Introduction
Pheochromocytoma (PC) is a rare neuroendocrine tumor
that originates from the adrenal medulla or chromaffin cells of the
sympathetic ganglia, and the tumor is characterized by the
excessive production of catecholamines (1,2). In
addition to catecholamines, PC may also participate in homeostatic
regulations by producing numerous peptides, including
adrenomedullin (ADM), which was first isolated from acid extracts
of human PC tissue and was subsequently found to be a circulating
hormone (3–5). The synthesis and release of ADM may be
affected by physical factors, including shear stress, ventricular
wall stress and hypoxia, and humoral factors, including cytokines,
endocrine hormones and paracrine hormones (6). Following the release from diverse
tissues, ADM acts as an autocrine or a paracrine hormone to play an
important role in the stress response, including the regulation of
vascular tone and blood pressure (5,7). ADM
exerts biological effects directly through cyclic adenosine
monophosphate (cAMP) and indirectly through endothelial nitric
oxide (8).
The natriuretic peptide system, including atrial
natriuretic peptide (ANP) and brain natriuretic peptide (BNP), may
locally and systemically regulate blood pressure and oxygen
transport by causing volume contraction through natriuresis,
diuresis and plasma shift, leading to hemoconcentration and an
increased oxygen-carrying capacity per unit volume of blood to
counteract hypoxic conditions (9–12). Certain
studies performed using cultured cardiomyocytes, particularly from
rodents, have demonstrated that the expression of the ANP and BNP
genes may be regulated by several factors, including catecholamines
and certain cytokines (13). Numerous
cardiovascular diseases, including chronic heart failure, systemic
hypertension, coronary disease and endothelial dysfunction, are
responsible for the raised secretion of ANP and BNP (14). ANP and BNP are mainly synthesized and
released by atrial and ventricular myocytes, respectively, and
exert biological actions through the accumulation of intracellular
cyclic guanosine monophosphate (15),
whereas the actions of ADM are mainly mediated by cAMP (16). The three peptides demonstrate similar
pathophysiological functions, despite differences in the
intracellular signaling systems, and the expression of the ANP and
BNP genes may be regulated by ADM. Therefore, it was hypothesized
that ADM functions along with ANP and BNP to counteract the
additional elevation of blood pressure in patients with PC.
To evaluate any changes in the plasma concentrations
of ADM, ANP and BNP in patients with PC, the plasma levels of the
three peptides were measured in untreated patients with PC and the
results were compared with those obtained from healthy control
individuals. The levels of ADM, ANP and BNP in hypertensive
patients with PC were measured subsequent to 4 weeks of effective
antihypertensive therapy. Laparoscopic adrenalectomy was then
performed on all PC patients, and the values were measured 2 weeks
later. The results prior to and following treatment were
compared.
Patients and methods
Patients
Between February 2006 and November 2013, 45 healthy
control individuals (mean age, 44.3±7.3 years; range, 31–59 years)
and 90 PC patients (mean age, 44.8±8.1 years; range, 32–59 years)
were recruited for the present study from Renmin Hospital of Wuhan
University (Wuhan, Hubei, China). The PC patients consisted of 20
normotensive patients (mean age, 45.0±6.6 years; range, 32–58
years), 30 borderline hypertensive patients (mean age, 43.0±7.5
years; range, 32–54 years) and 40 hypertensive patients (mean age,
46.0±9.1 years; range, 33–59 years). This study has been approved
by the Ethics Committee of Renmin Hospital of Wuhan University, and
was performed in adherence with the China Association for Ethical
Studies guidelines. All subjects agreed to participate in the
present study and provided written informed consent.
The routine laboratory and radiological assessments
of all patients were as follows: Assays of blood routine tests;
urinalysis; measurement of serum electrolytes and fasting blood
glucose levels; liver and kidney function tests; assessment of
plasma renin activity, aldosterone, catecholamine, cortisol and
thyroid hormone levels; measurement of the 24-h urine
vanillylmandelic acid (VMA) level; chest roentgenogram;
electrocardiogram; and B-scan ultrasonography of the abdomen,
including the liver, cholecyst, pancreas, spleen, kidneys and
adrenal glands. The glomerular filtration rate (GFR) was calculated
by measuring serum cystatin C, as previously described (17). Additional radiological studies
included one or more of the following diagnostic methods: Magnetic
resonance imaging; computed tomography (CT); positron emission
tomography (PET) imaging with fluorodeoxyglucose;
dihydroxyphenylalanine-PET-CT; Octreoscan; and
123I-metaiodo-benzylguanidine scintigraphy. A familial
history of multiple endocrine neoplasia syndrome was ruled out in
all patients. Symptomatic adrenal PC was definitely diagnosed on
the basis of clinical manifestations, such as hypertension,
tachycardia, dizziness, flushing, tremor, pallor, headache,
palpitations, sweating, feelings of panic or anxiety and excessive
catecholamine excretion, and the aforementioned imaging
examinations. Asymptomatic adrenal PC was an incidentaloma that was
identified during a checkup, and the diagnosis was confirmed by a
number of the aforementioned imaging examinations. In all PC
patients, imaging examinations revealed unilateral solid masses
with apparently benign features.
Post-operative histopathological findings of the
extirpated tumors at surgery were obtained. According to the
current guidelines (18), normal BP
was defined as a systolic pressure <140 mmHg and a diastolic
pressure <90 mmHg. Hypertension was defined as a systolic
pressure ≥160 mmHg or a diastolic pressure ≥100 mmHg, or the two
together. The term borderline hypertension was used to denote BP
values between the normal and hypertensive ranges, as
aforementioned. None of the present patients demonstrated clinical
evidence of cardiac or hepatic failure, diabetes, pulmonary
disease, angina pectoris, myocardial infarction, essential
hypertension or other diseases that may result in secondary
hypertension. No PC patients had undergone previous
antihypertensive drug treatment, or the antihypertensive therapy
had been discontinued at least 2 weeks prior to the present study.
The healthy control individuals were age- and gender-matched
normotensive subjects that had been hospitalized for a routine
checkup.
Subsequent to the initial evaluation, 40
hypertensive PC patients commenced antihypertensive therapy with
10–30 mg phenoxybenzamine twice a day. The plasma concentrations of
ADM, ANP and BNP were determined prior to the initiation of therapy
and subsequent to 4 weeks of effective antihypertensive treatment.
Transperitoneal laparoscopic adrenalectomy was then performed in
all PC subjects that were suitable for surgery. Subsequent to 2
weeks, the therapeutic effect was estimated with the normalization
of catecholamine hypersecretion and complete disappearance of
symptoms, as well as the reduction or abstention of
antihypertensive therapy in symptomatic PC patients. In addition,
the values of the three peptides were measured in all PC
patients.
Arterial blood pressure was measured using
ambulatory blood pressure monitoring for at least two weeks. The
results were analyzed using Microsoft Excel software, version 2007
(Microsoft Corporation, Redmond, WA, USA) and the mean of the blood
pressure measurements was used.
Preparations of human ADM, ANP and
BNP
Blood samples were obtained early in the morning,
between 08:00 and 09:00, following an overnight fast. An
intravenous catheter was inserted into the antecubital vein when
the subject was in the supine position. Plasma samples were
collected in tubes, centrifuged at 1,600 × g for 10 min at 4°C, and
then immediately frozen and stored in polypropylene tubes at −80°C
prior to use in the assays.
Hormone measurements
The plasma ADM concentrations were measured by a
specific radioimmunoassay (RIA; ADM RIA Shionogi; Shionogi
Pharmaceutical Co., Ltd., Osaka, Japan), as described previously
(19). Briefly, 2 ml of plasma was
applied to a Sep-Pak C18 cartridge (Waters Corporation, Milford,
MA, USA) and the column was sequentially washed using 5 ml isotonic
saline, 5 ml 0.1% trifluoroacetic acid (TFA) and 5 ml 20%
acetonitrile in 0.1% TFA. The ADM was then eluted with 4 ml 60%
acetonitrile in 0.1% TFA, lyophilized and then stored at −80°C
until the radioassay was performed. The residue was then dissolved
in 300 µl RIA buffer, 50 mmol/l sodium phosphate buffer (pH 7.4)
containing 0.5% bovine serum albumin (BSA), 0.5% Triton X-100, 80
mmol/1 sodium chloride, 25 mmol/l EDTA, 0.05% sodium azide and 500
KIU/ml aprotinin. In total, 100 µl of the dissolved plasma extract
was subjected to a specific RIA for human ADM, as previously
reported (19). According to the
manufacturer's instructions, the rabbit polyclonal antibody (cat
no. G-010-01, Phoenix Pharmaceuticals Inc., Burlingame, CA, USA)
against human ADM 1–52 at a final dilution of 1:3,200, cross-reacts
completely with human ADM(1-52), but not with rat ADM(1-50), human
amylin, human CGRP, calcitonin, α-human atrial natriuretic
peptide-(1-28), brain natriuretic peptide-32, C-type natriuretic
peptide-22 or neuropeptide Y. All assays were performed three
times, and the mean of the three measurements was used. The
detection limit was 0.5 pmol/l and the working range (CV <15%)
was 1–300 pmol/l. The interassay coefficient of variation was
3.4–7.8%, and the intraassay coefficient of variation was 5.6–8.9%.
All assays were performed in duplicate. Concentrations of ADM were
expressed in pmol/l.
The plasma ANP and BNP concentrations were measured
with specific immunoradiometric assays for human ANP and BNP
(ShionoRlA ANP and BNP kits; Shionogi Pharmaceutical Co., Ltd.).
The accuracies and detailed methods for these assays have been
described previously (20).
Statistical analysis
All continuous data were expressed as the mean ±
standard deviation and analyzed using SPSS software, version 19.0
(SPSS Inc., Chicago, IL, USA). Comparisons between the groups were
performed using analysis of variance, followed by the
Student-Newman-Keuls test. Categorical variables were assessed by
the χ2 test or Fisher's exact test. Stepwise multiple
linear regression analysis was used to identify the most important
determinant for blood pressure or serum catecholamines. The
correlations between variables were determined by simple linear
regression analysis and then confirmed using the Pearson
product-moment correlation coefficient. Comparisons between two
independent variables were performed using an unpaired
t-test. Comparisons between paired values were performed
using a paired t-test. Data that were not normally
distributed were assessed using the Mann-Whitney U test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Clinical characteristics of control
individuals and patients with PC
Table I reports the
clinical profiles of the control individuals and patients with PC.
There were no significant differences between the age and gender
distribution of the four groups. As expected, the mean SBP and DBP
values were significantly higher in borderline and hypertensive
patients with PC compared with the mean values of the control
individuals and normotensive patients with PC (P<0.05). In
addition, significant differences were present between the SBP and
DBP values of borderline and hypertensive patients. Similar changes
were observed between the mean serum epinephrine (E) and
norepinephrine (NE), urine VMA, left ventricular ejection fraction
(LVEF) and left ventricular mass index (LVMI) values in the four
groups. However, the mean blood urea nitrogen (BUN) and serum
creatinine (Scr) levels and GFR were only significantly higher in
the hypertensive patients with PC compared with the values in the
other three groups, between which no significant differences were
found.
 | Table I.Clinical profiles of control subjects
and patients with PC. |
Table I.
Clinical profiles of control subjects
and patients with PC.
|
|
| PC patient group |
|---|
|
|
|
|
|---|
| Parameters | Control | Normotensive | Borderline | Hypertensive |
|---|
| Total | 45 | 20 | 30 | 40 |
| Age, years | 44.3±7.3 | 45.0±6.6 | 43.0±7.5 | 46.0±9.1 |
| Gender,
male:female | 26:19 | 12:8 | 17:13 | 24:16 |
| SBP, mmHg | 121.0±10.0 | 120.0±9.0 |
150.0±6.0a,b |
177.0±10.0a–c |
| DBP, mmHg | 78.0±5.0 | 79.0±6.0 | 92.0±1.0a,b |
107.0±6.0a–c |
| BUN, mg/dl | 17.0±2.0 | 18.0±3.0 | 18.0±4.0 | 22.0±5.0a–c |
| Scr, mg/dl | 1.0±0.2 | 1.1±0.3 | 1.1±0.5 | 1.5±0.6a–c |
| GFR, ml/min | 98.0±9.0 | 96.0±8.0 | 95.0±10.0 |
82.0±14.0a–c |
| Serum E, pg/ml | 64.0±16.0 | 73.0±28.0 |
203.0±81.0a,b |
462.0±141.0a–c |
| Serum NE,
pg/ml | 208.0±64.0 | 246.0±123.0 |
751.0±238.0a,b |
1280.0±518.0a–c |
| Urine VMA, mg/24
h | 4.0±2.0 | 5.0±2.0 |
13.0±5.0a,b |
21.0±9.0a–c |
| LVEF, % | 84.0±7.0 | 82.0±6.0 |
77.0±8.0a,b |
72.0±10.0a–c |
| LVMI,
g/m2 | 114.0±6.0 | 117.0±7.0 |
128.0±10.0a,b |
140.0±12.0a–c |
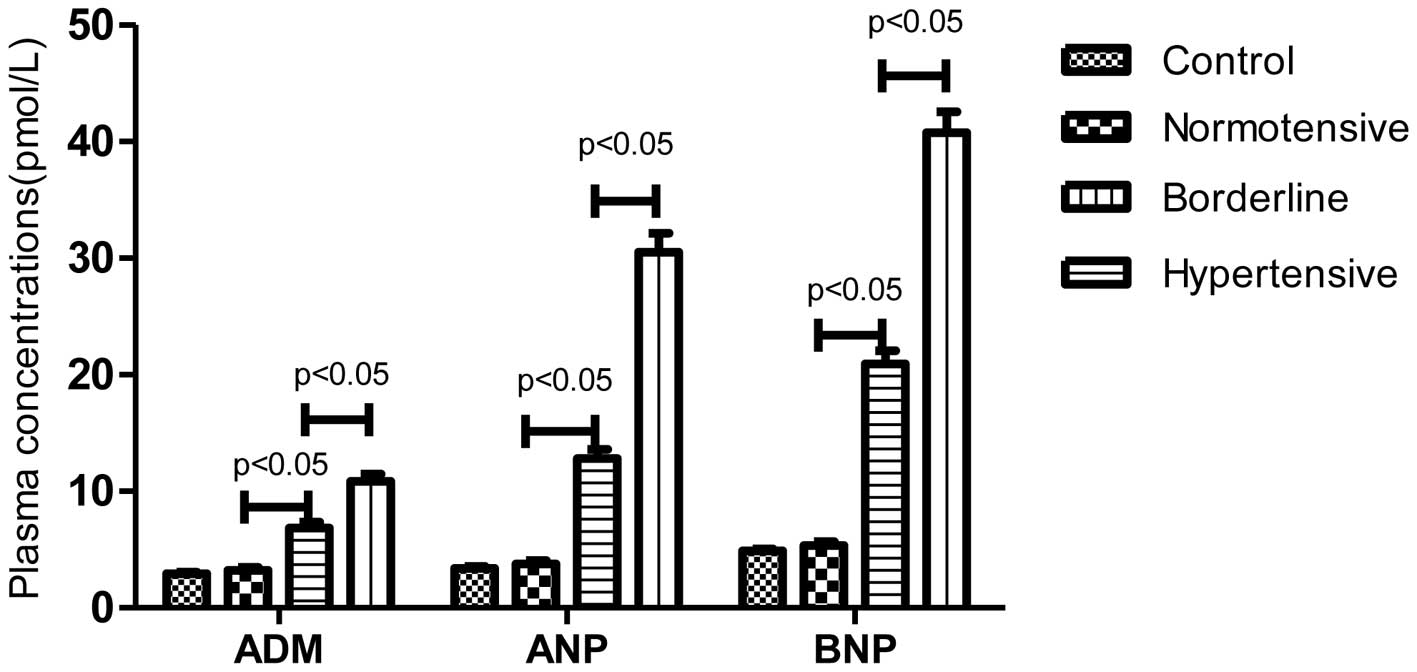
ADM, ANP and BNP concentrations in
subgroups of PC patients
The plasma concentrations of ADM, ANP and BNP in the
control and three PC subgroups are shown in Fig. 1. The mean ADM values were
significantly higher in the borderline and hypertensive patients
with PC (6.86±2.65 and 10.84±4.07 pmol/l, respectively) compared
with the values in the control individuals (2.93±0.74 pmol/l) and
normotensive PC patients (3.25±1.10 pmol/l) (P<0.05). There was
also a significant difference between the values in the borderline
and hypertensive patients (P<0.05). The mean ANP levels in the
control and 3 PC subgroups groups were 3.41±1.04, 3.77±1.24,
12.82±4.41, 30.52±10.39 pmol/l, respectively, while the mean BNP
levels were 4.91±1.02, 5.35±1.43, 20.92±6.34, 40.78±11.29 pmol/l,
respectively. Similar changes were observed between the mean ANP
and BNP levels in the four groups.
Association between plasma ADM
concentrations and clinical parameters
Table II reveals that
ADM was the most important factor associated with the blood
pressure, serum catecholamine levels or urine VMA levels in
hypertensive patients with PC. Stepwise multiple regression
analysis was performed on the independent parameters associated
with the SBP, DBP, serum E, serum NE and urine VMA values, to
identify which was the most important factor in hypertensive PC
patients.
 | Table II.Stepwise multiple regression analysis
of significant factors for SBP, DBP, serum E, serum NE and urine
VMA levels in hypertensive patients with PC. |
Table II.
Stepwise multiple regression analysis
of significant factors for SBP, DBP, serum E, serum NE and urine
VMA levels in hypertensive patients with PC.
|
| SBP | DBP | Serum E | Serum NE | Urine VMA |
|---|
|
|
|
|
|
|
|
|---|
| Variables | B | t | P-value | B | t | P-value | B | t | P-value | B | t | P-value | B | t | P-value |
|---|
| ADM | 0.998 | 2.221 | 0.033 |
0.733 |
2.425 | 0.020 | 20.695 | 3.926 | 0.000 | 103.077 | 6.161 | 0.000 | 1.137 | 2.883 | 0.007 |
| ANP | 0.201 | 1.400 | 0.170 | −0.060 | −0.625 | 0.536 |
2.582 | 1.534 | 0.134 |
0.078 | 0.015 | 0.988 | 0.163 | 1.297 | 0.203 |
| BNP | 0.097 | 0.690 | 0.494 |
0.151 |
1.597 | 0.119 |
1.412 | 0.858 | 0.396 |
2.987 | 0.572 | 0.571 | 0.055 | 0.445 | 0.659 |
The scatterplots in Fig.
2 demonstrate the association between plasma ADM concentrations
and BUN, Scr, serum E, serum NE and urine VMA levels and GFR in
hypertensive patients with PC. The plasma ADM concentrations were
not correlated with the BUN or Scr levels or GFR, but the
concentrations were associated with the serum E, serum NE and urine
VMA levels (P<0.05).
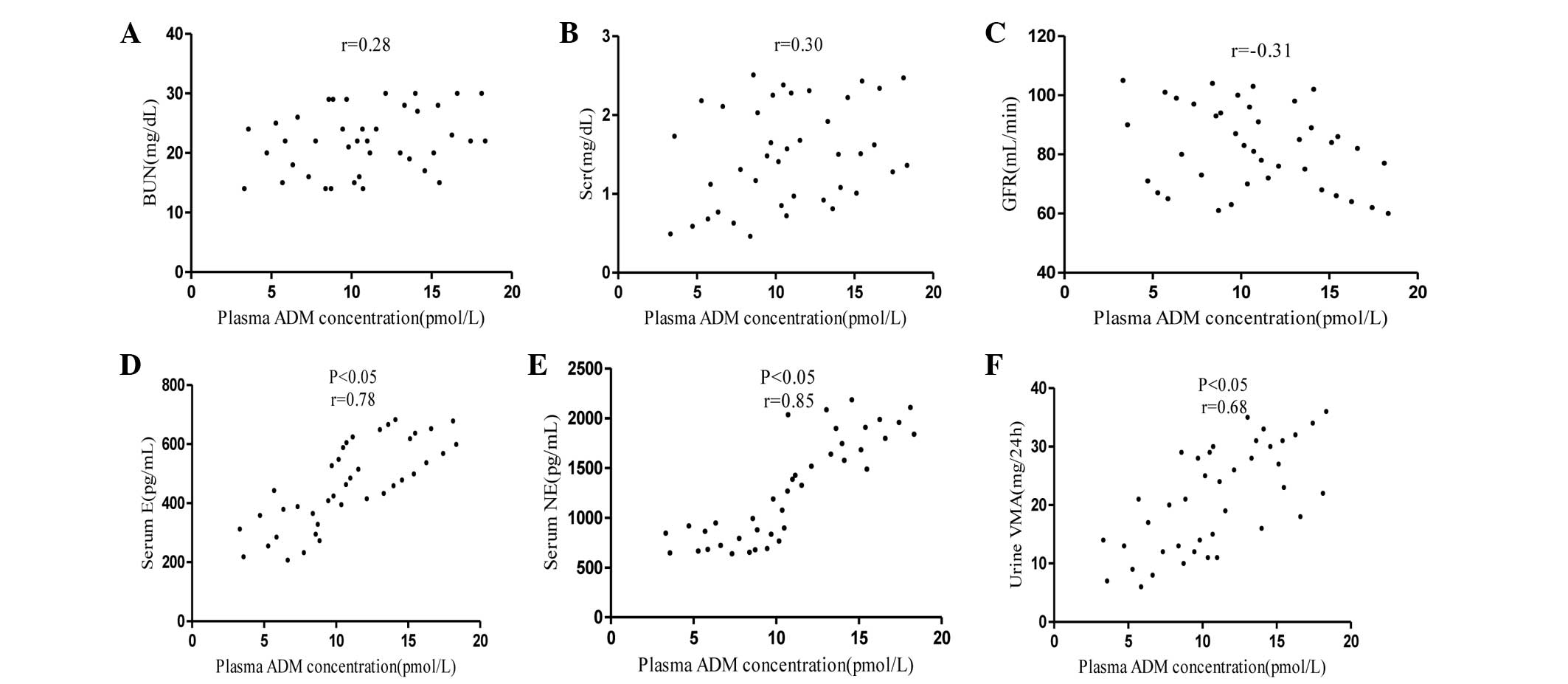 | Figure 2.Association between the plasma ADM
concentrations and (A) BUN, (B) Scr, (C) GFR, (D) serum E, (E)
serum NE and urine (F) VMA in hypertensive patients with
pheochromocytoma. BUN, blood urea nitrogen; Scr, serum creatinine;
GFR, glomerular filtration rate; E, epinephrine; NE,
norepinephrine; VMA, vanillylmandelic acid; ADM,
adrenomedullin. |
The scatterplots in Fig.
3 demonstrate the correlations between the plasma ADM
concentrations and the SBP, DBP, LVEF, LVMI, ANP and BNP values in
hypertensive PC patients. Plasma ADM concentrations were positively
correlated with the SBP, DBP, LVMI, ANP and BNP values, but
negatively correlated with the LVEF values (P<0.05).
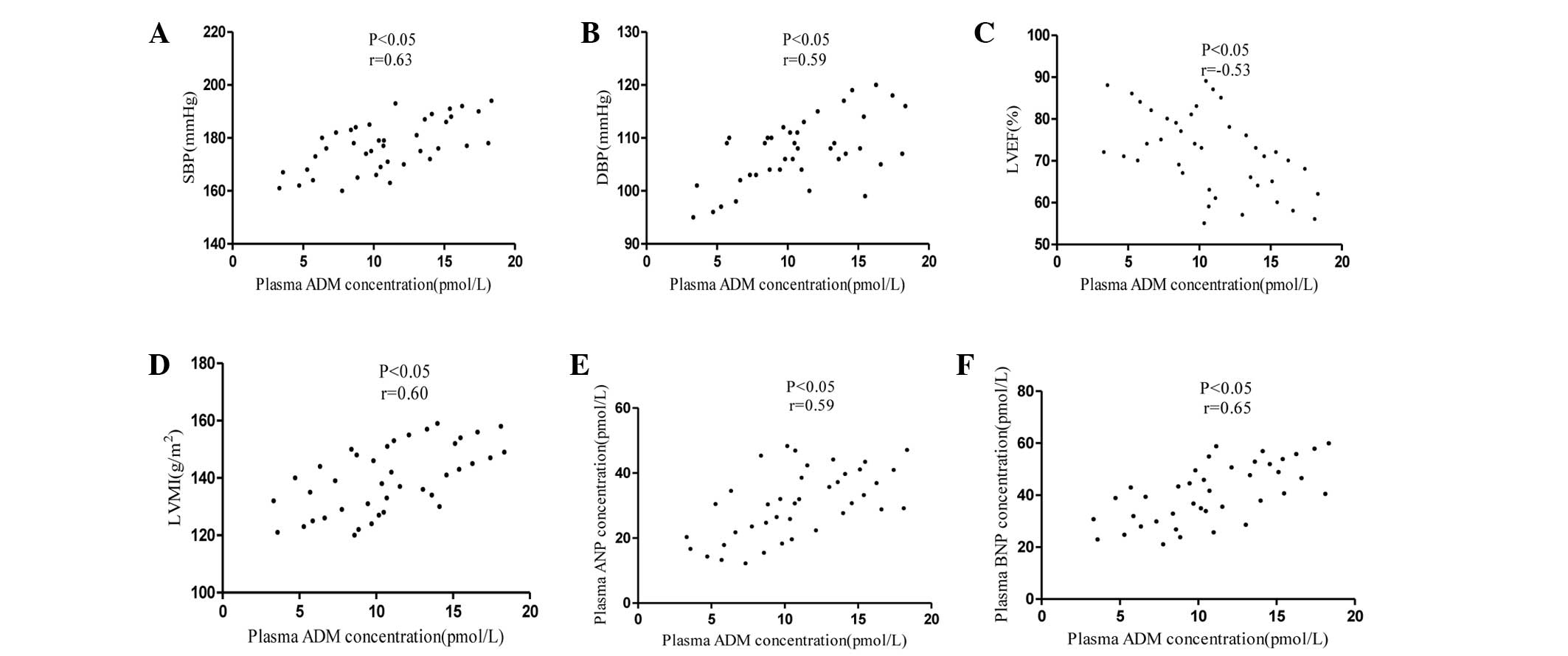 | Figure 3.Association between the plasma ADM
concentrations and (A) SBP, (B) DBP, (C) LVEF, (D) LVMI, (E) ANP
and (F) BNP levels in hypertensive patients with pheochromocytoma.
SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEF,
left ventricular ejection fraction; LVMI, left ventricular mass
index; ADM, adrenomedullin; ANP, atrial natriuretic peptide; BNP,
brain natriuretic peptide. |
Association between ADM, ANP and BNP
levels and renal function
The plasma concentrations of ADM, ANP and BNP were
also analyzed in hypertensive patients with PC, with or without
renal dysfunction. There were no significant differences in the
plasma concentrations between patients with renal dysfunction,
which was indicated by a Scr level ≥1.5 mg/dl or GFR ≤80 ml/min,
and subjects without renal dysfunction, which was indicated by a
Scr level <1.5 mg/dl or GFR >80 ml/min. However, the
concentrations of the three peptides in patients with or without
renal dysfunction were increased compared with the concentration in
the control individuals (P<0.05) (data not shown).
Hemodynamic parameters of hypertensive
PC patients
Table III reports
the clinical parameters of hypertensive patients with PC at the
time of diagnosis, subsequent to drug treatment and following
surgery. The SBP and DBP were significantly decreased subsequent to
drug treatment (P<0.05), but were within the normal range
following surgery (P<0.05; SBP <140 mmHg and DBP<90 mmHg).
The BUN and Scr levels and GFR were not significantly changed. The
serum E, serum NE, urine VMA, LVEF and LVMI values were not
significantly changed subsequent to drug treatment, but were within
the normal range following surgery (P<0.05; Table I).
 | Table III.Hemodynamic parameters of
hypertensive patients with PC (n=40). |
Table III.
Hemodynamic parameters of
hypertensive patients with PC (n=40).
|
|
| Time of sample
collection |
|---|
|
|
|
|
|---|
| Parameters | At diagnosis | After drugs | After surgery |
|---|
| SBP, mmHg | 177.0±10.0 |
150.0±4.0a |
120.0±10.0a,b |
| DBP, mmHg | 107.0±6.0 |
95.0±3.0a |
80.0±6.0a,b |
| BUN, mg/dl | 22.0±5.0 | 21.0±5.0 | 23.0±6.0 |
| Scr, mg/dl | 1.5±0.6 | 1.4±0.5 | 1.6±0.6 |
| GFR, ml/min | 82.0±14.0 | 84.0±15.0 | 80.0±12.0 |
| Serum E, pg/ml | 462.0±141.0 | 483.0±158.0 |
70.0±18.0a,b |
| Serum NE,
pg/ml | 1280.0±518.0 | 1345.0±603.0 |
226.0±104.0a,b |
| Urine VMA, mg/24
h | 21.0±9.0 | 22.0±10.0 |
4.0±1.0a,b |
| LVEF, % | 72.0±10.0 | 74.0±6.0 |
80.0±5.0a,b |
| LVMI,
g/m2 | 140.0±12.0 | 136.0±11.0 |
120.0±7.0a,b |
Changes in ADM, ANP and BNP
concentrations following drug treatment and surgery
The bars in Fig. 4
indicate the initial plasma concentrations of ADM, ANP and BNP and
the concentrations 4 weeks subsequent to effective antihypertensive
therapy or 2 weeks subsequent to surgery in the three PC subgroups.
The plasma concentrations of ADM, ANP and BNP in the normotensive
group were not significantly different prior to (3.25±1.10,
3.77±1.24 and 5.35±1.43 pmol/l, respectively) and following surgery
(3.02±1.01, 3.38±0.86 and 4.70±0.98 pmol/l, respectively). The
plasma concentrations in the borderline group were significantly
decreased subsequent to surgery (3.60±1.03, 5.55±2.05 and
13.82±4.86 pmol/l, respectively) compared with the concentrations
prior to surgery (6.86±2.65, 12.82±4.41 and 20.92±6.34,
respectively) (P<0.05). The values of the three peptides in the
hypertensive group were not significantly decreased subsequent to
drug treatment (9.52±3.60, 26.78±8.03 and 36.76±8.81 pmol/l,
respectively) compared with the values prior to drug treatment
(10.84±4.07, 30.52±10.39 and 40.78±11.29, respectively). However,
the concentrations were significantly decreased following surgery
(7.24±3.02, 18.73±5.86 and 23.73±7.27 pmol/l, respectively)
compared with the values prior to surgery (10.84±4.07, 30.52±10.39
and 40.78±11.29, respectively) (P<0.05).
Discussion
PC is a rare neuroendocrine tumor with a highly
variable clinical presentation, but this tumor most commonly
presents with episodes of hypertension due to the potent effects of
the catecholamines secreted by the tumor, such as dopamine, E and
NE, and this may result in high oxygen consumption (21–23).
Although hypertension is the hallmark of catecholamine release and
may result in serious and potentially lethal cardiovascular
complications, the amount, type and pattern of catecholamine
secretion is extremely variable (24). Blood pressure may also be consistently
normal, particularly in patients with adrenal incidentalomas or in
those with extremely small tumors (25). ADM is a peptide expressed in the
cardiovascular system that often demonstrates increased expression
prior to the development of hypertension (26,27).
Increased circulating levels of ADM have previously been associated
with the LVMI and left ventricular hypertrophy, and are considered
to be an independent risk factor for cardiovascular disease
(28–31). ANP and BNP are each similar to ADM in
cardiovascular effects, including natriuresis, diuresis,
hypotensive action and anti-hypertrophic action, thereby reducing
fluid volume and increasing oxygen transport (32,33).
Numerous previous studies have demonstrated that the plasma levels
of ADM, ANP and BNP are elevated in patients with essential
hypertension (27,34).
In the present study, significantly increased mean
BUN and Scr levels and decreased mean GFRs were noted in
hypertensive patients with PC compared with the control,
normotensive and borderline patients with PC. Therefore, it is
likely that secondary hypertension resulting from PC may lead to
renal dysfunction when hypertension is severe enough. Furthermore,
the BUN and Scr levels and GFRs were unchanged subsequent to the
administration of drugs or surgery. This may be due to certain
long-standing or elderly patients that possessed slightly
irreversible renal damage. Nevertheless, significantly decreased
LVEF and increased LVMI values were found in hypertensive patients
with PC compared with the values in control individuals and
normotensive and borderline hypertensive patients with PC. In
addition, the LVEF and LVMI values in the normotensive and
borderline hypertensive groups were significantly different. The
LVEF and LVMI values were unchanged subsequent to the
administration of drugs, but were significantly decreased following
surgery (27,34–36).
Therefore, the changes in cardiac function may not only be due to
secondary hypertension, but also due to catecholamine
cardiomyopathy. It is necessary to clarify these results in
additional studies.
In accordance with previous studies, the plasma
levels of ADM, ANP and BNP identified in the current study were
increased in hypertensive patients with PC compared with the levels
in the control individuals and normotensive and borderline
hypertensive patients with PC. The present data appear to be
compatible with the previous studies by Letizia et al and
Cotesta et al (35,36). In addition, significant differences
were found between the plasma levels of ADM, ANP and BNP in the
normotensive and borderline hypertensive patients with PC, but were
not observed between control individuals and normotensive patients
with PC. The most likely explanation is that normotensive PC
patients are challenging to diagnose, as there is no catecholamine
hypersecretion. Significant differences in the levels of serum E,
serum NE and urine VMA were found between borderline and
hypertensive PC patients, but were not observed between controls
and normotensive PC patients. By contrast, it may be inferred that
ADM, in addition to ANP and BNP, may participate in the
compensatory and protective mechanisms that counteract additional
elevation of blood pressure in the cardiovascular system, due to
the similar physiological roles. The plasma concentrations of ADM
were not only correlated with the SBP and DBP values, but were also
associated with the values of ANP and BNP in hypertensive PC
patients. Furthermore, ADM was identified as the most important
factor in hypertensive PC patients, which was confirmed by stepwise
multiple regression analysis of the independent parameters
associated with SBP, DBP, serum E, serum NE or urine VMA levels.
There may be an interactive balance mechanism of ADM and
catecholamines in the adrenal medulla. However, elevated values of
the three peptides in hypertensive PC patients were not associated
with renal function, which was confirmed by the lack of differences
between the peptide levels in patients with or without renal
dysfunction. In addition, ADM levels were not correlated with the
BUN level, Scr level or GFR of patients.
The present study revealed that the elevated values
of the peptides ADM, ANP and BNP were not significantly decreased
subsequent to anti-hypertensive treatment, but did significantly
decline following surgery in hypertensive PC patients. In addition,
the values significantly fell in borderline hypertensive PC
patients following surgery. It has been demonstrated that
catecholamines may increase myocardial oxygen consumption and lead
to cardiomyopathy (37). It is likely
that there is an ADM/catecholamine regulatory mechanism that may
also regulate the secretion of ANP and BNP. The plasma ADM
concentration may predict catecholamine hypersecretion. Plasma ADM
concentrations were not only correlated with the values of serum E,
serum NE and urine VMA, but were also associated with the ANP and
BNP levels. Furthermore, plasma ADM concentrations were correlated
with the values of LVEF and LVMI. Therefore, ADM may participate,
along with ANP and BNP, in the defense mechanisms in an attempt to
increase oxygen transport to satisfy the high oxygen consumption
that results from catecholamine hypersecretion and normalize the
state of cardiovascular health in patients with PC.
As aforementioned, a number of investigations have
revealed increased plasma ADM levels in patients with PC (35,36).
However, to the best of our knowledge, the present study is the
first to assess plasma ADM, ANP and BNP levels in patients with PC
that were classified into normotensive, borderline and hypertensive
groups, and also compared the concentrations of the three peptides
in hypertensive PC patients prior to and following drug
treatment.
In summary, the present study reveals that ADM, ANP
and BNP may participate in the mechanisms that counteract the
additional elevation of blood pressure and increase oxygen
transport to satisfy the high oxygen consumption in PC. However,
these peptides may be good predictors of catecholamine
hypersecretion and hypertension improvement in patients with PC. In
addition, there may be an ADM/catecholamine local regulatory
mechanism that is important for the control of adrenal medulla
functions. However, additional studies are required to identify the
specific pathophysiological significance of ADM, ANP and BNP in PC
and the exact pharmacokinetics of these peptides in patients with
PC.
Acknowledgements
This study was supported by grants from the National
Science Fund Project of China (grant no. 81200501) and the Doctor
Research Fund Project of Wuhan University of China (grant no.
2012302020203). Thanks to the Department of Urology in Renmin
Hospital of Wuhan University.
References
|
1
|
Shen WT, Grogan R, Vriens M, Clark OH and
Duh QY: One hundred two patients with pheochromocytoma treated at a
single institution since the introduction of laparoscopic
adrenalectomy. Arch Surg. 145:893–897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Domi R and Laho H: Management of
pheochromocytoma: Old ideas and new drugs. Niger J Clin Pract.
15:253–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thouennon E, Pierre A, Yon L and Anouar Y:
Expression of trophic peptides and their receptors in chromaffin
cells and pheochromocytoma. Cell Mol Neurobiol. 30:1383–1389. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitamura K, Kangawa K, Kawamoto M, Ichiki
Y, Nakamura S, Matsuo H and Eto T: Adrenomedullin: A novel
hypotensive peptide isolated from human pheochromocytoma. 1993.
Biochem Biophys Res Commun. 425:548–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong HK, Cheung TT and Cheung BM:
Adrenomedullin and cardiovascular diseases. JRSM Cardiovasc Dis.
1:ii2012.
|
|
6
|
Cheung BM and Tang F: Adrenomedullin:
Exciting new horizons. Recent Pat Endocr Metab Immune Drug Discov.
6:4–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimosawa T and Fujita T: Adrenomedullin
and its related peptide. Endocr J. 52:1–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishikimi T, Kuwahara K, Nakagawa Y,
Kangawa K and Nakao K: Adrenomedullin in cardiovascular disease: A
useful biomarker, its pathological roles and therapeutic
application. Curr Protein Pept Sci. 14:256–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arjamaa O and Nikinmaa M: Natriuretic
peptides in hormonal regulation of hypoxia responses. Am J Physiol
Regul Integr Comp Physiol. 296:R257–R264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arjamaa O and Nikinmaa M: Hypoxia
regulates the natriuretic peptide system. Int J Physiol Pharmacol.
3:191–201. 2011.
|
|
11
|
Arjamaa O and Nikinmaa M: Oxygen and
natriuretic peptide secretion from the heart. Int J Cardiol.
167:1089–1090. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arjamaa O: Physiology of natriuretic
peptides: The volume overload hypothesis revisited. World J
Cardiol. 6:4–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clerico A, Giannoni A, Vittorini S and
Passino C: Thirty years of the heart as an endocrine organ:
Physiological role and clinical utility of cardiac natriuretic
hormones. Am J Physiol Heart Circ Physiol. 301:H12–H20. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Federico C: Natriuretic Peptide system and
cardiovascular disease. Heart views. 11:10–15. 2010.PubMed/NCBI
|
|
15
|
Kuhn M: Endothelial actions of atrial and
B-type natriuretic peptides. Br J Pharmacol. 166:522–531. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu WW and Qi YF: Cardiovascular effects
and pathophysiological significance of adrenomedullin family
peptides. Sheng Li Ke Xue Jin Zhan. 44:177–182. 2013.(In Chinese).
PubMed/NCBI
|
|
17
|
Chollet-Dallon E, Stoermann-Chopard C and
Martin PY: Could cystatine C replace creatinine as a market of
glomerular filtration rate? Revue medicale suisse. 2:582–585.
2006.PubMed/NCBI
|
|
18
|
Mancia G, De Backer G, Dominiczak A,
Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE,
Laurent S, et al: 2007 Guidelines for the management of arterial
Hypertension: The task force for the management of arterial
hypertension of the european society of hypertension (ESH) and of
the European Society of Cardiology (ESC). J Hypertens.
25:1105–1187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohta H, Tsuji T, Asai S, Tanizaki S,
Sasakura K, Teraoka H, Kitamura K and Kangawa K: A simple
immunoradiometric assay for measuring the entire molecules of
adrenomedullin in human plasma. Clin Chim Acta. 287:131–143. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grzywa-Celińska A, Celiński R, Kwaśniewska
K, Dyczko M, Prystupa A and Mosiewicz J: The usefulness of
natriuretic peptides measurements in the diagnostics of chosen
cardiovascular diseases. Pol Merkur Lekarski. 34:232–234. 2013.(In
Polish). PubMed/NCBI
|
|
21
|
Lenders JW, Eisenhofer G, Mannelli M and
Pacak K: Phaeochromocytoma. Lancet. 366:665–675. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miller RA and Ohrt DW: Pheochromocytoma -
review and biochemical workup. S D Med. 66:267 269–270. 2013.
|
|
23
|
Marín MR, Arenas MF, Valverde FM, Garaulet
ET, Maderuelo MM, Avilés AM, Quirante FP and Blázquez AA:
Laparoscopic adrenalectomy for nonfamilial adrenal medullary
hyperplasia. JSLS. 17:433–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mannelli M, Lenders JW, Pacak K, Parenti G
and Eisenhofer G: Subclinical phaeochromocytoma. Best practice
& research. Best Pract Res Clin Endocrinol Metab. 26:507–515.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bovio S, Cataldi A, Reimondo G, Sperone P,
Novello S, Berruti A, Borasio P, Fava C, Dogliotti L, Scagliotti
GV, et al: Prevalence of adrenal incidentaloma in a contemporary
computerized tomography series. J Endocrinol Invest. 29:298–302.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bełtowski J and Jamroz A:
Adrenomedullin-what do we know 10 years since its discovery? Pol J
Pharmacol. 56:5–27. 2004.PubMed/NCBI
|
|
27
|
Kato J, Kitamura K and Eto T: Plasma
adrenomedullin level and development of hypertension. J Hum
Hypertens. 20:566–570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Al-Omari MA, Khaleghi M, Mosley TH Jr,
Turner ST, Morgenthaler NG, Struck J, Bergmann A and Kullo IJ:
Mid-regional pro-adrenomedullin is associated with pulse pressure,
left ventricular mass and albuminuria in African Americans with
hypertension. Am J Hypertens. 22:860–866. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bhandari SS, Davies JE, Struck J and Ng
LL: The midregional portion of proadrenomedullin is an independent
predictor of left ventricular mass index in hypertension.
Metabolism. 59:7–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coutinho T, Al-Omari M, Mosley TH Jr and
Kullo IJ: Biomarkers of left ventricular hypertrophy and remodeling
in blacks. Hypertension. 58:920–925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishida H, Horio T, Suzuki Y, Iwashima Y,
Kamide K, Kangawa K and Kawano Y: Plasma adrenomedullin as an
independent predictor of future cardiovascular events in high-risk
patients: Comparison with C-reactive protein and adiponectin.
Peptides. 29:599–605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sergeeva IA and Christoffels VM:
Regulation of expression of atrial and brain natriuretic peptide,
biomarkers for heart development and disease. Biochim Biophys Acta.
1832:2403–2413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saito Y: Roles of atrial natriuretic
peptide and its therapeutic use. J Cardiol. 56:262–270. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Soualmia H, Ayadi I, Omar S, Feki M,
Drissa H, Mebazaa A and Kaabachi N: Atrial natriuretic peptide and
brain natriuretic peptide release in human essential hypertension.
Clin Lab. 55:120–127. 2009.PubMed/NCBI
|
|
35
|
Letizia C, De Toma G, Caliumi C, Cerci S,
Massa R, Loria RD, Alo P, Marinoni EM, Diacinti D and D'Erasmo E:
Plasma adrenomedullin concentrations in patients with adrenal
pheochromocytoma. Horm Metab Res. 33:290–294. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cotesta D, Caliumi C, Alò P, Petramala L,
Reale MG, Masciangelo R, Signore A, Cianci R, D'Erasmo E and
Letizia C: High plasma levels of human chromogranin A and
adrenomedullin in patients with pheochromocytoma. Tumori. 91:53–58.
2005.PubMed/NCBI
|
|
37
|
Vindenes T, Crump N, Casenas R and Wood K:
Pheochromocytoma causing cardiomyopathy, ischemic stroke and acute
arterial thrombosis: A case report and review of the literature.
Conn Med. 77:95–98. 2013.PubMed/NCBI
|