Introduction
As the most commonly diagnosed cancer and the main
cause of cancer-related mortality among women, breast cancer
accounts for 23% of all cancer cases and 14% of the cancer-related
fatalities (1). Breast cancer is also
one of the most common malignant tumors in Chinese women, with
>100 new cases per 100,000 women aged 55–69 years estimated to
occur by 2021 (2). Advances in the
treatment of this disease using a multi-disciplinary approach with
improved combinations of surgery, radiotherapy, chemotherapy and
endocrine therapy have resulted in marked improvements in patient
outcomes. However, more than half a million women will continue to
succumb to breast cancer annually despite these advances (3).
Epithelial-mesenchymal transition (EMT) is an
essential process during cancer progression to tumor metastasis
(4,5).
Mesenchymal cell marker expression, such as that of vimentin, Snail
(6,7)
and E-cadherin (8), is considered a
hallmark for EMT. EMT can also be induced in mammary epithelial
cells by expression of various factors, such as the Twist or Snail
families (9,10). In addition, the upregulation of
nuclear factor-κB (NF-κB) is found in human breast tumor cell
lines, carcinogen-transformed mammary epithelial cells, and the
majority of primary human and rodent breast tumor tissue samples
(11). NF-κB has also been shown to
be a central mediator of EMT in a breast cancer progression mouse
model (12,13). Although activation of these processes
in breast cancer cells has greatly increased their invasive and
metastatic potential, the exact role of EMT in tumor metastasis
remains unknown (14).
Pigment epithelium-derived factor (PEDF) is a
potential independent prognostic marker for breast cancer, and
reduction in its expression levels is associated with the
progression of disease and a poor patient outcome (15,16). In
addition to its anti-angiogenic functions, PEDF also inhibits tumor
cell migration by promoting cell adhesion, inducing apoptosis and
regulating tumor cell differentiation (17). Specifically, PEDF induces endothelial
cell apoptosis via NF-κB activation and its downstream target Fas
ligand (18,19), Furthermore, PEDF expression is
significantly reduced in a wide range of tumor types, and its
recovered expression in these tumors delays the onset of primary
tumors and decreases metastasis (20). In the brain, PEDF acts as a metastatic
suppressor and a neuroprotectant, highlighting its role in limiting
brain metastasis and local consequences in primary breast tumors
(21). Thus, PEDF may play a critical
role in breast cancer development and progression. However, the
exact molecular mechanisms by which PEDF elicits its antitumor
effects remain unknown (22). The
effects of PEDF on tumor suppression and endothelial cell apoptosis
combined with its inhibition of tumor cell migration suggest that
it may have therapeutic value in the context of EMT. The present
study systemically investigated the association between PEDF levels
and EMT-related proteins in 119 cases of primary invasive ductal
breast cancer (IDC), and analyzed their correlation with
clinicopathological factors and patient survival to determine the
association between PEDF expression and EMT in breast cancer.
Materials and methods
Patients and tissue specimens
Paraffin-embedded surgical specimens were randomly
obtained from 119 non-consecutive breast cancer patients that
underwent modified radical masectomy at the Zhujiang Hospital
Affiliated to Southern Medical University (Guangzhou, Guangdong,
China) between 2006 and 2008. The 119 surgical specimens were
selected in accordance with the following criteria: Female patients
presenting with unilateral, primary IDC without a history of breast
cancer. Patients who received neoadjuvant chemotherapy prior to
surgery, presented with secondary breast cancer or exhibited
peritumorous carcinoma in situ in the tumor sample were
excluded. Tumor histology was determined according to the 2003
World Health Organization criteria (23), while disease stage was assessed
according to the Union for International Cancer Control (24). Tumors were graded according to Bloom
and Richardson, as modified by Elston and Ellis (25), and hormone receptor status was
assessed according to the scoring system developed by Remmele and
Stegner (26). Inclusion criteria for
the study were as follows: Female patients presenting with
unilateral, primary IDC, without a history of breast cancer.
Patients who received neo-adjuvant chemotherapy prior to surgery,
presented with secondary breast cancer or had peritumorous
carcinoma in situ present in the tumor sample were excluded.
Normal mammary parenchyma obtained from 30 women who underwent
breast reduction was also analyzed. Ethical approval was obtained
from the Medical Ethics Committee of Zhujiang Hospital Affiliated
to Southern Medical University and written informed consent was
obtained from all patients.
Immunohistochemical staining
Paraffin-embedded sections (5-µm thick) were
deparaffinized by immersion in dimethylbenzene for 20 min and then
rehydrated in graded concentrations of ethanol (100, 90, 80 and
70%; Beyotime Biotechnology, Haimen, China). The sections were then
subjected to immunohistochemical analysis, as previously described
by Zhang et al (27).
Subsequent to blocking endogenous peroxidase (3% hydrogen
peroxidase; Beyotime Biotechnology), the sections were incubated
with primary mouse anti-human monoclonal PEDF (1:100; Millipore,
Billerica, MA, USA), rabbit anti-human monoclonal E-cadherin
(1:500; Millipore), mouse anti-human monoclonal vimentin (1:100;
Cell Signaling Technology Inc., Danvers, MA, USA), goat anti-human
polyclonal Snail (1:50; Santa Cruz Biotechnology Inc., Dallas, TX,
USA) and rabbit anti-human monoclonal NF-κB (1:600; Cell Signaling
Technology Inc.) antibodies diluted in phosphate-buffered saline
containing 0.1% Tween-20 (PBST) and 5% bovine serum albumin
(Beyotime Biotechnology) overnight at 4°C. Subsequent to being
washed three times with PBST, the sections were incubated with
secondary antibodies (goat anti-mouse IgG/biotin, rabbit anti-goat
IgG/biotin or goat anti-rabbit IgG/biotin; 1:100),
avidin-biotin-peroxidase complex and DAB reagent (Wuhan Boster
Biological Technology, Ltd., Wuhan, China). Subsequently, all
sections were counterstained with hematoxylin (Beyotime
Biotechnology) and visualized by microscopy (DM40008; Leica, Solms,
Germany). Images were captured by Leica Application Suite 3.7
(Leica), and 5–10 photomicrographs were randomly selected from each
section.
Immunohistochemical evaluation
The expression levels of PEDF, E-cadherin, vimentin,
Snail and NF-κB were independently reviewed and scored by two
pathologists who were blinded to the clinical parameters. The
expression of Snail and NF-κB was observed in the cytoplasm,
nucleus or both; however, only nuclear expression was considered
immunopositive for Snail. Expression of PEDF, E-cadherin and
vimentin in the cytoplasm and/or plasma membrane were each
considered positive.
The semi-quantitative analysis of the distribution
of staining was scored according to the percentage of cells showing
immunoreactivity: Negative immunoreactivity indicated the absence
of staining or weak staining in 1% of the tumor cells; + indicated
focal staining in 1–10% of the tumor cells; ++ indicated positive
staining in 11–50% of the tumor cells; and +++ indicated positive
staining in >50% of the tumor cells. Tumors were defined as
immunopositive when >10% (++/+++) of tumor cells show
immunoreactivity. Thus, (+) is defined as low expression, whereas
(++/+++) is defined as high expression.
Statistical analysis
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA) was
used for all statistical analyses. The χ2 test was used
to analyze the correlation between PEDF, E-cadherin, vimentin,
Snail and NF-κB expression, and the clinicopathological features of
the IDC patients. Spearman's correlation coefficient analysis was
used to evaluate the correlations between the variables. The
Kaplan-Meier method and log-rank tests were used to evaluate the
correlation between marker expression and overall survival (OS).
The Cox proportional hazards model was used for the multivariate
analysis to identify independent prognostic factors. P<0.05 was
considered to indicate a statistically significant difference.
Results
PEDF, E-cadherin, vimentin, Snail and
NF-κB expression in IDC samples
The expression of PEDF, E-cadherin, vimentin, Snail
and NF-κB was analyzed in 119 IDC tissues by immunohistochemistry
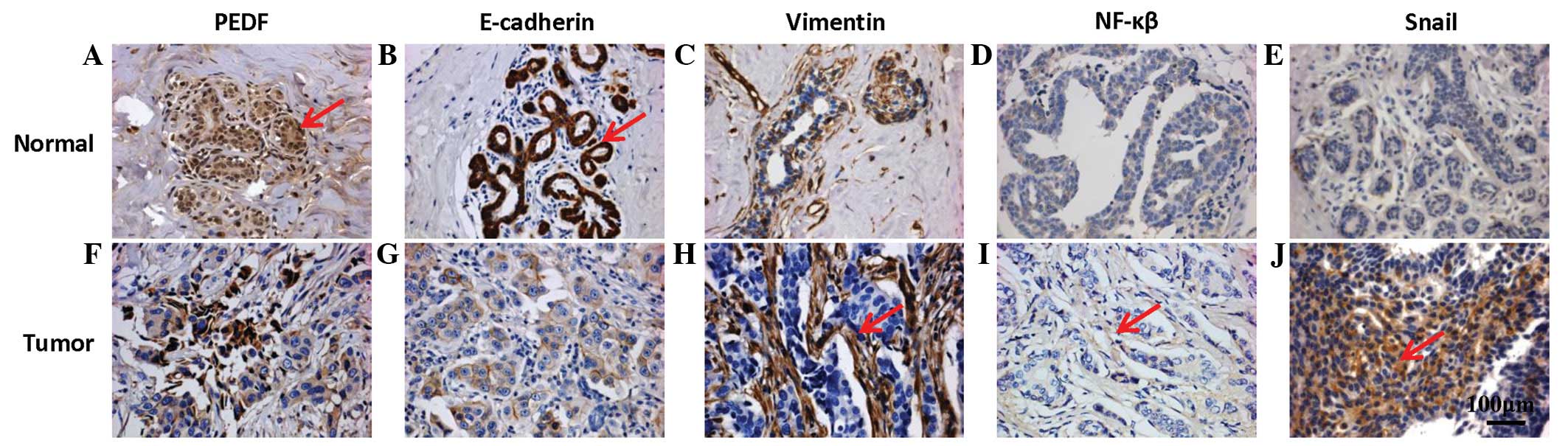
(Fig. 1). As shown in Fig. 1A, PEDF was detected in the cytoplasm
of the epithelial cells, and high levels of staining were observed
in 44.5% of the breast carcinoma tissues analyzed, which were
classified as PEDF-positive. Although E-cadherin was present in the
cell membranes of normal breast tissues, it was absent in the tumor
tissues (Fig. 1G). Approximately
49.6% (59/119) of the tumor sections exhibited an absence or
reduction in E-cadherin expression (Table
I). Reduced E-cadherin expression was observed in 20.9% (9/43)
of the late-stage (III/IV) and 49.1% (52/106) of the high-grade
tumors, which was significantly more than that observed for the
early-stage (I/II; 67.1%) and low-grade (61.5%) tumors (both
P=0.001) (Table I). Conversely,
vimentin and Snail were absent in the normal breast tissues
(Fig. 1C and E).
 | Table I.Correlation between PEDF, E-cadherin,
vimentin, Snail and NF-κB expression, and clinicopathological
features. |
Table I.
Correlation between PEDF, E-cadherin,
vimentin, Snail and NF-κB expression, and clinicopathological
features.
|
| PEDF, n |
| E-cadherin, n |
| Vimentin, n |
| Snail, n |
| NF-κB, n |
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Feature | (+) | (−) | P-value | (+) | (−) | P-value | (+) | (−) | P-value | (+) | (−) | P-value | (+) | (−) | P-value |
|---|
| Age, years |
|
| 0.809 |
|
|
0.929 |
|
|
0.407 |
|
|
0.775 |
|
|
0.246 |
|
<50 | 26 | 35 |
| 31 | 30 |
| 32 | 29 |
| 31 | 30 |
| 38 | 23 |
|
|
≥50 | 26 | 32 |
| 29 | 29 |
| 26 | 32 |
| 31 | 27 |
| 30 | 28 |
|
| Menopausal
status |
|
| 0.918 |
|
|
0.936 |
|
|
0.621 |
|
|
0.738 |
|
|
0.791 |
|
Premenopausal | 29 | 38 |
| 34 | 33 |
| 34 | 33 |
| 34 | 33 |
| 39 | 28 |
|
|
Postmenopausal | 23 | 29 |
| 26 | 26 |
| 24 | 28 |
| 28 | 24 |
| 29 | 23 |
|
| Lymph node
metastasis |
|
| 0.555 |
|
|
0.013 |
|
| <0.001 |
|
| <0.001 |
|
|
0.001 |
|
Negative | 22 | 32 |
| 34 | 20 |
| 16 | 38 |
| 18 | 36 |
| 22 | 32 |
|
|
Positive | 30 | 35 |
| 26 | 39 |
| 42 | 23 |
| 44 | 21 |
| 46 | 19 |
|
| Tumor size, cm |
|
| 0.039 |
|
|
0.004 |
|
|
0.017 |
|
| <0.001 |
|
|
0.001 |
|
≤2.0 | 26 | 21 |
| 32 | 15 |
| 16 | 31 |
| 15 | 32 |
| 18 | 29 |
|
|
>2.0 | 26 | 46 |
| 28 | 44 |
| 42 | 30 |
| 47 | 25 |
| 50 | 22 |
|
| Histopathological
grade |
|
| 0.113 |
|
|
0.126 |
|
|
0.005 |
|
|
0.672 |
|
|
0.001 |
| G1 | 3 | 10 |
| 8 | 5 |
| 4 | 9 |
| 6 | 7 |
| 4 | 9 |
|
|
G2/G3 | 49 | 57 |
| 52 | 54 |
| 54 | 52 |
| 56 | 50 |
| 64 | 42 |
|
| Pathological
stagea |
|
| 0.495 |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
I/II | 35 | 41 |
| 51 | 25 |
| 24 | 52 |
| 26 | 50 |
| 32 | 44 |
|
|
III/IV | 17 | 26 |
| 9 | 34 |
| 34 | 9 |
| 36 | 7 |
| 36 | 7 |
|
| ER status |
|
| 0.862 |
|
|
0.314 |
|
|
0.248 |
|
|
0.348 |
|
|
0.419 |
|
Positive | 31 | 41 |
| 39 | 33 |
| 32 | 40 |
| 35 | 37 |
| 39 | 33 |
|
|
Negative | 21 | 26 |
| 21 | 26 |
| 26 | 21 |
| 27 | 20 |
| 29 | 18 |
|
| PR status |
|
| 0.137 |
|
|
0.014 |
|
|
0.008 |
|
|
0.111 |
|
|
0.186 |
|
Positive | 32 | 32 |
| 39 | 25 |
| 24 | 40 |
| 29 | 35 |
| 33 | 31 |
|
|
Negative | 20 | 35 |
| 21 | 34 |
| 34 | 21 |
| 33 | 22 |
| 35 | 20 |
|
| Adjuvant
treatment |
|
| 0.304 |
|
|
0.216 |
|
|
0.173 |
|
|
0.388 |
|
|
0.910 |
|
None | 14 | 24 |
| 16 | 22 |
| 22 | 16 |
| 22 | 16 |
| 22 | 16 |
|
|
Therapyb | 38 | 43 |
| 44 | 37 |
| 36 | 45 |
| 40 | 41 |
| 46 | 35 |
|
However, vimentin was highly expressed in the tumor
tissues, and Snail was detected in 52.1% of the tumor tissues
analyzed (Fig. 1H and J; Table I). Finally, NF-κB was detected in the
cytoplasm of IDC cells in 57.1% of the tumors analyzed (Fig. 1I).
Correlation between PEDF, E-cadherin,
vimentin, Snail and NF-κB expression, and clinicopathological
features
The correlation analysis between the expression
levels of PEDF, E-cadherin, vimentin, Snail, NF-κB and
clinicopathological features is summarized in Table I. PEDF protein was detected in 40.7%
of lymph node-negative tumors and 46.2% of lymph node-positive
tumors (Table I). The cytoplasmic
expression of PEDF was significantly correlated with the tumor size
(P=0.039). Furthermore, a low level of E-cadherin expression was
correlated with a large tumor size (P=0.004), positive lymph node
metastasis status (P=0.013), early pathological stage (P<0.001)
and positive progesterone receptor (PR) status (P=0.014; Table I). High vimentin expression was
strongly associated with high pathological stage and large tumor
size (P<0.001 and P=0.009, respectively); it was also correlated
with positive lymph node status and negative PR status (P<0.001
and P=0.008, respectively) (Table I).
High nuclear Snail staining was significantly associated with a
large tumor size (P<0.001), positive lymph node metastasis
status (P<0.001) and late pathological stage (P=0.001). For
example, 83.7% of patients with late-stage tumors (III–IV)
expressed high levels of nuclear Snail, compared with 26.3% of
patients with early-stage (I–II) tumors. Positive nuclear
expression of NF-κB was also associated with all adverse
clinicopathological variables, namely tumor size, high tumor grade,
late tumor stage and lymph node positivity (all P≤0.001) (Table I). No significant association existed
between PEDF, E-cadherin, vimentin, Snail, NF-κB expression and
gender, age, menopausal status, adjuvant treatment and estrogen
receptor (ER) status.
Association between PEDF, E-cadherin,
vimentin, Snail and NF-κB expression in IDC samples
Next, the correlation between low level PEDF
expression levels and EMT-related proteins was analyzed in the
breast carcinoma tissues. In accordance with the protein changes
found during EMT, high expression levels of vimentin, Snail and
NF-κB were associated with the weak expression of membranous
E-cadherin (P<0.001; Table II).
In addition, a high level of vimentin expression was significantly
correlated with low level nuclear Snail expression (P<0.001,
r=0.428); a high level of Snail expression was also significantly
correlated with the low level expression of NF-κB (P=0.002,
r=0.291). These data indicated the presence of EMT in the IDC
tissues. Furthermore, a high level of PEDF expression was strongly
associated with a low level of E-cadherin expression (P<0.001,
r=0.496) and a high level of vimentin (P<0.001, r=–0.337), Snail
(P<0.001, r=0.34) and NF-κB (P<0.001, r=0.383) expression.
These data suggest that PEDF may be involved in the regulation of
EMT.
 | Table II.Association between PEDF, E-cadherin,
vimentin, Snail and NF-κB expression in invasive breast carcinoma
samples. |
Table II.
Association between PEDF, E-cadherin,
vimentin, Snail and NF-κB expression in invasive breast carcinoma
samples.
|
| E-cadherin, n |
|
| Vimentin, n |
|
| Snail, n |
|
| NF-κB, n |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Factor | (+) | (−) | P-value | r | (+) | (−) | P-value | r | (+) | (−) | P-value | r | (+) | (−) | P-value | r |
|---|
| PEDF |
|
| <0.001 | 0.496 |
|
| <0.001 | −0.337 |
|
| <0.001 | 0.340 |
|
| <0.001 |
0.383 |
|
Positive | 41 | 11 |
|
| 14 | 38 |
|
| 16 | 36 |
|
| 19 | 33 |
|
|
|
Negative | 19 | 48 |
|
| 45 | 22 |
|
| 46 | 21 |
|
| 50 | 17 |
|
|
| E-cadherin |
|
|
|
|
|
| <0.001 | −0.613 |
|
| <0.001 | −0.480 |
|
| <0.001 | −0.519 |
|
Positive |
|
|
|
| 11 | 49 |
|
| 17 | 43 |
|
| 19 | 41 |
|
|
|
Negative |
|
|
|
| 47 | 12 |
|
| 45 | 14 |
|
| 49 | 10 |
|
|
| Vimentin |
|
|
|
|
|
|
|
|
|
| <0.001 | 0.428 |
|
| <0.001 |
0.437 |
|
Positive |
|
|
|
|
|
|
|
| 42 | 16 |
|
| 46 | 12 |
|
|
|
Negative |
|
|
|
|
|
|
|
| 20 | 41 |
|
| 22 | 39 |
|
|
| Snail |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.002 |
0.291 |
|
Positive |
|
|
|
|
|
|
|
|
|
|
|
| 44 | 18 |
|
|
|
Negative |
|
|
|
|
|
|
|
|
|
|
|
| 24 | 33 |
|
|
Association between PEDF, E-cadherin,
vimentin, Snail and NF-κB expression, and patient survival
Next, the Nottingham prognostic index (NPI) was used
as an indicator of patient prognosis, as previously described
(28). As shown in Table III, the overexpression of
E-cadherin, vimentin, Snail and NF-κB was significantly associated
with NPI status, as determined by the Kruskal-Wallis test (all
P<0.001); however, PEDF expression was not associated with NPI.
Specifically, high NF-κB expression was more frequently observed in
patients with a high NPI (≥5.4; 37.7%) compared with those with a
low NPI (<5.4; 24.4%). The patients were then divided into two
groups on the basis of their prognosis. Patients were considered to
have a good prognosis (n=49) if they remained disease-free at the
5-year follow-up; those with a poor prognosis (n=70) included
patients who developed recurrence, metastasis to a distant site, or
those who had succumbed as a result of the breast cancer. As shown
in Table III, patients with poor
prognoses had low levels of PEDF and E-cadherin expression (P=0.014
and P<0.001, respectively) and high levels of vimentin, Snail
and NF-κB expression (P≤0.005).
 | Table III.Correlation of PEDF, E-cadherin,
vimentin, Snail and NF-κB expression with patient survival. |
Table III.
Correlation of PEDF, E-cadherin,
vimentin, Snail and NF-κB expression with patient survival.
| Factor | No. of cases | Positive, n | Negative, n | Score range | Median | P-value |
|---|
| PEDF |
|
|
|
|
|
|
| NPI |
|
|
|
|
|
0.701 |
| 1 | 24 | 9 | 15 | 2.2–4.4 | 3.3 |
|
| 2 | 52 | 24 | 28 | 2.6–6.4 | 4.4 |
|
| 3 | 43 | 19 | 24 | 3.5–7.2 | 5.8 |
|
| Survival
status |
|
|
|
|
|
0.014 |
|
Good | 49 | 28 | 21 |
|
|
|
|
Poor | 70 | 24 | 46 |
|
|
|
| E-cadherin |
|
|
|
|
|
|
| NPI |
|
|
|
|
| <0.001 |
| 1 | 24 | 20 | 4 | 2.2–4.4 | 3.3 |
|
| 2 | 52 | 29 | 23 | 2.6–6.4 | 4.4 |
|
| 3 | 43 | 11 | 32 | 3.5–7.2 | 5.8 |
|
| Survival
status |
|
|
|
|
| <0.001 |
|
Good | 49 | 39 | 10 |
|
|
|
|
Poor | 70 | 21 | 49 |
|
|
|
| Vimentin |
|
|
|
|
|
|
| NPI |
|
|
|
|
| <0.001 |
| 1 | 24 | 5 | 19 | 2.2–4.4 | 3.3 |
|
| 2 | 52 | 20 | 32 | 2.6–6.4 | 4.4 |
|
| 3 | 43 | 33 | 10 | 3.5–7.2 | 5.8 |
|
| Survival
status |
|
|
|
|
| <0.001 |
|
Good | 49 | 9 | 40 |
|
|
|
|
Poor | 70 | 49 | 21 |
|
|
|
| Snail |
|
|
|
|
|
|
| NPI |
|
|
|
|
| <0.001 |
| 1 | 24 | 6 | 18 | 2.2–4.4 | 3.3 |
|
| 2 | 52 | 24 | 28 | 2.6–6.4 | 4.4 |
|
| 3 | 43 | 32 | 11 | 3.5–7.2 | 5.8 |
|
| Survival
status |
|
|
|
|
|
0.005 |
|
Good | 49 | 18 | 31 |
|
|
|
|
Poor | 70 | 44 | 26 |
|
|
|
| NF-κB |
|
|
|
|
|
|
| NPI |
|
|
|
|
| <0.001 |
| 1 | 24 | 5 | 19 | 2.2–4.4 | 3.3 |
|
| 2 | 52 | 24 | 28 | 2.6–6.4 | 4.4 |
|
| 3 | 43 | 39 | 4 | 3.5–7.2 | 5.8 |
|
| Survival
status |
|
|
|
|
| <0.001 |
|
Good | 49 | 16 | 33 |
|
|
|
|
Poor | 70 | 52 | 18 |
|
|
|
Univariate and multivariate Cox
regression analysis of PEDF, E-cadherin, vimentin, Snail and NF-κB
expression, and clinicopathological variables
Univariate analyses of OS using Cox regression
analysis identified PEDF, vimentin, E-cadherin, Snail and NF-κB
expression (P=0.006, P<0.001, P<0.001, P=0.001 and
P<0.001, respectively), lymph node metastasis (P=0.015), tumor
size (P=0.012), pathological stage (P=0.012) and PR status
(P=0.003) as significant prognostic predicators (Table IV). To determine whether
PEDF-positive expression was an independent predictor of patient
survival, a multivariate analysis was performed using Cox
proportional regression models, together with vimentin, E-cadherin,
Snail and NF-κB expression, as well as basic patient and tumor
characteristics, such as age, tumor clinical stage, lymph node
metastasis, tumor size and ER/PR status. Cox multivariate analysis
showed that the expression of vimentin and E-cadherin were
independent prognostic factors associated with OS (P=0.016 and
P=0.004, respectively) and disease-free survival (DFS; P=0.012 and
P=0.005, respectively). Although PEDF expression was not correlated
with OS (P=0.51), a significant correlation with DFS was noted
(P=0.034). With the exception of tumor size (P=0.009), no
clinicopathological factors were independently predictive of
patient survival (Table V).
 | Table IV.Multivariate analysis of overall
survival of patients with invasive breast carcinoma. |
Table IV.
Multivariate analysis of overall
survival of patients with invasive breast carcinoma.
| Factor | Regression
coefficient | Standard error | Wald | RR | 95% CI | P-value |
|---|
| Age | −0.001 | 0.012 | 0.010 |
0.999 | 0.975–1.023 | 0.921 |
| Histopathological
grading |
0.472 | 0.516 | 0.838 |
1.604 | 0.583–4.410 | 0.360 |
| Lymph node
metastasis |
0.055 | 0.384 | 0.020 |
1.056 | 0.498–2.240 | 0.886 |
| Pathological
stage |
0.735 | 0.505 | 2.120 |
1.329 | 0.775–5.614 | 0.145 |
| Tumor size |
0.285 | 0.109 | 6.853 |
2.086 | 1.074–1.645 | 0.009 |
| ER status |
0.399 | 0.263 | 2.298 | 1.49 | 0.890–2.497 | 0.130 |
| PR status |
0.368 | 0.270 | 1.844 |
1.445 | 0.849–2.459 | 0.174 |
| PEDF | −0.232 | 0.351 | 0.435 |
0.793 | 0.398–1.579 | 0.510 |
| Vimentin | −0.847 | 0.353 | 5.751 |
0.429 | 0.215–0.857 | 0.016 |
| E-caherin |
1.186 | 0.406 | 8.517 |
3.274 | 1.476–7.262 | 0.004 |
| Snail | −0.001 | 0.328 | 0.000 |
0.999 | 0.525–1.900 | 0.997 |
| NF-κB | −0.609 | 0.364 | 2.796 |
0.544 | 0.267–1.110 | 0.094 |
 | Table V.Multivariate analysis of disease-free
survival of patients with invasive breast carcinoma. |
Table V.
Multivariate analysis of disease-free
survival of patients with invasive breast carcinoma.
| Factor | Regression
coefficient | Standard error | Wald | RR | 95% CI | P-value |
|---|
| Age | −0.001 | 0.013 | 0.003 | 0.999 | 0.975–1.025 | 0.959 |
| Histopathological
grading |
0.309 | 0.510 | 0.368 | 1.362 | 0.501–3.702 | 0.544 |
| Lymph node
metastasis | −0.201 | 0.380 | 0.281 | 0.818 | 0.388–1.723 | 0.596 |
| Pathological
stage |
0.528 | 0.505 | 1.095 | 1.696 | 0.631–4.563 | 0.295 |
| Tumor size |
0.219 | 0.108 | 4.134 | 1.245 | 1.008–1.537 | 0.042 |
| ER status |
0.404 | 0.268 | 2.270 | 1.497 | 0.886–2.531 | 0.132 |
| PR status |
0.257 | 0.270 | 0.907 | 1.294 | 0.762–2.197 | 0.341 |
| PEDF | −0.324 | 0.354 | 0.840 | 0.723 | 0.361–1.446 | 0.034 |
| Vimentin | −0.918 | 0.366 | 6.301 | 0.399 | 0.195–0.818 | 0.012 |
| E-caherin |
1.141 | 0.407 | 7.847 | 3.129 | 1.409–6.950 | 0.005 |
| Snail | −0.102 | 0.319 | 0.101 | 0.903 | 0.483–1.690 | 0.751 |
| NF-κB | −0.705 | 0.362 | 3.787 | 0.494 | 0.243–1.005 | 0.052 |
Kaplan-Meier survival analysis. Kaplan-Meier
survival curves are shown in Figs. 2
and 3. Among the 119 patients with
IDC analyzed, the patients with PEDF- and E-cadherin-positive
tumors exhibited higher OS rates compared with those with PEDF- and
E-cadherin-negative tumors (P=0.004 and P<0.001, respectively;
Fig. 2A and B). Patients with PEDF-
and E-cadherin-positive tumors also exhibited significantly higher
DFS rates (P=0.004 and P<0.001, respectively; Fig. 3A and B). By contrast, the patients
with vimentin-, Snail- and NF-κB-positive tumors exhibited lower OS
rates compared with the patients with vimentin-, Snail- and
NF-κB-negative tumors (all P≤0.001; Fig.
2C–E). Patients with vimentin-, Snail- and NF-κB-positive
tumors also exhibited significantly lower DFS rates (all P≤0.001;
Fig. 3C–E).
Discussion
PEDF is a 50-kDa protein found in the extracellular
matrix (ECM). It belongs to the serpin (serine protease inhibitor)
family, and contains heparin and collagen binding sites (29–31). PEDF
is a potent neurotrophic and angiogenesis-inhibiting factor with
tumor suppressor properties (32).
In vitro studies have demonstrated that the silencing of
PEDF may be a novel mechanism for the development of endocrine
resistance in breast cancer and that its expression may be a
predictive marker of endocrine sensitivity (33). In addition, previous studies have
shown that the expression of PEDF is significantly decreased in a
number of tumor types, including pancreatic adenocarcinoma
(34), glioblastoma (35) and ovarian carcinoma (36).
Vascular endothelial growth factor receptor-1
(VEGFR-1) regulates EMT for the promotion of breast cancer
progression and metastasis (37). In
various types of tumor cells, PEDF has been shown to decrease VEGF
levels (38–40); suppression of VEGF signaling by PEDF
may be a novel therapeutic target. In addition, Cai et al
(41) also identified two novel
pathways through which VEGF-induced angiogenesis is inhibited by
PEDF: Regulated intramembrane proteolysis and phosphorylation
inhibition. Therefore, we hypothesized that PEDF may represent a
potential biomarker of EMT in breast cancer and analyzed its
expression using immunohistochemistry analysis. In the present
study, high PEDF expression was strongly associated with low
E-cadherin expression, as well as with high vimentin, Snail and
NF-κB expression. These data suggest that PEDF may be involved in
the regulation of EMT. This is the first study to show that PEDF
may serve as a novel EMT suppressor and to reveal its potential as
a prognostic indicator in breast cancer.
EMT exhibits certain characteristic phenotypic
changes that are a result of complex genetic changes, which to a
certain degree are mediated by specific transcription factors that
are able to modulate E-cadherin expression and the expression of
numerous other EMT-associated genes in vitro. Vimentin is a
widely recognized EMT-like phenotype marker, and its expression has
been shown in a number of aggressive breast cancer cell lines
(42). In the present study, low
E-cadherin expression was associated with increased tumor size
(P=0.004), lymph node metastasis status (P=0.013) and the
pathological stage (P<0.001). In addition, high vimentin
expression was strongly associated with high-grade and late-stage
tumors (P<0.001), which is consistent with the results reported
by Lee et al (43). Notably,
vimentin overexpression and reduced E-cadherin expression were
significantly correlated with reduced survival and were independent
predictors in multivariate analysis. Moreover, multivariate
analysis showed that the expression of vimentin and E-cadherin were
independent prognostic factors correlated with shorter OS and DFS
times.
Several studies have demonstrated the expression of
Snail at the tumor-stroma interface and in invasive breast cancers
(44). As expected, high nuclear
Snail staining was significantly associated with large tumor size
(P<0.001), status of lymph node metastasis (P<0.001) and
pathological stage (P=0.001) in the present study. Moreover, it was
observed that positive nuclear NF-κB expression was associated with
all adverse clinicopathological variables analyzed, including large
tumor size, high tumor grade, late tumor stage and lymph node
positivity, confirming its significance in the development and
progression of cancer. Furthermore, low E-cadherin expression, and
high vimentin, Snail and NF-κB expression levels were associated
with shorter OS and DFS times, which is consistent with studies of
other cancers (45,46). Taken together, these results suggest
that the expression of certain transcription factors, including
NF-κB and Snail, is associated with a poor prognosis in a range of
different human cancer types (47).
Previous studies have reported that low PEDF
expression is associated with angiogenesis in breast cancer
(16). The present study also
demonstrated that PEDF was a statistically significant prognostic
factor in multivariate Cox regression analysis. Additionally,
although the association between EMT and metastasis in patients has
been indicated, the present study is the first to suggest a
possible mechanism by which PEDF may be capable of reversing tumor
growth and metastasis in breast cancer. To the best of our
knowledge, studies have rarely been focused on the PEDF and
EMT-related genes; Hirsch et al (48) concluded that PEDF upregulates PPARγ by
binding to PEDF receptor, resulting in the suppression of
NF-κB-mediated transcriptional activation in prostate cancer cells.
Therefore, further studies are required to elucidate the mechanisms
by which PEDF regulates EMT in breast cancer. Therapies targeting
PEDF may provide a novel therapeutic approach for untreatable
patients.
In summary, to the best of our knowledge, the
present study is the first to demonstrate a link between the
expression of PEDF in breast cancer and EMT-related genes. These
findings suggest that PEDF may be capable of reversing tumor growth
and metastasis in breast cancer; however, further studies are
necessary to elucidate the association of PEDF expression and EMT
in vitro and in vitro.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of China (nos. 81171824, 81371719 and 81402613),
the Natural Science Foundation of Guangdong province (nos.
S2012010009276, 2014A030310055 and 2014A030312013) and the Medical
Research Foundation of Guangdong province (no. B2014395).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Linos E, Spanos D, Rosner BA, et al:
Effects of reproductive and demographic changes on breast cancer
incidence in China: A modeling analysis. J Natl Cancer Inst.
100:1352–1360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Li X, Zhang N, Yang Q and Moran
MS: Low doses ionizing radiation enhances the invasiveness of
breast cancer cells by inducing epithelial-mesenchymal transition.
Biochem Biophys Res Commun. 412:188–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalluri R: EMT: When epithelial cells
decide to become mesenchymal-like cells. J Clin Invest.
119:1417–1419. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Emadi Baygi M, Soheili ZS, Schmitz I, et
al: Snail regulates cell survival and inhibits cellular senescence
in human metastatic prostate cancer cell lines. Cell Biol Toxicol.
26:553–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Kuiatse I, Lee AV, et al:
Sustained c-Jun-NH2-kinase activity promotes epithelial-mesenchymal
transition, invasion and survival of breast cancer cells by
regulating extracellular signal-regulated kinase activation. Mol
Cancer Res. 8:266–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tryndyak VP, Beland FA and Pogribny IP:
E-cadherin transcriptional down-regulation by epigenetic and
microRNA-200 family alterations is related to mesenchymal and
drug-resistant phenotypes in human breast cancer cells. Int J
Cancer. 126:2575–2583. 2010.PubMed/NCBI
|
|
9
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zavadil J and Böttinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 29:5764–5774.
2005. View Article : Google Scholar
|
|
11
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-kappaB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huber MA, Beug H and Wirth T:
Epithelial-mesenchymal transition: NF-kappaB takes center stage.
Cell Cycle. 3:1477–1480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huber MA, Azoitei N, Baumann B, et al:
NF-kappaB is essential for epithelial-mesenchymal transition and
metastasis in a model of breast cancer progression. J Clin Invest.
114:569–581. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial-mesenchymal
and mesenchymal-epithelial transitions in carcinoma progression. J
Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai J, Parr C, Watkins G, Jiang WG and
Boulton M: Decreased pigment epithelium-derived factor expression
in human breast cancer progression. Clin Cancer Res. 12:3510–3517.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou D, Cheng SQ, Ji HF, et al: Evaluation
of protein pigment epithelium-derived factor (PEDF) and microvessel
density (MVD) as prognostic indicators in breast cancer. J Cancer
Res Clin Oncol. 136:1719–1727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yasui N, Mori T, Morito D, et al:
Dual-site recognition of different extracellular matrix components
by antiangiogenic/neurotrophic serpin, PEDF. Biochemistry.
42:3160–3167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aurora AB, Biyashev D, Mirochnik Y, et al:
NF-kappaB balances vascular regression and angiogenesis via
chromatin remodeling and NFAT displacement. Blood. 116:475–484.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaichuk TA, Shroff EH, Emmanuel R, et al:
Nuclear factor of activated T cells balances angiogenesis
activation and inhibition. J Exp Med. 199:1513–1522. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoshina D, Abe R, Yamagishi SI and Shimizu
H: The role of PEDF in tumor growth and metastasis. Curr Mol Med.
10:292–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fitzgerald DP, Subramanian P, Deshpande M,
et al: Opposing effects of pigment epithelium-derived factor on
breast cancer cell versus neuronal survival: Implication for brain
metastasis and metastasis-induced brain damage. Cancer Res.
72:144–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manalo KB, Choong PF and Dass CR: Pigment
epithelium-derived factor as an impending therapeutic agent against
vascular epithelial growth factor-driven tumor-angiogenesis. Mol
Carcinog. 50:67–72. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
World Health Organization: World Health
Organization family of international classifications. The meeting
for heads of WHO collaborating centres for the family of
internation classifications (Cologne, Germany). October
19–25–2003.
|
|
24
|
Sobin LH and Wittekind C: TNM
Classification of Malignant Tumors (Fifth). New York, NY:
Wiley-Liss. 1997.
|
|
25
|
Elston EW and Ellis IO: Method for grading
breast cancer. J Clin Pathol. 46:189–190. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
27
|
Zhang M, Liu NY, Wang XE, et al: Activin B
promotes epithelial wound healing in vivo through Rhoa-JNK
signaling pathway. PLoS One. 6:e251432011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haybittle JL, Blamey RW, Elston CW, et al:
A prognostic index in primary breast cancer. Br J Cancer.
45:361–366. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Filleur S, Nelius T, de Riese W and
Kennedy RC: Characterization of PEDF: A multi-functional serpin
family protein. J Cell Biochem. 106:769–775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Setten GV and Abdiu O: Possible role of
pigment epithelium derived factor in angiogenesis. European
Opthalmic Rev. 10:64–67. 2009.
|
|
31
|
Kawaguchi T, Yamagishi SI and Sata M:
Structure-function relationships of PEDF. Curr Mol Med. 10:302–311.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Broadhead ML, Dass CR and Choong PF:
Cancer cell apoptotic pathways mediated by PEDF: Prospects for
therapy. Trends Mol Med. 15:461–467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jan R, Huang M and Lewis-Wambi J: Loss of
pigment epithelium-derived factor: A novel mechanism for the
development of endocrine resistance in breast cancer. Breast Cancer
Res. 14:R1462012. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Uehara H, Miyamoto M, Kato K, et al:
Expression of pigment epithelium-derived factor decreases liver
metastasis and correlates with favorable prognosis for patients
with ductal pancreatic adenocarcinoma. Cancer Res. 64:3533–3537.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guan M, Yam HF, Su B, et al: Loss of
pigment epithelium derived factor expression in glioma progression.
J Clin Pathol. 56:277–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheung LW, Au SC, Cheung AN, et al:
Pigment epithelium-derived factor is estrogen sensitive and
inhibits the growth of human ovarian cancer and ovarian surface
epithelial cells. Endocrinology. 147:4179–4191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ning Q, Liu C, Hou L, et al: Vascular
endothelial growth factor receptor-1 activation promotes migration
and invasion of breast cancer cells through epithelial-mesenchymal
transition. PLoS One. 8:e652172013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Seki R, Yamagishi S, Matsui T, et al:
Pigment epithelium-derived factor (PEDF) inhibits survival and
proliferation of VEGF-exposed multiple myeloma cells through its
anti-oxidative properties. Biochem Biophys Res Commun. 431:693–697.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang J, Chen S, Huang X, et al: Growth
suppression of cervical carcinoma by pigment epithelium-derived
factor via anti-angiogenesis. Cancer Biol Ther. 9:967–974. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Han J, Yang X, et al: Pigment
epithelium-derived factor inhibits angiogenesis and growth of
gastric carcinoma by down-regulation of VEGF. Oncol Rep.
26:681–686. 2011.PubMed/NCBI
|
|
41
|
Cai J, Jiang WG, Grant MB and Boulton M:
Pigment epithelium-derived factor inhibits angiogenesis via
regulated intracellular proteolysis of vascular endothelial growth
factor receptor 1. J Biol Chem. 281:3604–3613. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kokkinos MI, Wafai R, Wong MK, et al:
Vimentin and epithelial-mesenchymal transition in human breast
cancer-observations in vitro and in vivo. Cells Tissues Organs.
185:191–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee J, Hahm ER, Marcus AI and Singh SV:
Withaferin A inhibits experimental epithelial-mesenchymal
transition in MCF-10A cells and suppresses vimentin protein level
in vivo in breast tumors. Mol Carcinog. 30:102013.
|
|
44
|
Blanco MJ, Moreno-Bueno G, Sarrio D, et
al: Correlation of Snail expression with histological grade and
lymph node status in breast carcinomas. Oncogene. 21:3241–3246.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Luo WR, Li SY, Cai LM and Yao KT: High
expression of nuclear Snail, but not cytoplasmic staining, predicts
poor survival in nasopharyngeal carcinoma. Ann Surg Oncol.
19:2971–2979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alkatout I, Wiedermann M, Bauer M, et al:
Transcription factors associated with epithelial-mesenchymal
transition and cancer stem cells in the tumor centre and margin of
invasive breast cancer. Exp Mol Pathol. 94:168–173. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hirsch J, Johnson CL, Nelius T, Kennedy R,
Riese WD and Filleur S: PEDF inhibits IL8 production in prostate
cancer cells through PEDF receptor/phospholipase A2 and regulation
of NFκB and PPARγ. Cytokine. 55:202–210. 2011. View Article : Google Scholar : PubMed/NCBI
|