Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide and small-cell lung cancer
(SCLC) accounts for ~15% of all primary lung cancers (1). Notably, SCLC and non-small cell lung
cancer (NSCLC) differ in terms of tumor biology, clinical
presentation and response to treatment. Mediastinal or hilar
lymphadenopathies, which are associated with a cough and dyspnea,
are more common on clinical presentation of SCLC than NSCLC
(2). Platinum doublet therapy with
etoposide or irinotecan is the standard treatment for SCLC and
>60% of SCLC patients respond to treatment (3,4). The
five-year survival rate of SCLC patients is <5% (4). Recently, advances in high-throughput
technologies, such as next generation sequencing, have led to the
identification of a number of mutations, including RICTOR, KIT and
PIK3CA, which are considered to exert a crucial role in SCLC tumor
biology (5). Treatment response is
evaluated in order to determine the therapeutic effect and to
modify the treatment regimen when progressive disease is observed
in a variety of cancers. Heterogeneous radiological response (HRR)
among tumor lesions, where certain lesions decrease in size or
disappear and other lesions increase in size, is usually observed
following chemotherapy or radiation treatment in cancer patients
and is estimated to occur in 15–36% of cancer cases (6,7). With
advances in high-throughput technologies, such as next-generation
sequencing, intra- or inter-tumoral genomic heterogeneity has been
highlighted in the area of molecular oncology and can improve our
understanding of this response (8).
For example, inter-tumoral heterogeneity of an EGFR mutation has
been well described in adenocarcinoma (9). Therefore, the HRR leads clinicians to
switch to another anticancer regimen due to the clinically high
suspicion of resistance, as histological confirmation is not
feasible in the majority of cases. However, when the response is
presented, a non-tumor diagnosis should be considered where its
relevant treatment is required. The present study reports the case
report of a patient with limited-stage small cell lung cancer who
presented with an HRR following concurrent chemoradiation therapy.
Written informed consent was obtained from the patient.
Case report
In March 2013, a previously healthy 62-year-old male
was referred to Inha University Hospital (Incheon, South Korea) due
to small cell lung cancer diagnosed from a specimen obtained by
bronchoscopic biopsy. The patient was a current smoker with no
history of tuberculosis (TB). The Eastern Cooperative Oncology
Group performance score (10) was
zero. Smears and cultures for acid-fast bacilli were negative in a
bronchial washing specimen. A mass on the lower lobe of the left
lung and bulky lymphadenopathies on the left hilum, and mediastinal
and supraclavicular areas, were noted on
[18F]fluorodeoxyglucose positron emission
tomography/computed tomography (18FDG-PET-CT) scans
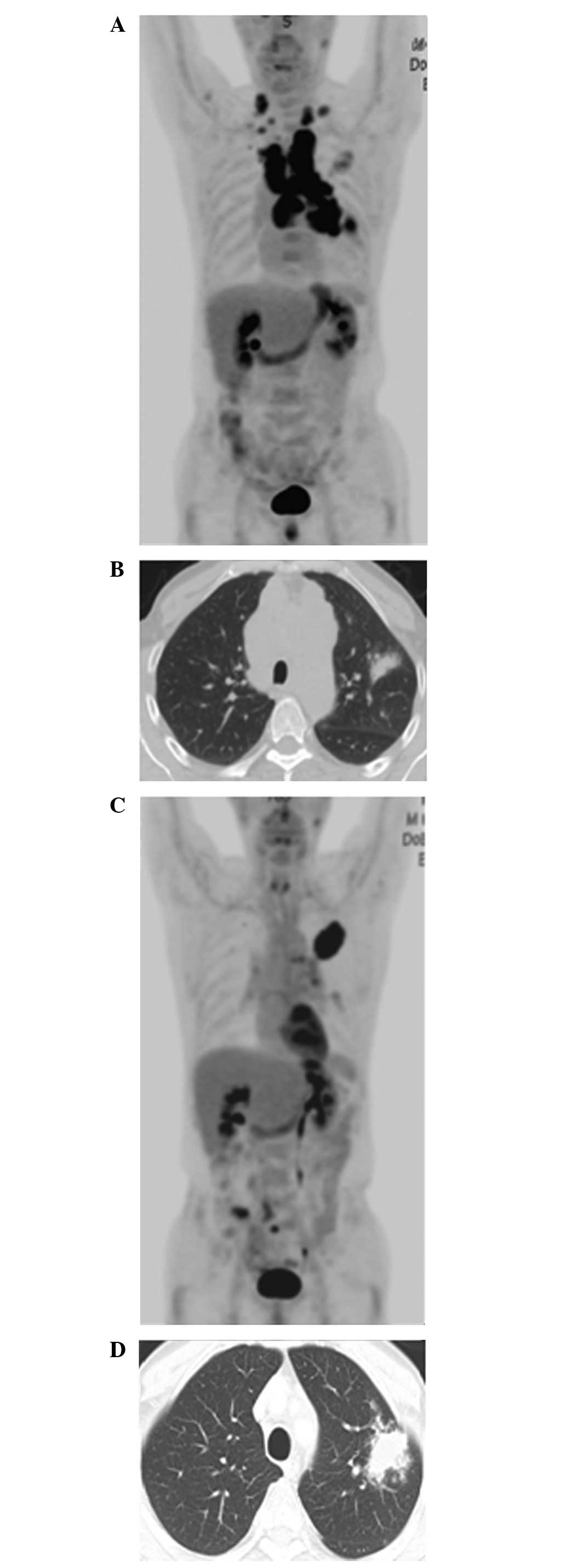
(Fig. 1A). A consolidative mass was
observed on the upper lobe of the left lung (Fig. 1B). No evidence for metastasis was
observed in other imaging studies, including bone scans and brain
magnetic resonance imaging. Finally, the disease was classified as
limited-stage disease, T4N3M0, using the seventh edition TNM
staging system (11). Radiation
therapy consisting of a total of 5,400 cGy was administered
concurrently to the patient with chemotherapy for 1.5 months. All
the aforementioned lesions were included in the radiation field.
Chemotherapy consisted of etoposide (100 mg/m2 on days
1, 2 and 3) and cisplatin (60 mg/m2 on day 1) every
three weeks. Once the treatment was completed, response evaluation
revealed that the lymphadenopathies and mass on the lower lobe of
the left lung were apparently decreased in size (Fig. 1C), while the mass on the upper lobe of
the left lung was significantly increased in size (Fig. 1D). The patient experienced no fever,
coughing, sputum production or dyspnea. A blood examination showed
a white blood cell count of 5.5×103/ml (normal range,
4–10×103/ml), with 70% neutrophils and 17% lymphocytes,
a C-reactive protein level of 1.18 mg/dl (normal range, 0-–0.3
mg/dl) and an erythrocyte sedimentation rate of 52 mm/h (normal
range for male individuals, 1–15 mm/h). A repeat bronchoscopic
examination was performed for the evaluation of the aggravated
lesion. No endobronchial lesions were found. Smears and polymeric
chain reaction for acid-fast bacilli, and examinations for
bacteria, fungi and malignancy were negative in a bronchial washing
specimen. A CT-guided percutaneous needle biopsy was performed on
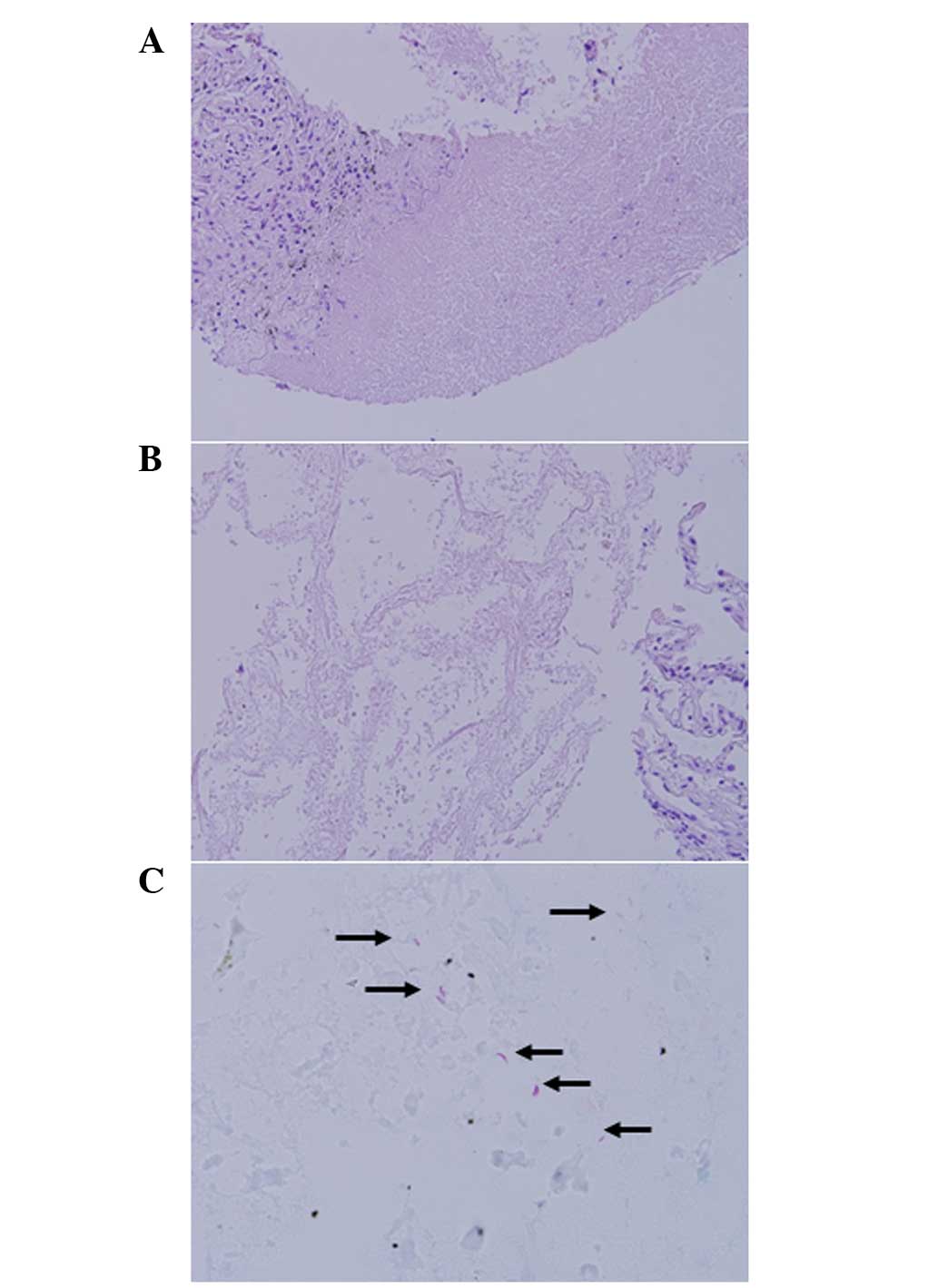
the lesion. Pathological examination showed nodular necrosis with a
fibrotic rim (Fig. 2A) and
infarct-like necrosis of the normal lung tissue without definite
granuloma formation (Fig. 2B).
Several acid-fast bacilli in the necrotic area were identified on
Ziehl-Neelsen stain (Fig. 2C). Next,
anti-TB treatment [oral isoniazid (400 mg) rifampin (600 mg) and
ethambutol (800 mg) plus pyrazinamide (1,500 mg)] was administered
to the patient. One month later, cultures for acid-fast bacilli
were positive in a bronchial washing specimen. Drug susceptibility
test results of Mycobacterium tuberculosis isolates showed
sensitivity to all anti-TB drugs. The initial anti-TB regimen was
administered for two months, and then isoniazid (400 mg), rifampin
(600 mg) and ethambutol (800 mg) were administered in the following
four months. Then, his tuberculous lesion was lessened and
stabilized. His lung cancer was not recurred until April 2014 when
he was lost to be followed up.
Discussion
The coincidence of active TB and lung cancer is an
unusual clinical situation that is reported to occur in 0.3–0.6% of
patients with lung cancer in TB endemic areas (12,13). The
question of whether chemotherapy or radiation therapy can
reactivate latent M. tuberculosis infection in patients with
lung cancer remains elusive (14).
For instance, radiation causes a significant decrease in numbers of
lymphocytes, which can led to the development of TB reactivation,
while a clinical dose of radiation can directly kill ~60% of M.
tuberculosis (15,16). Therefore, treatment can interact with
TB in the fashion of a double-edged sword. No differences in
clinical manifestation and outcome of anti-TB treatment were
observed between patients with active TB occurring during
chemotherapy in a previous study (17). With regard to the survival of patients
with lung cancer, it has been reported that concomitant active TB
can enhance local T-cell immunity and prolong survival (18). For these reasons, testing for latent
TB is not recommended in these patients; clinicians are required to
pay less attention to coincidence.
HRR is an existing concept that has witnessed a
recent resurgence in use, as rapid advances in cancer genomics and
functional imaging have facilitated our understanding of biological
heterogeneity and its clinical application in the evaluation of
treatment response. Consequently this can be of use in guiding
further treatment and predicting survival in cancer patients
(6,7,9).
The TB diagnosis for the lesion on the upper lobe of
the left lung in the current patient was so unlikely due to an
absence of typical radiological patterns for TB on
18FDG-PET-CT scan and negative results for acid-fast
bacilli at the time of diagnosis. Therefore, the lesion was
included in the radiation field during treatment on the assumption
that this was malignant. In general, the response to concurrent
chemoradiation therapy was expected to be higher, as this is
reported to be 70–90% in limited-stage small cell lung cancer
(19). Thus, it was not easy to
predict the reason for this response when an HRR was obtained in
the patient without histological confirmation.
In conclusion, when an HRR is observed, aggressive
diagnostic approaches, including tissue biopsy for suspicious
lesions, are useful in differentiating a non-tumor diagnosis from
tumor heterogeneity and should be considered when the treatment
provided is expected to have a higher response rate, particularly
in TB endemic countries.
Acknowledgements
This study was supported by the National Research
Foundation of Korea grant funded by the Korean government (Ministry
of Science, ICT and Future Planning; no. NRF-2014R1A5A2009392), and
the Basic Science Research Program through the National Research
Foundation of Korea funded by the Ministry of Education, Science
and Technology (no. 2013R1A1A2007537).
References
|
1
|
Vallières E, Shepherd FA, Crowley J, et
al: International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals regarding the
relevance of TNM in the pathologic staging of small cell lung
cancer in the forthcoming (seventh) edition of the TNM
classification for lung cancer. J Thorac Oncol. 4:1049–1059. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rivera MP, Mehta AC and Wahidi MM:
Establishing the diagnosis of lung cancer: Diagnosis and management
of lung cancer, 3rd ed: American College of Chest Physicians
evidence-based clinical practice guidelines. Chest. 143(Suppl 5):
eS142–S65. 2013. View Article : Google Scholar
|
|
3
|
Noda K, Nishiwaki Y, Kawahara M, et al:
Japan Clinical Oncology Group: Irinotecan plus cisplatin compared
with etoposide plus cisplatin for extensive small-cell lung cancer.
N Engl J Med. 346:85–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chute JP, Chen T, Feigal E, Simon R and
Johnson BE: Twenty years of phase III trials for patients with
extensive-stage small-cell lung cancer: Perceptible progress. J
Clin Oncol. 17:1794–1801. 1999.PubMed/NCBI
|
|
5
|
Ross JS, Wang K, Elkadi OR, Tarasen A,
Foulke L, Sheehan CE, Otto GA, Palmer G, Yelensky R, Lipson D, et
al: Next-generation sequencing reveals frequent consistent genomic
alterations in small cell undifferentiated lung cancer. J Clin
Pathol. 67:772–776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Kessel CS, Samim M, Koopman M, van den
Bosch MA, Rinkes Borel IH, Punt CJ and van Hillegersberg R:
Radiological heterogeneity in response to chemotherapy is
associated with poor survival in patients with colorectal liver
metastases. Eur J Cancer. 49:2486–2493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Win T, Miles KA, Janes SM, Ganeshan B,
Shastry M, Endozo R, Meagher M, Shortman RI, Wan S, Kayani I, et
al: Tumor heterogeneity and permeability as measured on the CT
component of PET/CT predict survival in patients with non-small
cell lung cancer. Clin Cancer Res. 19:3591–3599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gerlinger M, Rowan AJ, Horswell S, et al:
Intratumor heterogeneity and branched evolution revealed by
multiregion sequencing. N Engl J Med. 366:883–892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen ZY, Zhong WZ, Zhang XC, Su J, Yang
XN, Chen ZH, Yang JJ, Zhou Q, Yan HH, An SJ, et al: EGFR mutation
heterogeneity and the mixed response to EGFR tyrosine kinase
inhibitors of lung adenocarcinomas. Oncologist. 17:978–985. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shepherd FA, Crowley J, Van Houtte P,
Postmus PE, Carney D, Chansky K, Shaikh Z and Goldstraw P:
International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions: The
International Association for the Study of Lung Cancer lung cancer
staging project: Proposals regarding the clinical staging of small
cell lung cancer in the forthcoming (seventh) edition of the tumor
node, metastasis classification for lung cancer. J Thorac Oncol.
2:1067–1077. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu CY, Hu HY, Pu CY, Huang N, Shen HC, Li
CP and Chou YJ: Aerodigestive tract, lung and haematological
cancers are risk factors for tuberculosis: An 8-year
population-based study. Int J Tuberc Lung Dis. 15:125–130.
2011.PubMed/NCBI
|
|
13
|
Kim HR, Hwang SS, Ro YK, Jeon CH, Ha DY,
Park SJ, Lee CH, Lee SM, Yoo CG, Kim YW, et al: Solid-organ
malignancy as a risk factor for tuberculosis. Respirology.
13:413–419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nair R, Prabhash K, Sengar M, Bakshi A,
Gujral S, Gupta S and Parikh P: The effect of short-term intensive
chemotherapy on reactivation of tuberculosis. Ann Oncol.
18:1243–1245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stratton JA, Byfield PE, Byfield JE, Small
RC, Benfield J and Pilch Y: A comparison of the acute effects of
radiation therapy, including or excluding the thymus, on the
lymphocyte subpopulations of cancer patients. J Clin Invest.
56:88–97. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zack MB, Stottmeier K, Berg G and Kazemi
H: The effect of radiation on microbiologic characteristics of M
tuberculosis. Chest. 66:240–243. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim DK, Lee SW, Yoo CG, Kim YW, Han SK,
Shim YS and Yim JJ: Clinical characteristics and treatment
responses of tuberculosis in patients with malignancy receiving
anticancer chemotherapy. Chest. 128:2218–2222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuo CH, Lo CY, Chung FT, Lee KY, Lin SM,
Wang CH, Heh CC, Chen HC and Kuo HP: Concomitant active
tuberculosis prolongs survival in non-small cell lung cancer: A
study in a tuberculosis-endemic country. PLoS One. 7:e332262012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edelman MJ, Chansky K, Gaspar LE, Leigh B,
Weiss GR, Taylor SA, Crowley J, Livingston R and Gandara DR: Phase
II trial of cisplatin/etoposide and concurrent radiotherapy
followed by paclitaxel/carboplatin consolidation for limited
small-cell lung cancer: Southwest oncology group 9713. J Clin
Oncol. 22:127–132. 2004. View Article : Google Scholar : PubMed/NCBI
|