Introduction
Gastric cancer (GC) is the second most common cause
of cancer-related mortality globally (1). α-fetoprotein (AFP)-producing GC (AFPGC)
is a special type of stomach cancer, due to its rareness, and its
aggressive and malignant features. AFPGC was initially described by
Bourreille et al in 1970 (2)
in gastric cancer patients that exhibited elevated levels of serum
AFP and simultaneous liver metastases. AFPGC is characterized by
elevated levels of serum AFP and thus, this forms the basis for
diagnosis of the disease. However, at present, the exact definition
of AFPGC remains unclear (2). We
hypothesize that histopathological examination is important for the
diagnosis of AFPGC, as other malignancies, such as hepatitis,
cirrhosis, hepatocellular carcinoma and germ cell malignancies,
also produce AFP and thus, must be excluded. According to the
English-language literature, AFPGC accounts for 1.3–15% of GC cases
worldwide (3–8). To the best of our knowledge, no standard
therapy is currently available for patients with AFPGC and the
prognosis remains extremely poor. In 1965, the Lauren
classification of gastric carcinoma (9) was established, which may be applied to
guide treatment choices and predict prognosis of gastric cancer
patients. The present study reports the case of an AFPGC patient
with simultaneous liver metastases who ultimately achieved an
overall survival time of 30 months following multi-modal
therapy.
Case report
A 59-year-old male with upper abdominal bloating
presented to a local hospital in January 2012. The patient had no
history of hepatitis and there was no significant relevant family
history. Endoscopy revealed a primary lesion located in the gastric
cardia. Subsequent pathological examination of biopsy specimens
determined the lesion to be an adenocarcinoma. Ultrasound and
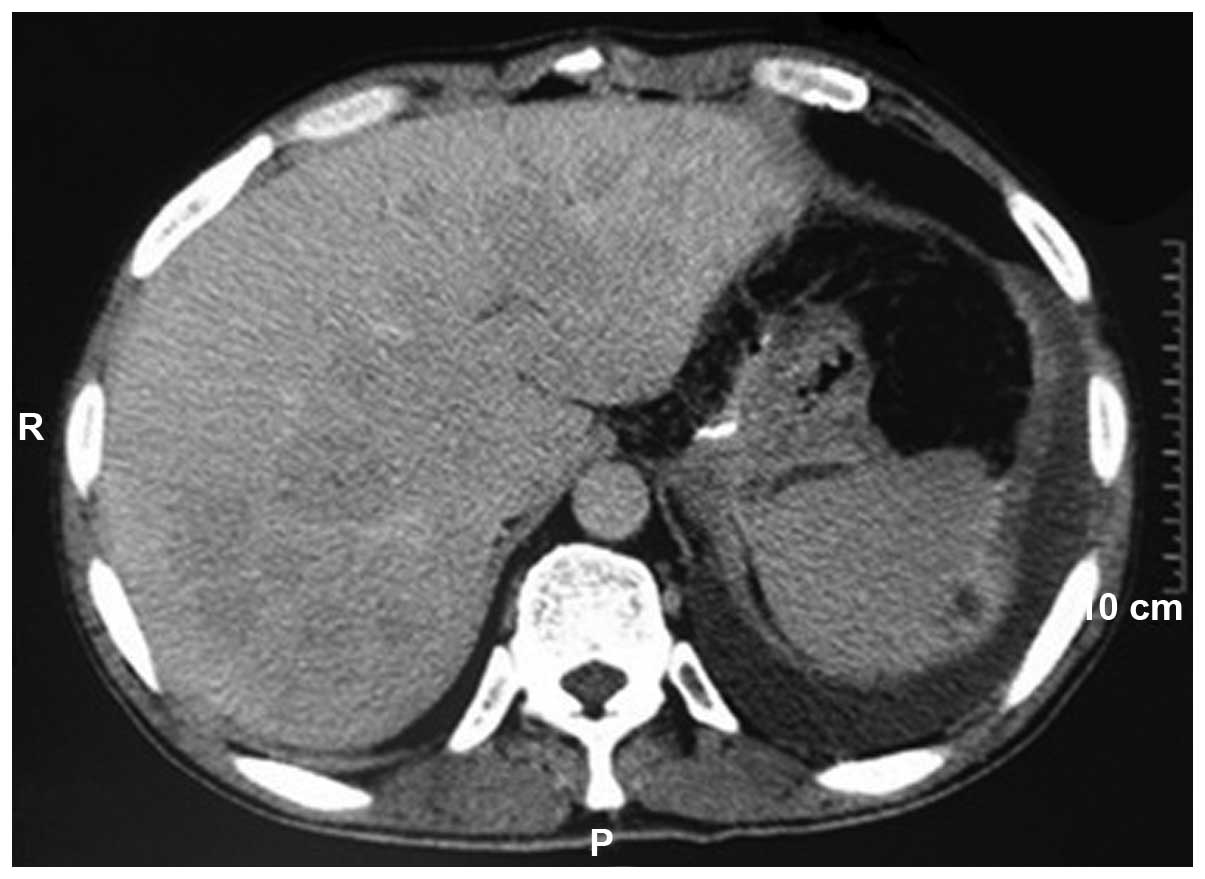
computed tomography revealed multiple lesions in the liver. The
liver metastases lesions were defined as unresectable lesions
(Fig. 1). The patient underwent a
proximal gastrectomy plus liver nodule biopsy in January 2012.
Histopathological examination demonstrated
moderately-differentiated adenocarcinoma (Fig. 2A), diffuse type (Lauren
classification) (9). Three lymph node
metastases were detected in 28 retrieved lymph nodes. Cancer tissue
was found in the liver nodules. Immunohistochemical analysis
revealed that epidermal growth factor receptor protein and AFP
(Fig. 2B) were positively expressed.
However, the tumor cells were negative for vascular endothelial
growth factor (VEGF) and C-met. Fluorescence in situ
hybridization revealed that HER-2 gene amplification was negative.
Individual tumor target detection revealed that VEGF receptor 1
(VEGFR1) mRNA expression was low [≥35.3% (low, 0.0–40.0%; moderate,
40.1–80.0%; high, 80.1–100.0%)], that VEGFR2 mRNA expression was
low [≥14.0% (low, 0.0–40.0%; moderate, 40.1–80.0%; high,
80.1–100.0%)] and that VEGFR3 mRNA expression was moderate [≥50.3%
(low, 0.0–40.0%; moderate, 40.1–80.0%; high, 80.1–100.0%)]. The
post-operative period of the patient was uneventful and the
bloating in the upper abdomen disappeared.
At 1 month post-diagnosis, the patient was referred
to the 81st Hospital of the People's Liberation Army (Nanjing,
Jiangsu, China) for chemotherapy. The serum level of
carcinoembryonic antigen (CEA) was 173.3 µg/l (normal range, 0–9.9
µg/l) and the serum AFP was not checked. Other data obtained from
laboratory investigations were within the normal ranges. The
patient was treated with a paclitaxel (135
mg/m2)/leucovorin calcium (200
mg/m2)/fluorouracil (2.4 g/m2) regimen (TLF)
as first-line treatment. This regimen was repeated every 3 weeks
for 6 cycles. During each course of this chemotherapy, the patient
suffered from grade 1 leucopenia, according to the Common
Terminology Criteria for Adverse Events (version 3.0) (10). Subsequent to 6 cycles of treatment,
compared with the lesions in the baseline period, the liver lesions
were markedly smaller. According to the Response Evaluation
Criteria in Solid Tumors criteria (version 1.1) (11), the evaluation of efficacy indicated a
partial response (PR) (Fig. 3). The
serum CEA level returned to normal. Thereafter, 1 cycle of Xeloda
monotherapy (1,250 mg/m2 on prescription, days 1–14) was
administered as maintenance treatment.
At 9 months post-diagnosis, the serum AFP level was
recorded at 43.9 µg/l (normal range, 0–10.0 µg/l). To treat the
liver metastases, 4 cycles of transhepatic arterial chemotherapy
and embolization (TACE)-oxaliplatin (150 mg)/S-1 (50
mg/m2 on prescription, days 1–28) (oral) was
administered. No significant adverse effects were observed and no
gastrointestinal or bone marrow toxicities were detected. Following
2 cycles of treatment, the serum AFP level had decreased to normal.
Computed tomography evaluation showed that the efficacy was being
maintained as a PR.
At 19 months post-diagnosis, the serum level of AFP
had increased to 186.8 µg/l. Computed tomography revealed
retroperitoneal lymph node swelling and new metastases to the right
lung. The efficacy was now evaluated as progressive disease (PD).
The patient accepted CPT-11/leucovorin calcium/fluorouracil regimen
(FOLFIRI) chemotherapy. Following 2 cycles of treatment, the serum
AFP level had decreased to a normal level. However, subsequent to 4
cycles of treatment, computed tomography revealed that the liver
metastases had increased in size by ~10% compared with previously,
and the serum AFP level had increased to 340.4 µg/l. The disease
was considered to be undergoing slow progression and 1 cycle of an
oxaliplatin/capecitabine regimen was administered as palliative
chemotherapy.
At 22 months post-diagnosis, the serum AFP level had
increased to 998.8 µg/l. The patient required active treatment. By
searching the literature, a single study was found on AFPGC
patients who were orally administered sorafenib to extend their
survival time and improve their quality of life (12). Therefore at 22 months post-diagnosis,
the patient commenced treatment with oral sorafenib (400 mg/day and
200 mg/day, alternately) monotherapy due to refusal to undertake
other chemotherapies. The serum AFP level had decreased to 739.1
µg/l 2 weeks later. At 24 months post-diagnosis, upper abdominal
magnetic resonance imaging scans demonstrated that the liver
lesions were increased in size and revealed a tumor thrombus in the
left branch of the portal vein. At the same time, the serum AFP
level had increased to 837.0 µg/l. The evaluation of efficacy was
PD. Sorafenib treatment was stopped. The only adverse event
observed was grade 1 blood pressure.
At 27 months post-diagnosis, the patient complained
of upper abdominal pain and bloating. The evaluation of efficacy
was PD by upper abdominal magnetic resonance imaging scans. The
serum AFP level had increased to 20,624.6 µg/l. Abraxane (75
mg/m2 on days 1 and 8) monotherapy was administered.
Following the first cycle of treatment, the serum AFP level had
decreased to 9,392.2 µg/l. The symptoms of upper abdominal pain and
bloating were also alleviated. Thereafter, a second cycle of
treatment was performed.
At 29 months post-diagnosis, the patient presented
with jaundice and a fever. Laboratory examination results were as
follows: Total bilirubin, 132.1 µmol/l (normal range, 5.1–19.0
µmol/l); direct bilirubin, 114.8 µmol/l (normal range, 1.7–6.8
µmol/l); albumin, 29.3 g/l (normal range, 40.0–55.0 g/l); aspartate
aminotransferase, 139.0 IU/l (normal range, 0–39.0 IU/l); and
urobilirubin and urobilinogen were positive. Abdominal ultrasound
revealed biliary sludge. A diagnosis of hepatocellular jaundice
with cholestatic jaundice was formed. Unexpectedly, the serum AFP
level had decreased to 4,225.73 µg/l. Magnetic resonance
cholangiopancreatography demonstrated that compared with
previously, the hepatic nodular lesions were smaller, the right
hepatic duct was dilated, and the right lung nodules were smaller
(Fig. 4). Following symptomatic and
supportive treatment, such as magnesium isoglycyrrhizinate, reduced
glutathione and Kuhuang injection, the bilirubin progressively
increased and the indication for chemotherapy was lost.
Multidisciplinary consultation recommended that the patient should
undergo endoscopic retrograde cholangiopancreatography plus stent
implantation. However, the patient refused and went home to
recuperate. The patient succumbed to the disease at 30 months
post-diagnosis.
Discussion
AFPGC is a special type of GC, accounting for only
1.3–15% of GCs in the English-language literature (3). AFPGC was first described in 1970 by
Bourreille et al (2). Elevated
serum AFP level is the basis for the diagnosis of AFPGC, while it
is also required to rule out other possible diseases, such as
hepatitis, cirrhosis, hepatocellular carcinoma and germ cell
malignancy. This type of GC is prone to liver and lymph node
metastasis, and has a poor prognosis. There is no effective means
to treat AFPGC, particularly in the field of internal medicine.
Currently, the disease is treated with reference to common cancer,
but the prognosis remains extremely poor. The median survival time
of AFPGC patients is significantly shorter than for those with
normal GC (13).
The present study reports the case of an
AFP-positive GC patient with simultaneous liver metastases. The
detection of serum AFP level was not considered at first. This also
suggests that attention should be focused on the disease in the
future process of diagnosis and treatment. The detection of serum
AFP level should be applied as a routine examination in GC
patients, particularly those with liver metastases. In the
post-treatment process of the present study, the serum AFP level
was constantly monitored, and the rise and fall in serum AFP levels
were found to be positively correlated with the patient's
condition. This is therefore an important means that can be used to
evaluate condition changes of a patient.
According to studies, the different Lauren
classifications of GC have different sensitivity to
chemotherapeutic drugs. The patients who are of the diffuse type
according to the Lauren classification can benefit more from drugs
such as paclitaxel, irinotecan and S-1 (14–17).
Fukuda et al reported that of the eight AFPGC patients that
received chemotherapy, two patients who received cisplatin plus
paclitaxel therapy exhibited a PR (18). Of the six remaining patients, who
received oral TS-1, in combination with either cisplatin or
camptothecin, the best response achieved was stable disease
(18). In the present case, the
patient' disease was of diffuse type. First-line chemotherapy with
a paclitaxel-based TLF regimen was selected and the efficacy
achieved was a PR. Following the use of this type of drug for 2
years, Abraxane (paclitaxel) was used, which again shrank the tumor
and decreased the serum AFP level. The drug exhibited good
antitumor effect. This encourages us not to give up when treating
AFPGC patients. In addition, the application of irinotecan for
AFPGC patients has also been reported (19). In this case, the patients were treated
with irinotecan-based FOLFIRI regimen, which was also effective.
Paclitaxel-based chemotherapy or irinotecan-based chemotherapy are
valid for use in such patients. This situation, whether associated
with the Lauren classification or affected by other factors,
requires further study.
The liver metastatic lesions were defined as
unresectable lesions in the present case. TACE-oxaliplatin/S-1
(oral) therapy was performed and achieved tumor shrinkage and AFP
level reduction. This shows the value of interventional therapy. A
survival time of >5 years has also been reported in an AFPGC
patient receiving hepatic arterial infusion therapy (12). It was suggested that hepatic arterial
infusion therapy may improve the prognosis compared with the use of
systemic chemotherapies in AFPGC patients with multiple liver
metastases (12).
Sorafenib is a multi-kinase inhibitor, but its
specific targets are not fully understood. On the one hand, it can
inhibit the activity of the hepatocyte growth factor/c-Met pathway
and the downstream RAF/mitogen-activated protein kinase
kinase/extracellular signal-regulated kinases signaling pathway,
resulting in inhibition of tumor growth. On the other hand, it can
block tumor angiogenesis by inhibiting VEGFR and platelet-derived
growth factor receptor to indirectly inhibit the growth of tumor
cells. The drug has achieved impressive efficacy in hepatocellular
carcinoma (20,21). During an Oriental Studies subgroup
analysis, in which AFP-positive HCC patients (serum AFP level, ≥40
µg/l) were treated with sorafenib and placebo, respectively, the
median survival time was prolonged by 1.8 months in the sorafenib
group compared with the placebo group (5.9 vs. 4.1 months; hazard
ratio, 0.65) (22). AFPGC and
AFP-positive HCC are similar with regard to the elevated serum AFP
level. Studies have reported the use of sorafenib for the treatment
of advanced GC (23), and even AFPGC
(12), revealing a certain extension
in the patient's survival time and improvement to their quality of
life. In the present case, immunohistochemistry revealed that c-Met
and VEGF were negative, and that the VEGFRs were mainly expressed
at a low level. This may be one of the reasons why sorafenib
monotherapy only reduced the level of AFP and a decrease in tumor
size was not observed. Therefore, we believe that sorafenib in
AFPGC may be effective. Thus, investigations should be performed to
identify the gastric cancer patient population most receptive to
sorafenib treatment. In addition, the effect may be more
significant if combining other chemotherapies with sorafenib.
In summary, AFPGC is a rare, aggressive and
malignant tumor. In the present case, it was treated with
multimodal therapy, including surgery, chemotherapy, interventional
therapy and molecular targeted therapy. The use of a
paclitaxel-based TLF regimen, a irinotecan-based FOLFIRI regimen,
sorafenib and intervention therapy was valid. The study showed that
antitumor therapy is active and effective. The choice of
chemotherapy regimen according to the Lauren classification and the
use of oral sorafenib are likely to be novel and effective
treatments for this type of stomach cancer. However, investigations
should be performed to identify the gastric cancer patient
population most receptive to sorafenib treatment. In addition,
combined chemotherapy and molecular targeting treatment require
further study to determine if there is a synergistic effect. This
case study may indicate a potential treatment option for this rare
disease. Consideration of this type of cancer should be ensured in
future clinical practice.
Acknowledgements
The authors are grateful to the patient and his
family for their permission to report the present case study. This
study was financed by a Grant-in-aid for Gastric Cancer Research
from the Chinese Gastrointestinal Oncology Group (no.
20130101002).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bourreille J, Metayer P, Sauger F, Matray
F and Fondimare A: Existence of alpha fetoprotein during
gastric-origin secondary cancer of the liver. Presse Med.
78:1277–1278. 1970.(In French). PubMed/NCBI
|
|
3
|
Hirajima S, Komatsu S, Ichikawa D, Kubota
T, Okamoto K, Shiozaki A, Fujiwara H, Konishi H, Ikoma H and Otsuji
E: Liver metastasis is the only independent prognostic factor in
AFP-producing gastric cancer. World J Gastroenterol. 19:6055–6061.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kono K, Amemiya H, Sekikawa T, Iizuka H,
Takahashi A, Fujii H and Matsumoto Y: Clinicopathologic features of
gastric cancers producing alpha-fetoprotein. Dig Surg. 19:359–365.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang YC, Nagasue N, Kohno H, Taniura H,
Uchida M, Yamanoi A, Kimoto T and Nakamura T: Clinicopathologic
features and long-term results of alpha-fetoprotein-producing
gastric cancer. Am J Gastroenterol. 85:1480–1485. 1990.PubMed/NCBI
|
|
6
|
Chun H and Kwon SJ: Clinicopathological
characteristics of alpha-fetoprotein-producing gastric cancer. J
Gastric Cancer. 11:23–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long
Z, Zhu H and Wang Y: Clinicopathologic features and prognostic
factors in alpha-fetoprotein-producing gastric cancers: Analysis of
104 cases. J Surg Oncol. 102:249–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McIntire KR, Waldmann TA, Moertel CG and
Go VL: Serum alpha-fetoprotein in patients with neoplasms of the
gastrointestinal tract. Cancer Res. 35:991–996. 1975.PubMed/NCBI
|
|
9
|
Lauren P: The two histological main types
of gastric carcinoma: diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
10
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events v3.0 (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdfAccessed.
June 16–2014
|
|
11
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koneri K, Hirono Y, Fujimoto D, Sawai K,
Morikawa M, Murakami M, Goi T, Iida A, Katayama K and Yamaguchi A:
Five-year survival of alpha-fetoprotein producing gastric cancer
with synchronous liver metastasis: A case report. J Gastric Cancer.
13:58–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lew DH, Jung WT, Kim HJ, Min HJ, Ha CY,
Kim HJ, Kim TH and Ko GH: Clinicopathological characteristics and
prognosis of alpha-fetoprotein producing gastric cancer. Korean J
Gastroenterol. 62:327–335. 2013.(In Korean). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamaguchi K, Tada M, Horikoshi N, Otani T,
Takiuchi H, Saitoh S, Kanamaru R, Kasai Y, Koizumi W, Sakata Y, et
al: Phase II study of paclitaxel with 3-h infusion in patients with
advanced gastric cancer. Gastric Cancer. 5:90–95. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Emi Y, Yamamoto M, Takahashi I, Orita H,
Kakeji Y, Kohnoe S and Maehara Y: Phase II study of weekly
paclitaxel by one-hour infusion for advanced gastric cancer. Surg
Today. 38:1013–1020. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Narahara H, Iishi H, Imamura H, Tsuburaya
A, Chin K, Imamoto H, Esaki T, Furukawa H, Hamada C and Sakata Y:
Randomized phase III study comparing the efficacy and safety of
irinotecan plus S-1 with S-1 alone as first-line treatment for
advanced gastric cancer (study GC0301/TOP-002). Gastric Cancer.
14:72–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ajani JA, Rodriguez W, Bodoky G,
Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A,
Lang I and Falcon S: Multicenter phase III comparison of
cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced
gastric or gastroesophageal adenocarcinoma study: The FLAGS trial.
J Clin Oncol. 28:1547–1553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukuda K, Ito S, Shimizu K, Mikami E,
Sakuraba S, Shiroki T, Ikehata A, Murakami A, Ono S, Sakuma T and
Kato S: Retrospective analysis concerning AFP-producing gastric
cancer. Gan To Kagaku Ryoho. 40:191–195. 2013.(In Japanese).
PubMed/NCBI
|
|
19
|
Shiochi H, Yamada M, Kishina M, Murawaki
Y, Miura M, Azumi T, Yuuki T, Tanaka S, Kono M, Yoshimura T, et al:
A case of AFP-producing gastric cancer responding to the
combination of systemic chemotherapy, transcatheter arterial
embolization and hepatic infusion chemotherapy. Gan To Kagaku
Ryoho. 36:843–846. 2009.(In Japanese). PubMed/NCBI
|
|
20
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng AL, Guan Z, Chen Z, Tsao CJ, Qin S,
Kim JS, Yang TS, Tak WY, Pan H, Yu S, et al: Efficacy and safety of
sorafenib in patients with advanced hepatocellular carcinoma
according to baseline status: Subset analyses of the phase III
Sorafenib Asia-Pacific trial. Eur J Cancer. 48:1452–1465. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim C, Lee JL, Choi YH, Kang BW, Ryu MH,
Chang HM, Kim TW and Kang YK: Phase I dose-finding study of
sorafenib in combination with capecitabine and cisplatin as a
first-line treatment in patients with advanced gastric cancer.
Invest New Drugs. 30:306–315. 2012. View Article : Google Scholar : PubMed/NCBI
|