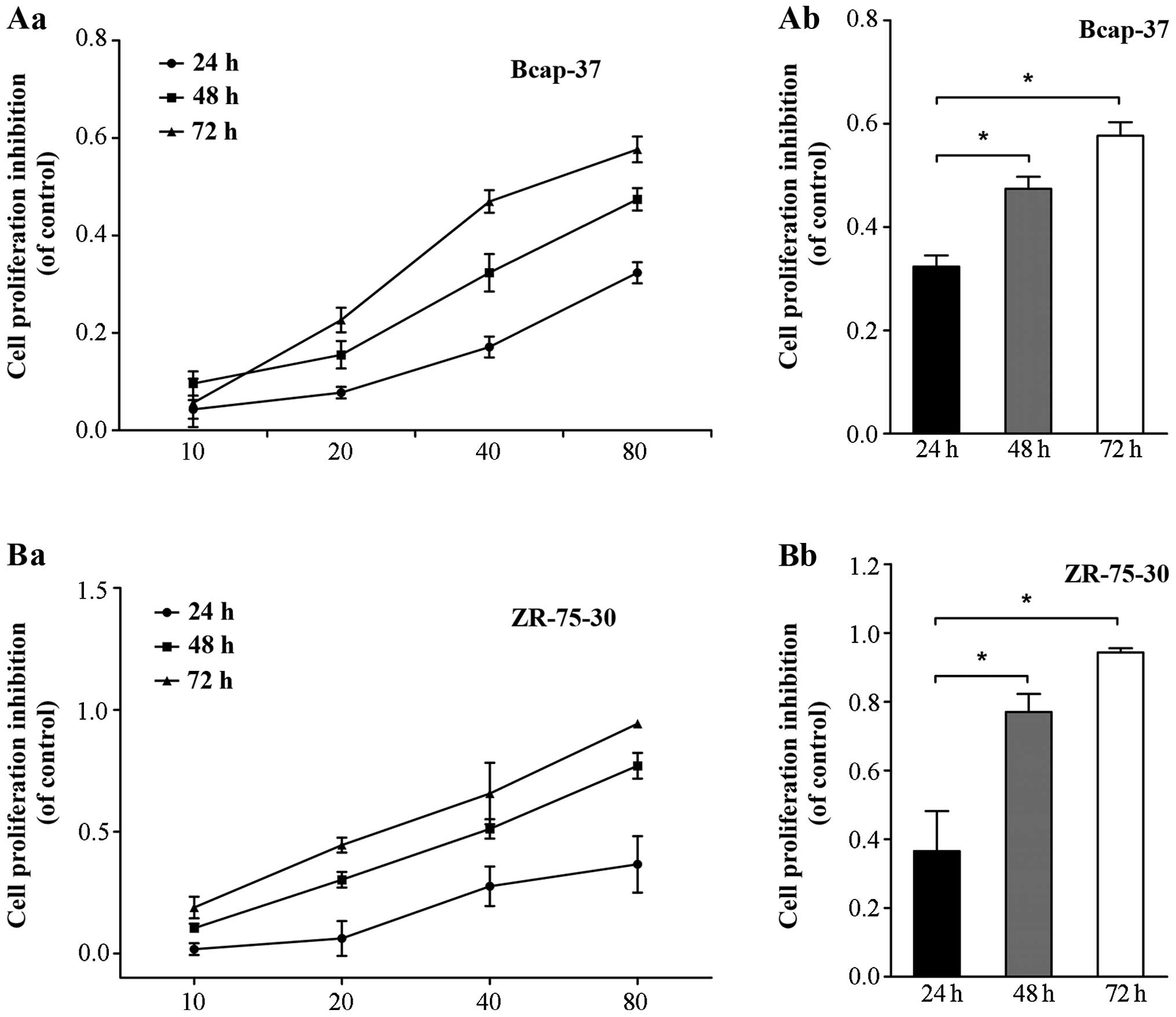
The effects of emodin on the growth of Bcap-37 and
ZR-75-30 cells were measured by MTT assay. As shown in Fig. 1, emodin significantly inhibited the
growth of Bcap-37 and ZR-75-30 cells in a time- and dose-dependent
manner. The highest rates of decreased growth were observed using
at least 10 µmol/l emodin for 72 h (Fig.
1Aa and Ba; P<0.05). This result suggests that emodin
inhibited the proliferation of Bcap-37 and ZR-75-30 cells.
Effects of emodin on apoptosis
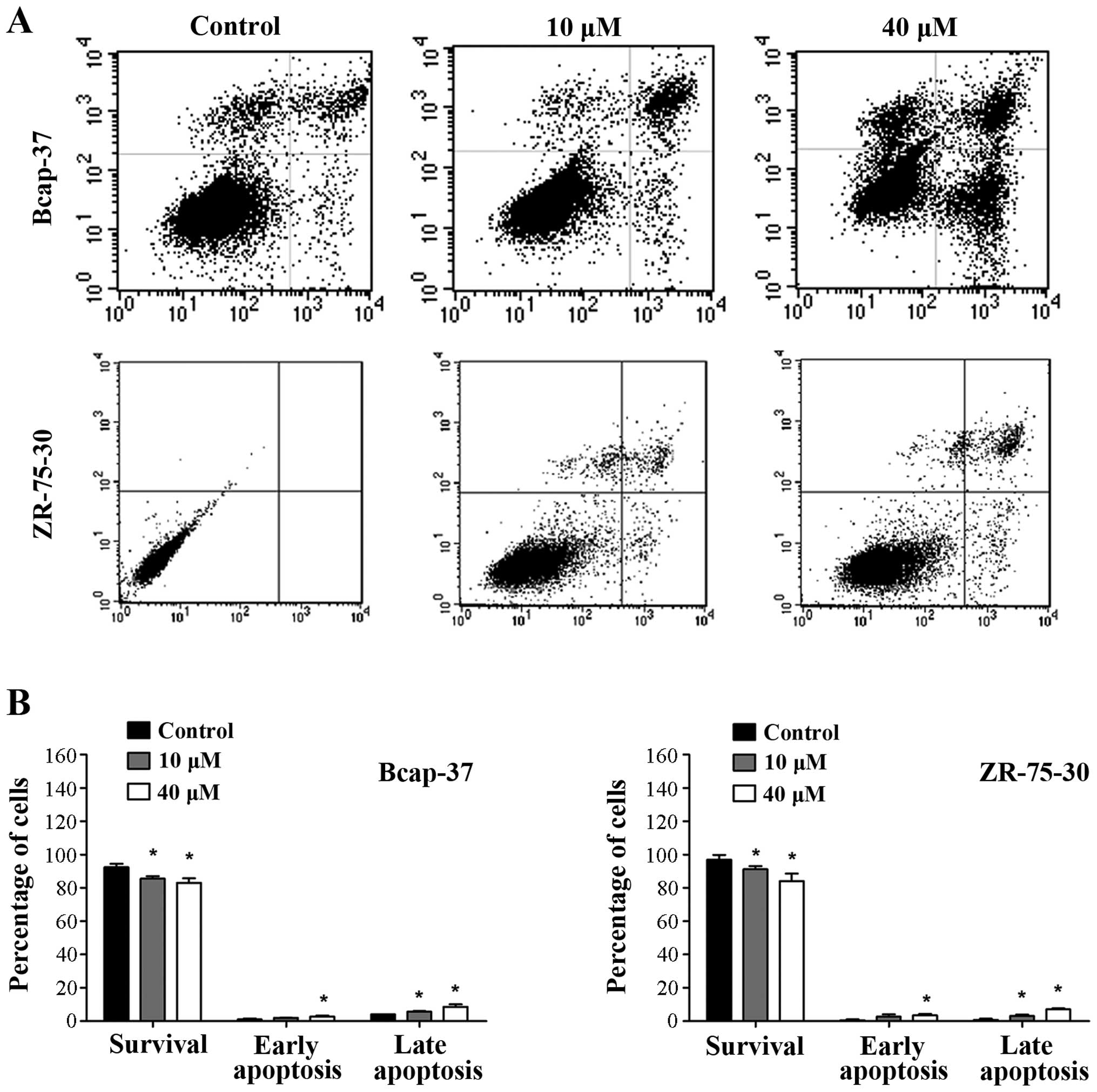
To determine whether the growth inhibition by emodin
is associated with apoptosis, flow cytometry analysis was
performed. Significant apoptosis induced by emodin was observed in
Bcap-37 and ZR-75-30 cells in a concentration-dependent manner
(Fig. 2; P<0.05). Apoptotic rates
were highest when using emodin at 40 µM. These results indicate
that emodin induced apoptosis of breast cancer cells.
Effects of emodin on caspase
activation and Bcl-2 family members
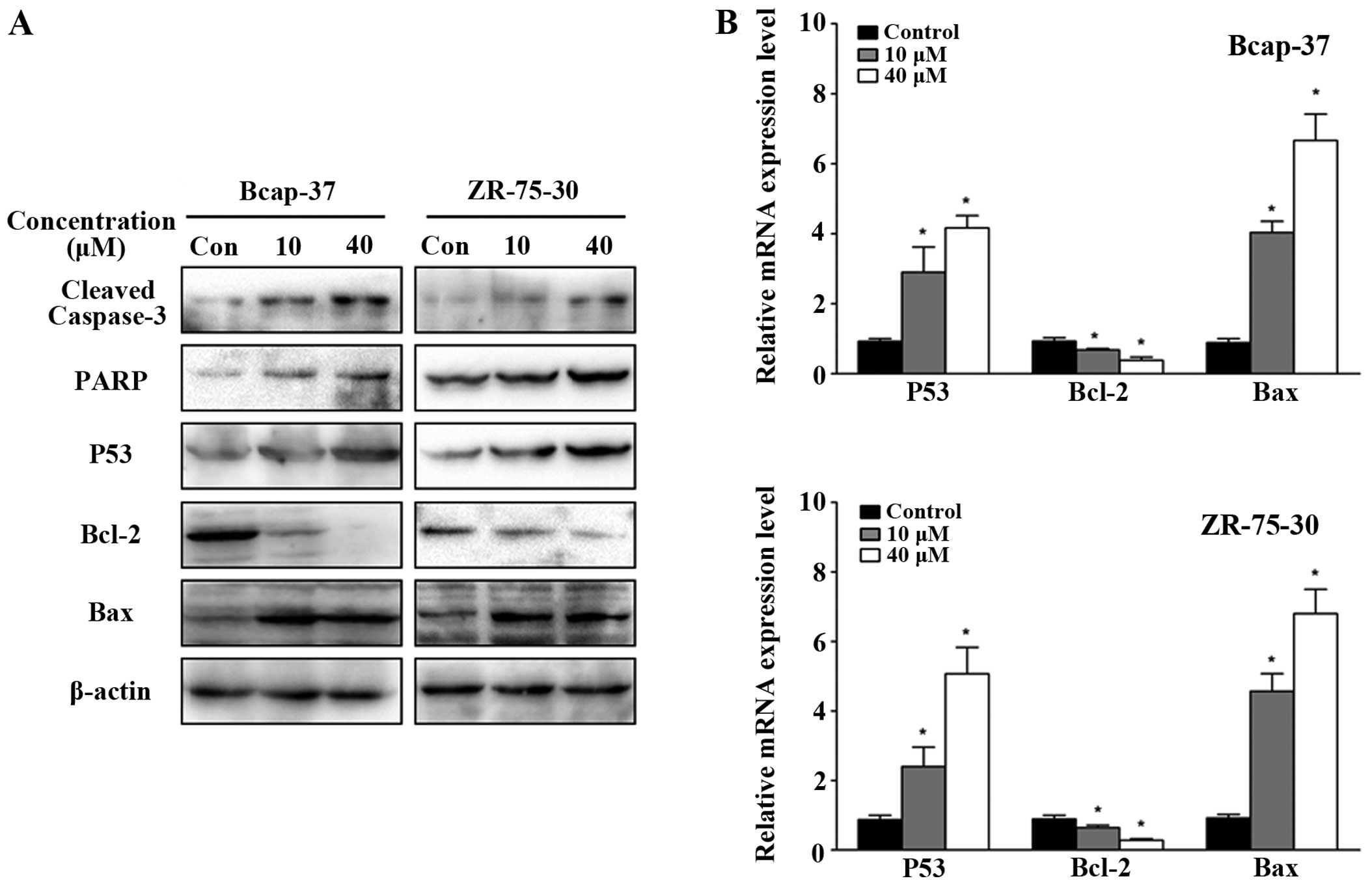
To investigate the effects of emodin on the
molecular mechanisms associated with apoptosis in breast cancer
cells, the expression of apoptosis-related proteins was analyzed by
western blot analysis. Emodin treatment downregulated Bcl-2
expression while increasing caspase-3 activation, PARP, p53 and Bax
levels in breast cancer cells in a concentration-dependent manner
(Fig. 3A). Similar observations were
made with respect to the mRNA level by qPCR, with significant
increases in Bax levels, and decreases in p53 and Bcl-2 (Fig. 3B; P<0.05). These observations
provide further support that emodin induces apoptosis of breast
cancer cells.
Effects of emodin on cell
invasion
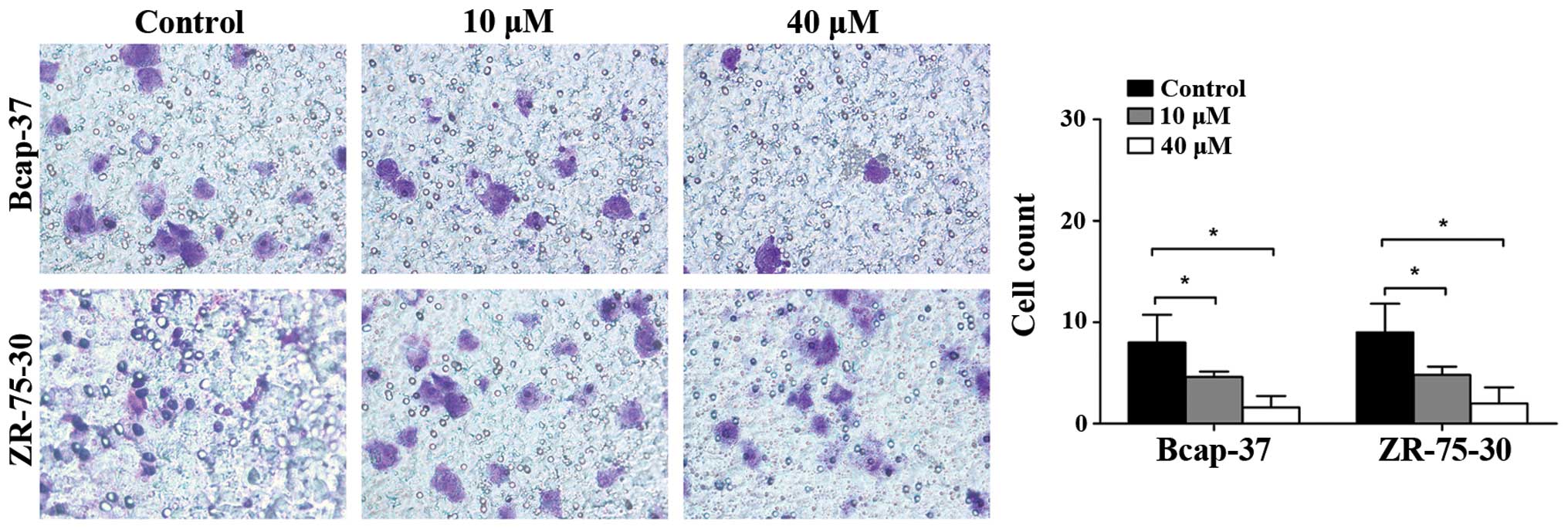
The effects of emodin on the invasiveness of breast
cancer cells were assessed by the Matrigel invasion assay. As shown
in Fig. 4, emodin decreased the
number of Bcap-37 and ZR-75-30 cells invading through the Matrigel
at 24 h in a concentration-dependent manner. Transwell assay
demonstrated that the invasiveness of Bcap-37 and ZR-75-30 cells
was suppressed by emodin.
Discussion
Emodin is a natural anthraquinone and an inhibitor
of tyrosine kinase (14). Emodin is
derived from rhubarb and numerous other plants, and has
demonstrated antibacterial, antiviral, anti-inflammatory and
anticancer effects (15). However,
previous studies have revealed that emodin inhibits cell growth in
several types of cancer cells by regulating genes related to
apoptosis, oncogenesis, proliferation and cancer cell invasion and
metastasis (10,16,17).
Notably, emodin inhibited epithelial-mesenchymal transition,
suggesting that emodin may have anticancer effects (18). Additionally, a study demonstrated that
emodin enhanced anticancer effects when used in combination with
gemcitabine (19). However, little is
known about the effects of emodin in breast cancer cells. In this
study, we assessed the effects of emodin on the growth, apoptosis
and invasiveness of Bcap-37 and ZR-75-30 breast cancer cells.
In this study, emodin induced apoptosis of Bcap-37
and ZR-75-30 cells in a dose- and time-dependent manner.
Furthermore, flow cytometric analysis demonstrated a significant
increase in the number of Bcap-37 and ZR-75-30 cells in the early
and late apoptosis phase. Emodin also inhibited the invasiveness of
these cells, as demonstrated by the Matrigel invasion assay. These
findings suggest that emodin functions by inhibiting the growth and
invasion of tumor cells.
Cell apoptosis is an autonomous cell death process
that regulates the development and homeostasis of multicellular
organisms (20). A key strategy for
drug development is to induce apoptosis of cancer cells while
minimizing the damage to normal and healthy cells (21). The induction of apoptosis in cancer
cells has been described as a key mechanism in anticancer therapy
(22,23).
Apoptosis is regulated by the Fas and mitochondrial
signaling pathways (24). The tumor
suppressor gene p53 plays a significant role in genomic stability,
anticancer activity, protection against malignant transformation,
and induction of apoptosis (25,26). p53
is a transcriptional factor that upregulates the expression of
downstream genes involved in cell cycle arrest, senescence,
autophagy and apoptosis. The p53 protein is also involved in
apoptosis, partly by inducing Bax expression (27). Bcl-2 is an inhibitor of the
mitochondrial apoptotic pathway, which prevents the release of
cytochrome c and caspase activation by blocking the effects
of pro-apoptotic proteins (28).
Among the Bcl-2 gene family, the apoptosis-promoting protein Bax
and the anti-apoptotic protein Bcl-2 play a crucial role in
regulating cell apoptosis (29,30), which
depends on the occurrence and severity of apoptosis (30). In our study, a dose-dependent decrease
in Bcl-2 expression and an increase in Bax expression was observed
following emodin treatment in Bcap-37 and ZR-75-30 cells. These
observations demonstrate that emodin-induced apoptosis of Bcap-37
and ZR-75-30 cells was triggered by the downregulation of Bcl-2 and
the upregulation of Bax. Previous studies have demonstrated that
the mitochondrial membrane potential stimulates the opening of the
mitochondrial membrane, resulting in the release of cytochrome
c into the cytoplasm, caspase activation and degradation of
key intracellular proteins, which leads to apoptosis (31,32).
Caspase-3 exists in the cytoplasm as an inactive
zymogen. When activated by the external apoptotic signals,
caspase-3 induces the inactivation of a number of key proteases in
the cytoplasm, cell nucleus and cytoskeleton, which subsequently
induces apoptosis (33). The cleavage
of apoptosis-related proteins, caspase-9, caspase-3 and PARP is
accompanied by cytochrome c release. In this study, the
cleavage of caspase-3 expression and the alteration of PARP were in
accordance with the tendency of cell apoptosis (34).
In summary, we have demonstrated in the present
study that emodin significantly inhibited the growth and
invasiveness, and induced the apoptosis of human breast cancer
cells in a dose- and time-dependent manner. These processes were
also associated with caspase and PARP activation, increased levels
of p53 and Bax, and decreased levels of Bcl-2. Further studies are
needed in the future in order to elucidate the biological and
molecular mechanisms of emodin for the treatment of breast cancer.
Overall, this study suggests that emodin is a potential therapeutic
agent for the treatment of breast cancer.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81172199 and
81272920).
References
|
1
|
Sanguinetti A, Polistena A, Lucchini R,
Monacelli M, Avenia S, Conti C, Barillaro I, Rondelli F,
Bugiantella W and Avenia N: Breast cancer in older women: What
factors affect the treatment? Int J Surg. 12(Suppl 2): S177–S180.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay JSI, Ervik M, Dikshit R, Eser S,
Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: GLOBOCAN 2012
v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No.
11. (Lyon, France). International Agency for Research on Cancer.
2013.http://globocan.iarc.frAccessed. December
12–2013
|
|
3
|
Zeng H, Zheng R, Zhang S, Zou X and Chen
W: Female breast cancer statistics of 2010 in China: Estimates
based on data from 145 population-based cancer registries. J Thorac
Dis. 6:466–470. 2014.PubMed/NCBI
|
|
4
|
Lin G, Zhu W, Yang L, Wu J, Lin B, Xu Y,
Cheng Z, Xia C, Gong Q, Song B and Ai H: Delivery of siRNA by
MRI-visible nanovehicles to overcome drug resistance in MCF-7/ADR
human breast cancer cells. Biomaterials. 35:9495–9507. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amirzada MI, Ma X, Gong X, Chen Y, Bashir
S and Jin J: Recombinant human interleukin 24 reverses Adriamycin
resistance in a human breast cancer cell line. Pharmacol Rep.
66:915–919. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bright-Gbebry M, Makambi KH, Rohan JP,
Llanos AA, Rosenberg L, Palmer JR and Adams-Campbell LL: Use of
multivitamins, folic acid and herbal supplements among breast
cancer survivors: the black women's health study. BMC Complement
Altern Med. 11:302011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spazzapan S, Crivellari D, Bedard P,
Lombardi D, Miolo G, Scalone S and Veronesi A: Therapeutic
management of breast cancer in the elderly. Expert Opin
Pharmacother. 12:945–960. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li WY, Chan SW, Guo DJ, Chung MK, Leung TY
and Yu PH: Water extract of Rheum officinale Baill. induces
apoptosis in human lung adenocarcinoma A549 and human breast cancer
MCF-7 cell lines. J Ethnopharmacol. 124:251–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Wu X, Chen M, Duan W, Sun L, Yan M
and Zhang L: Emodin induces apoptosis through caspase 3-dependent
pathway in HK-2 cells. Toxicology. 231:120–128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li WY, Ng YF, Zhang H, Guo ZD, Guo DJ,
Kwan YW, Leung GP, Lee SM, Yu PH and Chan SW: Emodin elicits
cytotoxicity in human lung adenocarcinoma A549 cells through
inducing apoptosis. Inflammopharmacology. 22:127–134. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li WY, Chan RY, Yu PH and Chan SW: Emodin
induces cytotoxic effect in human breast carcinoma MCF-7 cell
through modulating the expression of apoptosis-related genes. Pharm
Biol. 51:1175–1181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sui JQ, Xie KP, Zou W and Xie MJ: Emodin
inhibits breast cancer cell proliferation through the
ERα-MAPK/Akt-cyclin D1/Bcl-2 signaling pathway. Asian Pac J Cancer
Prev. 15:6247–6251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pooja T and Karunagaran D: Emodin
suppresses Wnt signaling in human colorectal cancer cells SW480 and
SW620. Eur J Pharmacol. 742:55–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei WT, Lin SZ, Liu DL and Wang ZH: The
distinct mechanisms of the antitumor activity of emodin in
different types of cancer (Review). Oncol Rep. 30:2555–2562.
2013.PubMed/NCBI
|
|
15
|
Xu JD, Liu S, Wang W, Li LS, Li XF, Li Y,
Guo H, Ji T, Feng XY, Hou XL, et al: Emodin induces chloride
secretion in rat distal colon through activation of mast cells and
enteric neurons. Br J Pharmacol. 165:197–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue H, Chen Y, Cai X, Zhao L, He A, Guo K
and Zheng X: The combined effect of survivin-targeted shRNA and
emodin on the proliferation and invasion of ovarian cancer cells.
Anticancer Drugs. 24:937–944. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yaoxian W, Hui Y, Yunyan Z, Yanqin L, Xin
G and Xiaoke W: Emodin induces apoptosis of human cervical cancer
hela cells via intrinsic mitochondrial and extrinsic death receptor
pathway. Cancer Cell Int. 13:712013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Way TD, Huang JT, Chou CH, Huang CH, Yang
MH and Ho CT: Emodin represses TWIST1-induced
epithelial-mesenchymal transitions in head and neck squamous cell
carcinoma cells by inhibiting the β-catenin and Akt pathways. Eur J
Cancer. 50:366–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin SZ, Wei WT, Chen H, Chen KJ, Tong HF,
Wang ZH, Ni ZL, Liu HB, Guo HC and Liu DL: Antitumor activity of
emodin against pancreatic cancer depends on its dual role:
Promotion of apoptosis and suppression of angiogenesis. PLoS One.
7:e421462012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muppidi J, Porter M and Siegel RM:
Measurement of apoptosis and other forms of cell death. Curr Protoc
Immunol. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kelly PN and Strasser A: The role of Bcl-2
and its pro-survival relatives in tumourigenesis and cancer
therapy. Cell Death Differ. 18:1414–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Strasser A, Cory S and Adams JM:
Deciphering the rules of programmed cell death to improve therapy
of cancer and other diseases. EMBO J. 30:3667–3683. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heath RM, Jayne DG, O'Leary R, Morrison EE
and Guillou PJ: Tumour-induced apoptosis in human mesothelial
cells: A mechanism of peritoneal invasion by Fas Ligand/Fas
interaction. Br J Cancer. 90:1437–1442. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geng QQ, Dong DF, Chen NZ, Wu YY, Li EX,
Wang J and Wang SM: Induction of p53 expression and apoptosis by a
recombinant dual-target MDM2/MDMX inhibitory protein in wild-type
p53 breast cancer cells. Int J Oncol. 43:1935–1942. 2013.PubMed/NCBI
|
|
26
|
Harris SL and Levine AJ: The p53 pathway:
positive and negative feedback loops. Oncogene. 24:2899–2908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leard LE and Broaddus VC: Mesothelial cell
proliferation and apoptosis. Respirology. 9:292–299. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang CC, Yen CJ, Tsai TJ, Chen RH, Lee PH
and Tomino Y: Antibiotics induce apoptosis of human peritoneal
mesothelial cells. Nephrology (Carlton). 8:142–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Korsmeyer SJ, Shutter JR, Veis DJ, Merry
DE and Oltvai ZN: Bcl-2/Bax: a rheostat that regulates an
anti-oxidant pathway and cell death. Semin Cancer Biol. 4:327–332.
1993.PubMed/NCBI
|
|
30
|
Lindsay J, Esposti MD and Gilmore AP:
Bcl-2 proteins and mitochondria-specificity in membrane targeting
for death. Biochim Biophys Acta. 1813:532–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma L and Li W: Emodin inhibits LOVO
colorectal cancer cell proliferation via the regulation of the
Bcl-2/Bax ratio and cytochrome c. Exp Ther Med. 8:1225–1228.
2014.PubMed/NCBI
|
|
32
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
33
|
Liu TY, Tan ZJ, Jiang L, Gu JF, Wu XS, Cao
Y, Li ML, Wu KJ and Liu YB: Curcumin induces apoptosis in
gallbladder carcinoma cell line GBC-SD cells. Cancer Cell Int.
13:642013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Wu N, Ma LN, Zhong JT, Liu G, Zheng
LH and Lin XK: p38 MAPK signaling mediates mitochondrial apoptosis
in cancer cells induced by oleanolic acid. Asian Pac J Cancer Prev.
15:4519–4525. 2014. View Article : Google Scholar : PubMed/NCBI
|