Introduction
Surgical resection is currently the optimal curative
treatment in esophageal cancer patients without distant metastases.
However, even after curative resection by esophagectomy with
extended three-field lymphadenectomy, the recurrence of cancer is
found in ~50% of patients (1). Nodal
status, including the metastatic lymph node number and extent, is
the strongest prognostic factor in esophageal cancer patients who
have undergone esophagectomy (1).
Accurate assessment of lymph node status is essential. However,
pre-operative evaluation of lymph node metastasis is difficult, as
smaller lymph nodes cannot be detected by radiographic examination.
Indeed, the pre-operative diagnostic accuracy of lymph node
metastasis by computed tomography or endoscopic ultrasonography is
60–80% and remains unsatisfactory (2,3).
The intraoperative diagnosis of lymph node
metastasis is currently based on histopathological examination of
frozen tissue specimens stained with hematoxylin and eosin.
Recently, genetic analysis, such as polymerase chain reaction of
molecular markers specific to cancer cells, has been used to detect
lymph node metastasis (4,5). However, these methods are complicated
and time-consuming, and not suitable for examining a large number
of specimens (4,5). Therefore, the establishment of a simple
and rapid intraoperative diagnostic method of lymph node metastasis
is important.
Photodynamic diagnosis using 5-aminolevulinic acid
(ALA-PDD) has recently emerged as a promising technique for
detecting cancer nests. ALA is the natural precursor of the heme
pathway. Exogenous application of ALA causes a selective
accumulation of a heme precursor, protoporphyrin IX (PPIX), in
cancer cells due to an increase in porphobilinogen deaminase
activity and a decrease in ferrochelatase activity. PPIX is a
fluorescent substrate that emits a red fluorescence at ~635 nm on
blue light excitation (6). ALA-PDD
has proven useful in various types of malignancies. In early
bladder cancer, the introduction of ALA cystoscopy has increased
the recurrence-free survival rate compared with white-light
cystoscopy after the transurethral resection of superficial bladder
cancer (7,8). In gastric cancer, our previous study
reported that staging laparoscopy using ALA improved the detection
sensitivity of peritoneal metastasis (9). In malignant glioma, intraoperative
ALA-induced fluorescence guidance was useful for the complete
removal of tumors and improved progression-free survival (10). However, to the best of our knowledge,
the usefulness of ALA-PDD in human esophageal cancer has not been
investigated. The present study evaluated the feasibility of
ALA-PDD of lymph node metastasis in patients with esophageal
cancer.
Materials and methods
Uptake of ALA in a cell line
First, ALA-induced PPIX red fluorescence was
confirmed in an esophageal cancer cell line. Human esophageal
squamous cell cancer TE-1 cells were cultured in RPMI-1640 medium
(Gibco Life Technologies, Tokyo, Japan) in a 6-well plate at a
concentration of 140,000/ml and incubated at 37°C in a humidified
atmosphere of 5% CO2 for one night. Next, the medium was
changed to RPMI-1640 containing 0, 0.25, 0.5 or 1.0 mg/ml ALA
hydrochloride (Cosmo Bio Co., Ltd., Tokyo, Japan), and the cells
were incubated at 37°C for 60 min. Once the medium had been changed
to RPMI-1640 containing no ALA, 5 µl Hoechst 33342 (Sigma-Aldrich,
Tokyo, Japan) was added to each dish. Finally, the TE-1 cells were
subjected to observation under a fluorescence microscope (BZ-8100;
Keyence, Tokyo, Japan). An OP-66834 BZ fluorescence filter
(excitation, 360 nm; emission, 460 nm; dichroic mirror, 400 nm;
Keyence) and an OP-66838 BZ fluorescence filter (excitation, 560
nm; emission, 630 nm; dichroic mirror, 595 nm, Keyence) were used
for Hoechst and PPIX observations, respectively.
Fluorescence microscopy of metastatic
lymph nodes
Second, the study confirmed whether the distribution
of ALA-induced PPIX red fluorescence agreed with the presence of
cancer nests in apparently metastatic lymph nodes in patients with
esophageal squamous cell cancer at Osaka Medical Center for Cancer
and Cardiovascular Diseases (Osaka, Japan). Between June 2010 and
August 2010, ALA hydrochloride (1 g/body; Cosmo Bio Co., Ltd.) was
dissolved in 20 ml of a 5% glucose solution and orally administered
to 2 patients with esophageal squamous cell cancer 3 h prior to
esophagectomy. Patients were protected from direct sunlight for 24
h to avoid phototoxic reactions. Surgical therapy consisted of en
bloc esophagectomy via a right thoracotomy with two- or three-field
lymphadenectomy, and reconstruction using the stomach, jejunum or
colon. Excised metastatic lymph nodes were immediately embedded in
TissueTek OCT medium (Sakura, Tokyo, Japan) and frozen at −80°C for
10 min. In a dark room, 2 cryosections of an 8-µm thickness were
prepared. One section was stained with hematoxylin and eosin to
confirm the presence of cancer. Another section was stained with
hematoxylin and Hoechst 33342 (Sigma-Aldrich, Tokyo, Japan). Each
section was subjected to observation by fluorescence microscopy
(BZ-8100; Keyence). An OP-66834 BZ fluorescence filter (excitation,
360 nm; emission, 460 nm; dichroic mirror, 400 nm; Keyence) and an
OP-66838 BZ fluorescence filter (excitation, 560 nm; emission, 630
nm; dichroic mirror, 595 nm; Keyence) were used for Hoechst and
PPIX observation, respectively.
Intraoperative PDD of lymph node
metastasis
Next, intraoperative PDD of lymph node metastasis
was performed in 8 patients with esophageal squamous cell cancer
between June 2010 and February 2012. The administration of ALA and
surgical treatment were performed as aforementioned. Excised lymph
nodes were sliced through the center and subjected to PDD.
ALA-induced PPIX fluorescence was analyzed using a spectrometer
(VLD-M1; M&M Co., Ltd., Tokyo, Japan) and its accessory
software (BWSpec V3.24; B&W Tek, Inc., Newark, DE, USA). Lymph
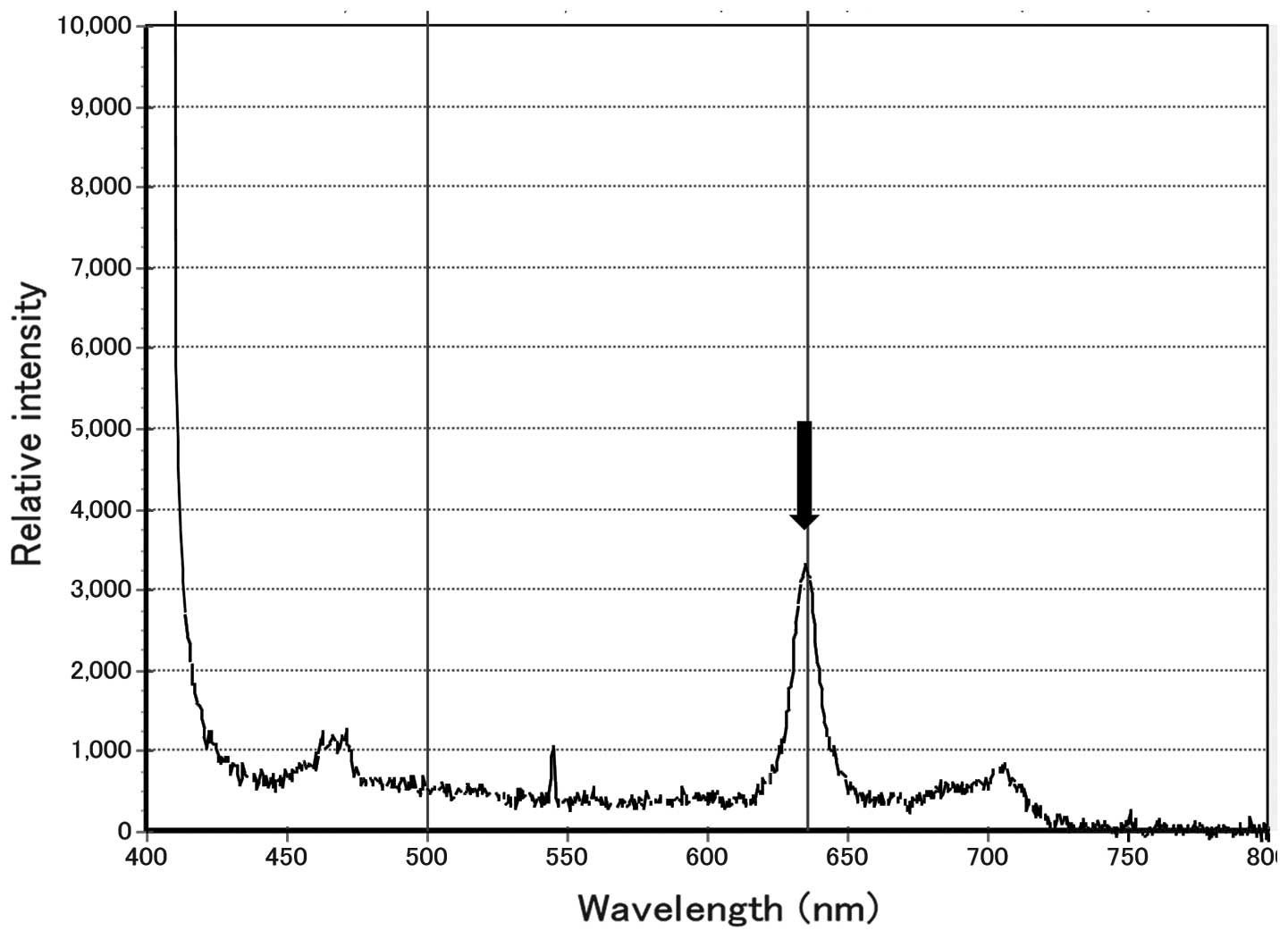
nodes were exposed to a laser light that exhibited a peak
wavelength of 405 nm, and those which exhibited a peak wavelength
of 635 nm were defined as metastasis-positive on PDD, as shown in
Fig. 1. Following PDD, each lymph
node was fixed with 10% formalin and subjected to a routine
histopathological examination, which was regarded as the gold
standard diagnostic procedure. The study protocol was approved by
the Human Ethics Review Committee of Osaka Medical Center for
Cancer and Cardiovascular Diseases. Written informed consent was
obtained from all patients.
Results
PPIX fluorescence in a cell line
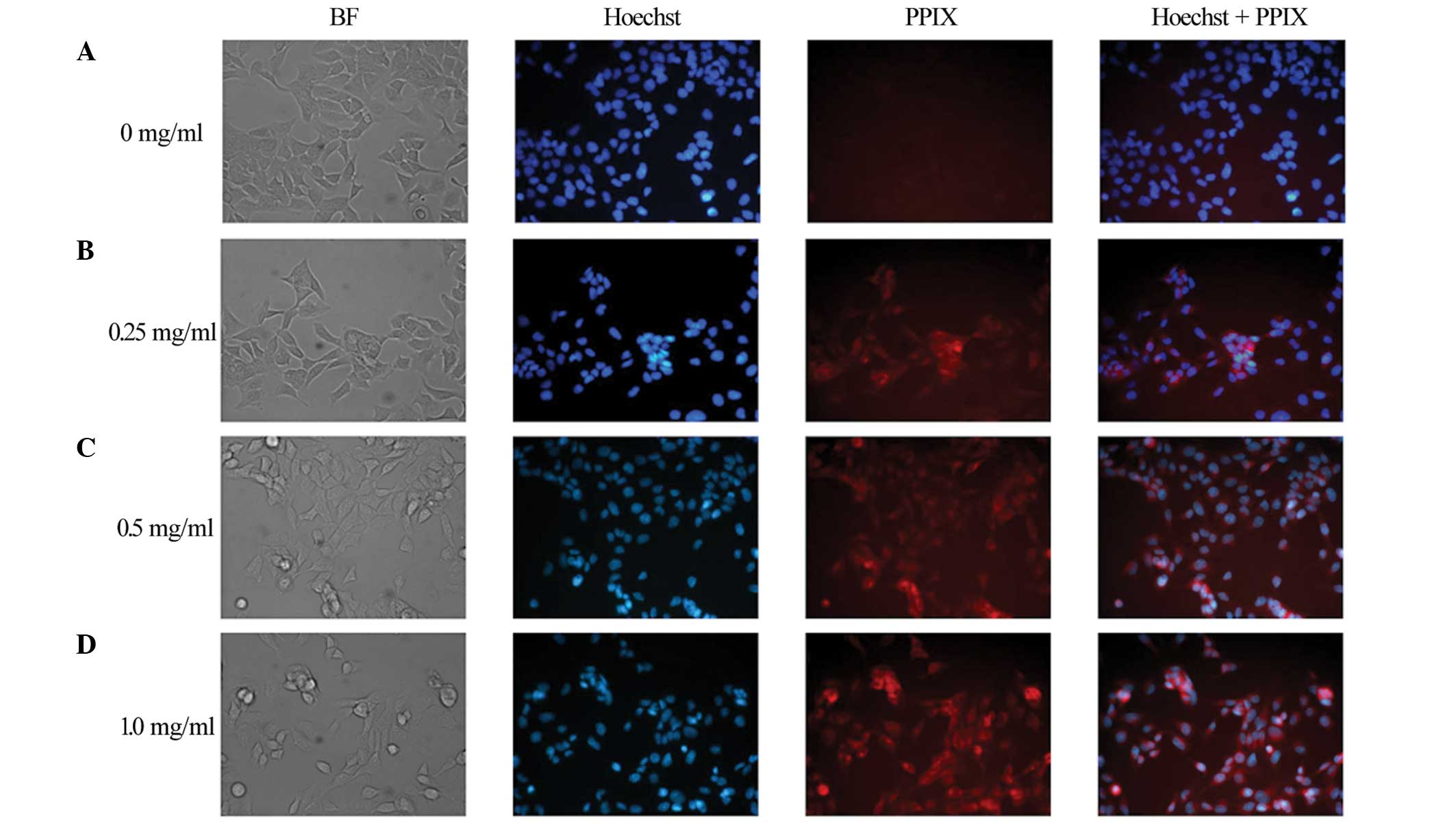
ALA-induced PPIX red fluorescence was observed in
the TE-1 cells incubated with 0.25, 0.5 and 1.0 mg/ml ALA, but not
in the TE-1 cells incubated without ALA (Fig. 2).
Distribution of PPIX in metastatic
lymph nodes
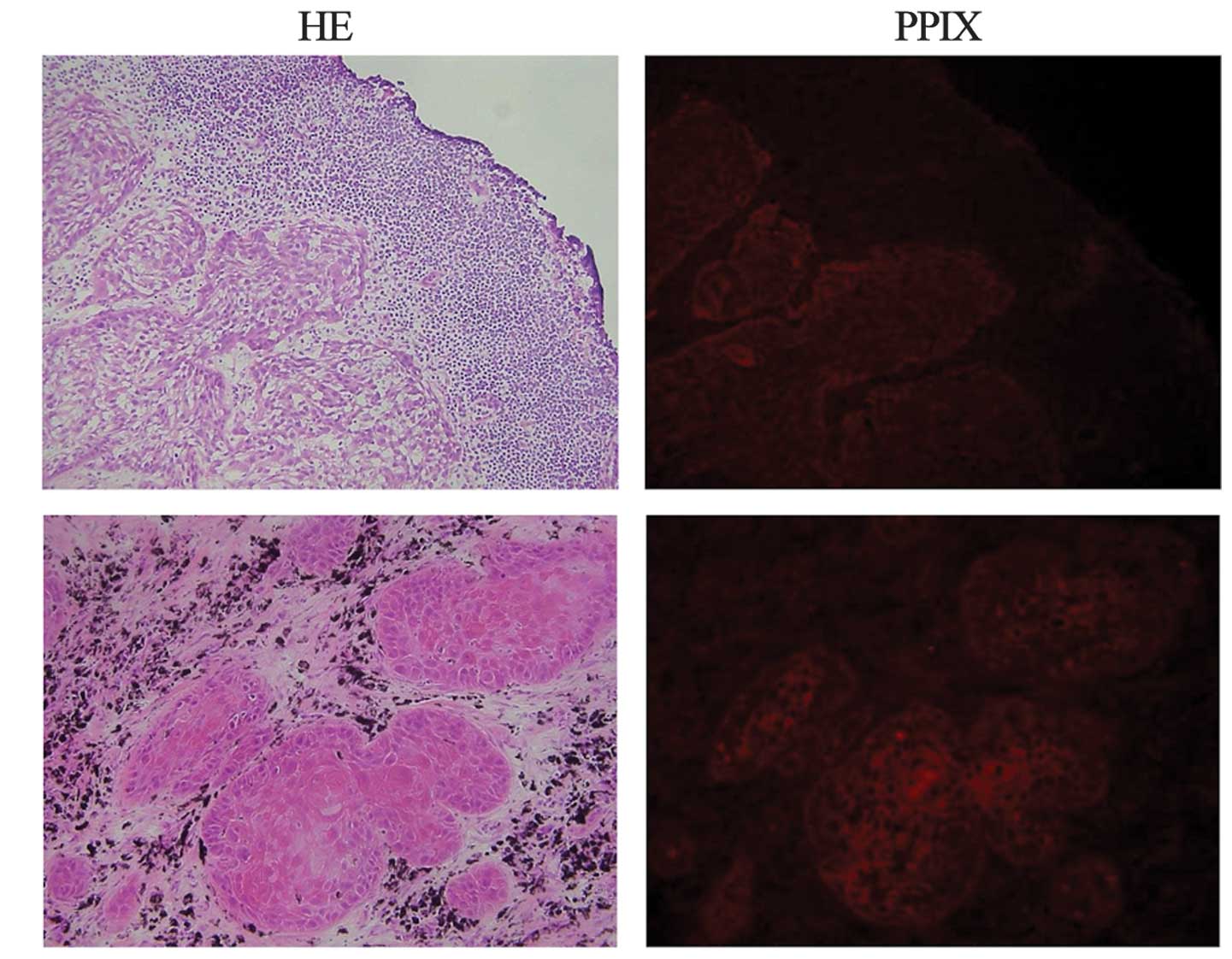
The distribution of ALA-induced PPIX red
fluorescence in apparently metastatic lymph nodes was analyzed in 5
patients with esophageal squamous cell cancer. The distribution of
red fluorescence was identical to that of the metastatic focus
(Fig. 3).
Comparison of PDD of lymph node
metastasis with pathological diagnosis
Patient characteristics are shown in Table I. Tumor location, depth of tumor and
lymph node metastasis were based on the 7th edition of the Union
for International Cancer Control tumor-node-metastasis
classification guidelines (11). A
total of 3 patients were diagnosed with clinical T2 disease and 5
patients with clinical T3 disease. While 2 patients were clinically
node-negative, 6 patients were node-positive. All patients were
pathologically node-positive. Of all the 292 lymph nodes, 19 nodes
were pathologically metastatic and the remaining 273 nodes were
pathologically non-metastatic. Table
II shows the comparison of PDD with the pathological diagnosis.
The sensitivity and specificity of PDD were 84.2% (16/19) and 98.2%
(268/273), respectively. Furthermore, PDD was compared with the
clinical diagnosis in pathologically metastatic nodes. All the
clinically-positive nodes were correctly diagnosed by PDD. Among 10
clinically-negative but pathologically-positive nodes, 7 nodes were
diagnosed as positive by PDD and the remaining 3 nodes were
diagnosed as negative by PDD (Table
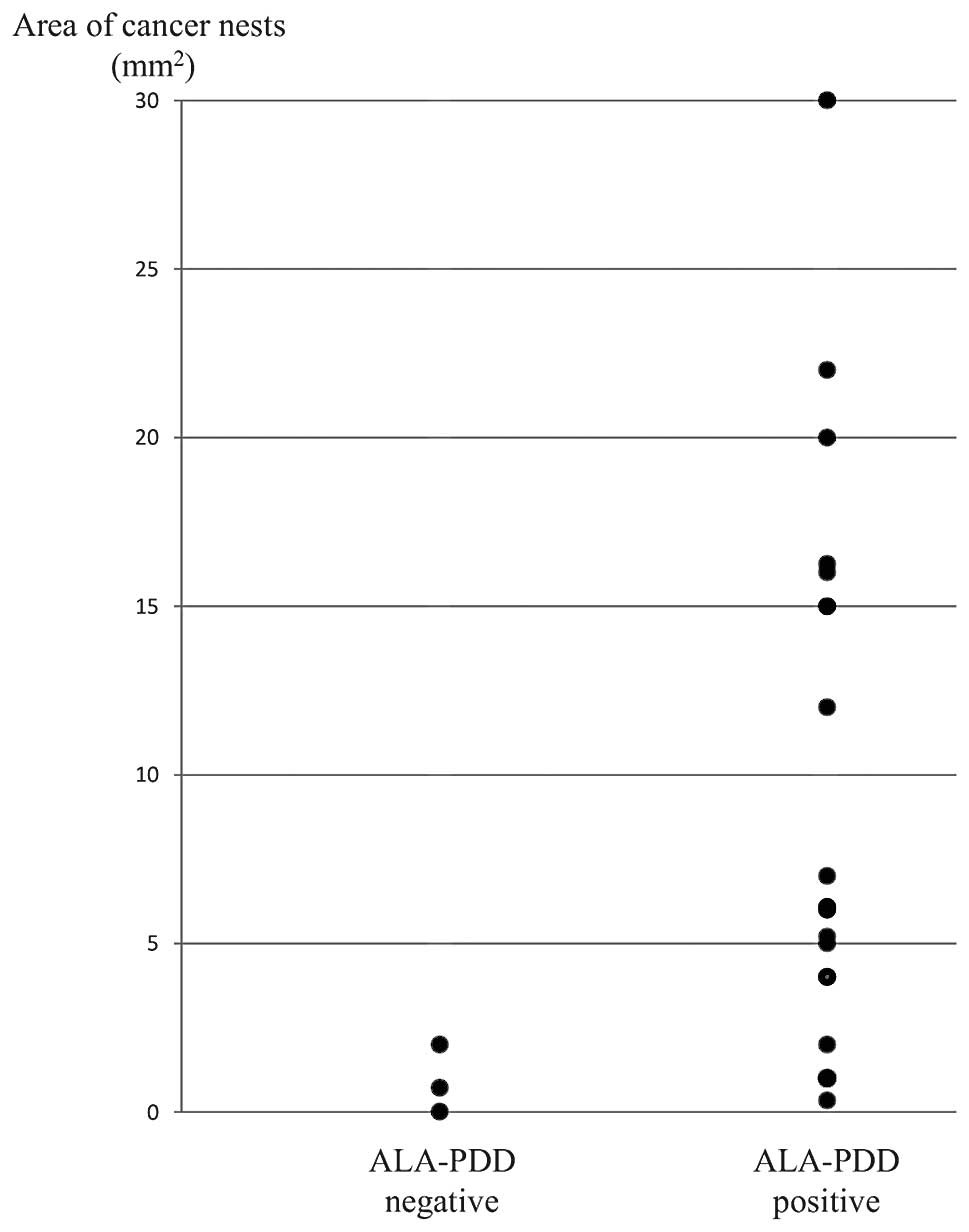
III). Fig. 4 shows the area of
cancer nests in the pathologically metastatic lymph nodes. The
metastasis size of the PDD-negative lymph nodes was <2
mm2. Metastatic lymph nodes, including cancer nests
>4 mm2, were correctly diagnosed by ALA-PDD.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Value |
|---|
| Gender, n |
|
Male/female | 7/1 |
| Median age (range),
years | 65 (52–78) |
| Tumor location,
n |
|
Upper/middle/lower | 2/4/2 |
| Depth of tumor,
n |
|
cT2/cT3 | 3/5 |
| Lymph node
metastasis, n |
|
cN0/cN1/cN2/cN3 | 2/3/2/1 |
 | Table II.Comparison of the PDD of lymph nodes
with the pathological diagnosis. |
Table II.
Comparison of the PDD of lymph nodes
with the pathological diagnosis.
|
| Histology
|
|
|---|
| ALA-PDD | Positive | Negative | Total |
|---|
| Positive | 16 |
5 | 21 |
| Negative | 3 | 268 | 271 |
| Total | 19 | 273 | 292 |
 | Table III.Comparison of the PDD of lymph nodes
with the clinical diagnosis. |
Table III.
Comparison of the PDD of lymph nodes
with the clinical diagnosis.
|
| Clinical diagnosis
|
|
|---|
| ALA-PDD | Positive | Negative | Total |
|---|
| Positive | 3 | 0 | 3 |
| Negative | 7 | 9 | 16 |
| Total | 10 | 9 | 19 |
Discussion
This is the first study demonstrating that ALA-PDD
of lymph node metastasis in patients with esophageal squamous cell
cancer is feasible. The present study determined that the time
required for diagnosing the metastatic status of one lymph node by
ALA-PDD is only a few minutes. When compared with conventional
histopathological examination of frozen tissue specimens, ALA-PDD
is simple and useful for examining a large number of lymph nodes.
ALA is metabolized and excreted within 24–48 h, and the time of
shielding from strong light is short compared with other
photosensitizers, such as hematoporphyrin and Photofrin. In this
study, no adverse events, including cutaneous photosensitization,
were observed. Therefore, ALA-PDD is a safe and promising method of
intraoperative diagnosis of lymph node metastasis.
In the present study, the excised lymph nodes were
sliced prior to performing PDD. Usually, lymph nodes are surrounded
by a number of connective tissues. The penetration depth of red
light ranges from 0.2 to 2 cm, with a mean depth of ~0.6 cm, and
that of blue light is even shallower (12,13). The
penetration depth of excitation blue light is too short to reach
metastatic lesions buried in tissues. Previously, we performed PDD
without cutting the lymph nodes, but the detection rate of PPIX
fluorescence was poor (unpublished data). In gastric cancer,
Koizumi et al performed fluorescence imaging of the cut
surface of lymph nodes (14), as did
the present study. Further examination is necessary for
intraoperative PDD of lymph node metastasis without excision of the
lymph nodes.
In the present study, all the clinically-positive
nodes were correctly diagnosed by PDD. False-negative results were
observed in 3 clinically-negative nodes. The size of the metastatic
focus of these 3 lymph nodes was <2 mm2 and
relatively small. It is considered that the smaller the cancer
nest, the smaller the amount of accumulated PPIX. Furthermore, a
small cancer nest may be easily affected by photobleaching, a
phenomenon whereby the photosensitizer is photochemically destroyed
by light. To induce higher intracellular PPIX levels, chemical
modifications of ALA, such as esterification with aliphatic
alcohols, may be useful for developing a more sensitive method of
PDD (15).
Additionally, a number of false-positive cases were
observed in the present study. A non-metastatic lymph node was
observed, in which PPIX red fluorescence was observed in the
marginal sinus by fluorescence microscopy observation (data not
shown). This may cause a false-positive result. Koizumi et
al reported that they observed some non-metastatic lymph nodes
presenting accumulation of PPIX red fluorescence in normal lymphoid
follicles (14). This phenomenon may
have also occurred in the present study. However, fluorescence
microscopy examination was only performed on a small number of
lymph nodes. Previous studies demonstrated that PPIX tends to
accumulate at an inflamed site (16).
The detailed mechanism of PPIX accumulation in non-cancerous
lesions is unclear.
For the further application of ALA-induced
fluorescence in esophageal cancer surgery, intraoperative PDD of
resection margins, such as in the aorta, trachea and recurrent
laryngeal nerve, are considered to be important. Preliminary
ALA-PDD was performed in the present study for evaluating remnant
cancer in the surgical margins around the trachea, but the
evaluation was extremely difficult. One of the problems of
evaluating the surgical margin is hemorrhage, as hemoglobin absorbs
blue excitation light. Several problems remain to be resolved prior
to applying ALA-PDD to the surgical margin of esophageal cancer.
ALA-induced fluorescence has been used not only for diagnosis but
also for treatment. In nodular basal cell carcinoma, PD therapy
(PDT) using methyl aminolevulinate cream is effective (17). The complete response rate of
clinically assessed lesion clearance did not differ significantly
between the PDT group and the surgically-treated group (17). Pech et al reported that the
excellent long-term results of ALA-PDT in patients with superficial
Barrett's esophageal cancer or high-grade intraepithelial neoplasia
and ALA-PDT may be an alternative treatment to esophagectomy and
endoscopic resection, particularly in cases with multifocal
Barrett's neoplasia (18). In
patients with advanced esophageal cancer, intraoperative ALA-PDT
for surgical margins where microscopic cancer nests may be left has
potential as a novel treatment option.
In conclusion, the present study demonstrated that
ALA-PDD of lymph node metastasis in patients with esophageal cancer
is feasible. Further investigation would make this method a simple
and rapid intraoperative diagnostic tool.
Acknowledgements
The authors would like to thank Ms. Noriko Kanto for
providing expert technical assistance.
References
|
1
|
Akiyama H, Tsurumaru M, Udagawa H and
Kajiyama Y: Radical lymph node dissection for cancer of the
thoracic esophagus. Ann Surg. 220:364–372. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu LF, Wang BZ, Feng JL, Cheng WR, Liu GR,
Xu XH and Zheng ZC: Preoperative TN staging of esophageal cancer:
Comparison of miniprobe ultrasonography, spiral CT and MRI. World J
Gastroenterol. 9:219–224. 2003.PubMed/NCBI
|
|
3
|
Nishimaki T, Tanaka O, Ando N, Ide H,
Watanabe H, Shinoda M, Takiyama W, Yamana H, Ishida K, Isono K, et
al: Evaluation of the accuracy of preoperative staging in thoracic
esophageal cancer. Ann Thorac Surg. 68:2059–2064. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshioka S, Fujiwara Y, Sugita Y, Okada Y,
Yano M, Tamura S, Yasuda T, Takiguchi S, Shiozaki H and Monden M:
Real-time rapid reverse transcriptase-polymerase chain reaction for
intraoperative diagnosis of lymph node micrometastasis: Clinical
application for cervical lymph node dissection in esophageal
cancers. Surgery. 132:34–40. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyata H, Yano M, Doki Y, Yasuda T,
Yoshioka S, Sugita Y, Takiguchi S, Fujiwara Y and Monden M: A
prospective trial for avoiding cervical lymph node dissection for
thoracic esophageal cancers, based on intra-operative genetic
diagnosis of micrometastasis in recurrent laryngeal nerve chain
nodes. J Surg Oncol. 93:477–484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hinnen P, de Rooij FW, van Velthuysen ML,
Edixhoven A, van Hillegersberg R, Tilanus HW, Wilson JH and
Siersema PD: Biochemical basis of 5-aminolaevulinic acid-induced
protoporphyrin IX accumulation: A study in patients with
(pre)malignant lesions of the oesophagus. Br J Cancer. 78:679–682.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daniltchenko DI, Riedl CR, Sachs MD,
Koenig F, Daha KL, Pflueger H, Loening SA and Schnorr D: Long-term
benefit of 5-aminolevulinic acid fluorescence assisted
transurethral resection of superficial bladder cancer: 5-year
results of a prospective randomized study. J Urol. 174:2129–2133.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Denzinger S, Burger M, Walter B, Knuechel
R, Roessler W, Wieland WF and Filbeck T: Clinically relevant
reduction in risk of recurrence of superficial bladder cancer using
5-aminolevulinic acid-induced fluorescence diagnosis: 8-year
results of prospective randomized study. Urology. 69:675–679. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kishi K, Fujiwara Y, Yano M, Inoue M,
Miyashiro I, Motoori M, Shingai T, Gotoh K, Takahashi H, Noura S,
et al: Staging laparoscopy using ALA-mediated photodynamic
diagnosis improves the detection of peritoneal metastases in
advanced gastric cancer. J Surg Oncol. 106:294–298. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stummer W, Pichlmeier U, Meinel T,
Wiestler OD, Zanella F and Reulen HJ: ALA-Glioma Study Group:
Fluorescence-guided surgery with 5-aminolevulinic acid for
resection of malignant glioma: A randomised controlled multicentre
phase III trial. Lancet Oncol. 7:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: TNM classification of malignant tumors (7th). Wiley-Blackwell.
Oxford, UK: 2009.
|
|
12
|
Webber J, Herman N, Kessel D and Fromm D:
Current concepts in gastrointestinal photodynamic therapy. Ann
Surg. 230:12–23. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Braathen LR, Szeimies RM, Basset-Seguin N,
Bissonnette R, Foley P, Pariser D, Roelandts R, Wennberg AM and
Morton CA: International Society for Photodynamic Therapy in
Dermatology: Guidelines on the use of photodynamic therapy for
nonmelanoma skin cancer: an international consensus. International
society for photodynamic therapy in dermatology, 2005. J Am Acad
Dermatol. 56:125–143. 2007.
|
|
14
|
Koizumi N, Harada Y, Murayama Y, Harada K,
Beika M, Yamaoka Y, Dai P, Komatsu S, Kubota T, Ichikawa D, et al:
Detection of metastatic lymph nodes using 5-aminolevulinic acid in
patients with gastric cancer. Ann Surg Oncol. 20:3541–3548. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaullier JM, Berg K, Peng Q, Anholt H,
Selbo PK, Ma LW and Moan J: Use of 5-aminolevulinic acid esters to
improve photodynamic therapy on cells in culture. Cancer Res.
57:1481–1486. 1997.PubMed/NCBI
|
|
16
|
Messmann H, Knuchel R, Bäumler W, Holstege
A and Schölmerich J: Endoscopic fluorescence detection of dysplasia
in patients with Barrett's esophagus, ulcerative colitis, or
adenomatous polyps after 5-aminolevulinic acid-induced
protoporphyrin IX sensitization. Gastrointest Endosc. 49:97–101.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rhodes LE, de Rie M, Enström Y, Groves R,
Morken T, Goulden V, Wong GA, Grob JJ, Varma S and Wolf P:
Photodynamic therapy using topical methyl aminolevulinate vs
surgery for nodular basal cell carcinoma: results of a multicenter
randomized prospective trial. Arch Dermatol. 140:17–23. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pech O, Gossner L, May A, Rabenstein T,
Vieth M, Stolte M, Berres M and Ell C: Long-term results of
photodynamic therapy with 5-aminolevulinic acid for superficial
Barrett's cancer and high-grade intraepithelial neoplasia.
Gastrointest Endosc. 62:24–30. 2005. View Article : Google Scholar : PubMed/NCBI
|