Introduction
Ovarian cancer is the most common invasive
malignancy of the female genital tract in the USA, with an
estimated 22,240 cases diagnosed annually. Approximately 14,030
females succumb every year to ovarian cancer, representing the most
common cause of mortality among females with gynecological
malignancies (1). Surgical resection
and platinum-based combination regimens offer a modest but
significant survival advantage in ovarian cancer patients with
advanced or metastatic disease, although most patients eventually
experience disease progression (2).
These data highlight the need to identify new approaches that,
along with the current treatments, may assist in bringing about a
better outcome for ovarian cancer patients.
Protein kinases and phosphatases work together to
control cellular processes and signaling pathways (3,4). Although
much more is known about protein kinases and their relevant
substrates compared with protein phosphatases (5–7), the
significance of studying protein phosphatase enzymes and their
targets has been demonstrated for disease states attributed in part
to malfunctioning protein phosphatase enzymes (8). Protein phosphatase 5 (PP5; gene name,
PPP5C) is a ubiquitously expressed serine/threonine protein
phosphatase related to PP1, PP2A and PP2B (9). Structural analysis has revealed that PP5
contains a C-terminal catalytic domain and three N-terminal
tetratricopeptide repeats (TPRs) that are unique in the
phosphoprotein phosphatase family (10). PP5 is auto-inhibited by intramolecular
interactions with its TPR domain (11).
PP5 has been implicated in numerous cellular
processes, including MAPK-mediated growth and differentiation
(12), cell cycle arrest and DNA
damage repair via the p53, ataxia telangiectasia mutated (ATM) and
ATM and Rad3-related (ATR) pathways (13,14),
regulation of ion channels via the membrane receptor for atrial
natriuretic peptide (15), cellular
heat shock response as mediated by the heat shock transcription
factor, and steroid receptor signaling, in particular
glucocorticoid receptor (16,17). Notably, another steroid, estradiol,
upregulates PP5 expression in MCF-7 breast cancer cells (18). PP5 was observed to promote
proliferation of breast cancer cells and growth of tumors in a
mouse xenograph model (19). Until
now, the issue of whether PP5 is involved in other types of
gynecological cancer, including ovarian cancer, has been unclear.
Therefore, this study was designed to investigate the the
biological role of PP5 in human ovarian cancer. Herein, we
successfully silenced PPP5C mRNA and protein expression in
CAOV-3 ovarian cancer cells using RNA interference (RNAi)
technology. Functional analysis revealed that PP5 knockdown
significantly inhibited the proliferation and colony formation of
CAOV-3 ovarian cancer cells, as well as G0/G1 phase cell cycle
arrest. This study provides new evidence that PP5 plays a
significant role in ovarian cancer development.
Materials and methods
Cell lines and reagents
The human ovarian mucinous adenocarcinoma cancer
cell line CAOV-3 and the human embryonic kidney cell line 293T were
obtained from the Cell Bank of the Chinese Academy of Science
(Shanghai, China). The CAOV-3 cell line was cultured in RPMI-1640
(Hyclone, Pittsburgh, PA, USA) supplemented with 10% fetal bovine
serum (FBS; Biowest, Nuaillé, France) at 37°C with 5%
CO2. The HEK293T cell line was cultured in Dulbecco's
modified Eagle's medium (Hyclone) with 10% FBS at 37°C with 5%
CO2. Short hairpin RNA (shRNA) expression vector pFH-L
and helper plasmids pVSVG-I and pCMVΔR8.92 were purchased from
Shanghai Hollybio (Shanghai, China). Lipofectamine 2000 and TRIzol
were purchased from Invitrogen Life Technologies (Carlsbad, CA,
USA). M-MLV reverse transcriptase was purchased from Promega
(Madison, WI, USA). AgeI, EcoRI and SYBR-Green master
mix kits were purchased from Takara (Dalian, China). All other
chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
The antibodies used were as follows: anti-PP5 (1:3,000 dilution;
Abcam, Cambridge, UK), anti-GAPDH (1:10,000 dilution; Proteintech
Group, Inc., Chicago, IL, USA), and anti-rabbit horseradish
peroxidase (HRP; 1:5,000 dilution; Santa Cruz Biotechnology, Inc.
Dallas, TX, USA).
Construction of PPP5C shRNA containing
lentivirus and transduction into ovarian cancer cells
CAOV-3 cells were transduced with PPP5C shRNA
following the manufacturer's instructions. To create a PPP5C
shRNA-silenced sub-cell line, we used the following shRNA sequence
designed against the PPP5C gene: 5′-GAGACAGAGAAGATTACAGTACTCGAG
TACTGTAATCTTCTCTGTCTCTTTTT-3′. The control shRNA sequence was
5′-GCGGAGGGTTTGAAAGAATAT CTCGAGATATTCTTTCAAACCCTCCGCTTTTTT-3′. Each
nucleotide sequence was inserted into the pFH-L shRNA expressing
vector. Lentiviruses were generated by triple transfection of 80%
confluent 293T cells with modified pFH-L plasmid and pVSVG-I and
pCMVΔR8.92 helper plasmids using Lipofectamine 2000 according to
the manufacturer's instructions. Then the lentiviral particles were
harvested by ultra-centrifugation (4,000 × g at 4°C) for 10 min,
filtered through a 45-µm filter and centrifuged again (4,000 × g at
4°C) for 15 min.
For cell infection, CAOV-3 cells were seeded in a
volume of 2 ml at a density of 5×104 cells/well in
six-well plates and transduced with the constructed lentiviruses
containing PP5 shRNA (shPPP5C) and non-silencing shRNA (shCon) at a
multiplicity of infection of 20. The infection efficiency was
observed after 96 h through a fluorescence microscope for the green
fluorescence protein expression.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from cells using TRIzol
reagent and synthesized into cDNA by M-MLV reverse transcriptase
according to the manufacturer's instructions. qPCR was performed on
a Connect real-time PCR platform (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) using a SYBR-Green master mix kit. In brief,
each PCR reaction mixture containing 10 µl 2X SYBR Premix Ex Taq,
0.8 µl sense and antisense primers (2.5 µM), 5 µl cDNA and 4.2 µl
ddH2O was run for 40 cycles with initial denaturation at
95°C for 1 min, denaturation at 95°C for 5 sec and extension at
60°C for 20 sec. β-actin was used as an internal control. Relative
gene expression levels were calculated using 2−ΔΔCT
analysis. The primers were as follows: PPP5C (forward),
5′-CCCAACTACTGCGACCAGAT-3′; PPP5C (reverse),
5′-CCCGTCACCTCACATCATTC-3′; β-actin (forward),
5′-GTGGACATCCGCAAAGAC-3′; β-actin (reverse),
5′-AAAGGGTGTAACGCAACTA-3′.
Western blot analysis
CAOV-3 cells were collected 7 days after lentivirus
infection and lysed in 2X sodium dodecyl sulphate (SDS) sample
buffer [100 mM Tris-HCl (pH 6.8), 10 mM EDTA, 4% SDS and 10%
glycine). The protein content was measured using the Lowry method.
To detect target proteins, equal amounts of protein samples were
separated by SDS-polyacrylamide gel electrophoresis and transferred
to polyvinylidenedifluoride membranes. The membranes were incubated
with Tris-buffered saline and Tween-20 (TBST; 25 mM Tris, pH 7.4,
150 mM NaCl and 0.1% Tween-20) containing 5% nonfat dry milk at
room temperature for 1 h. After washing with TBST three times, the
membranes were probed with the primary antibody (anti-PP5 rabbit
mAb or anti-GAPDH rabbit mAb) at 4°C overnight followed by
incubation with goat anti-rabbit IgG, HRP-linked antibody for 1 h
at room temperature. The blots were detected with an enhanced
chemiluminescence detection kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA) according to the manufacturer's instructions.
GAPDH was used as the reference control.
Cell viability assay
Following lentivirus infection, CAOV-3 cells were
seeded in a volume of 200 µl at a density of 4×103
cells/well in 96-well plates. Following incubation for 1, 2, 3, 4
and 5 days, respectively, 20 µl 3-(4,5-dimethyl
thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 5.0 mg/ml) was
added into each well and incubated with cells for 4 h. Then 100 µl
acidic isopropanol (10% SDS, 5% isopropanol and 0.01 mol/l HCl) was
added to each well after removing the medium and MTT from the
wells. The absorbance was measured using a microplate reader
(BioTek, Winooski, VT, USA) at 595 nm.
Colony formation assay
Following lentivirus infection, CAOV-3 cells were
were seeded in a volume of 2 ml at a density of 1.5×103
cells/well in six-well plates. The medium was changed every three
days. After nine days of culture, the cells were washed with
phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde.
The fixed cells were stained purple with freshly prepared crystal
violet staining (Sigma-Aldrich) for 20 min. The colony formation
was observed through a light/fluorescence microscope to obtain the
colony numbers.
Cell cycle analysis
The cell cycle distribution was analyzed by flow
cytometry using propidium iodide (PI) staining. Following
lentivirus infection for 6 days, CAOV-3 cells were seeded in a
volume of 5 ml at a density of 2×105 cells/well in 6-cm
dishes. Cells were harvested and fixed in 70% ice-cold ethanol for
12 h at 4°C. After washing with PBS three times, cells were stained
for DNA content by use of 300 µl PBS containing 50 µg/ml PI and 50
µg/ml preboiled RNase A. The suspension was incubated in the dark
at room temperature for 30 min and then subjected to FACSCalibur
flow cytometry (BD Biosciences, San Jose, CA, USA). Data were
analyzed with the ModFit DNA analysis program (Verity Software
House, Maine, USA).
Statistical analysis
Data were presented as the mean ± standard deviation
from at least three independent experiments. Statistical analysis
was performed using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Lentivirus-mediated RNAi suppresses
PP5 expression in CAOV-3 ovarian cancer cells
To examine the function of PP5 in ovarian cancer
cells, we firstly applied lentivirus-mediated RNAi to specifically
suppress PPP5C in the ovarian cancer cell line CAOV-3 long term. As
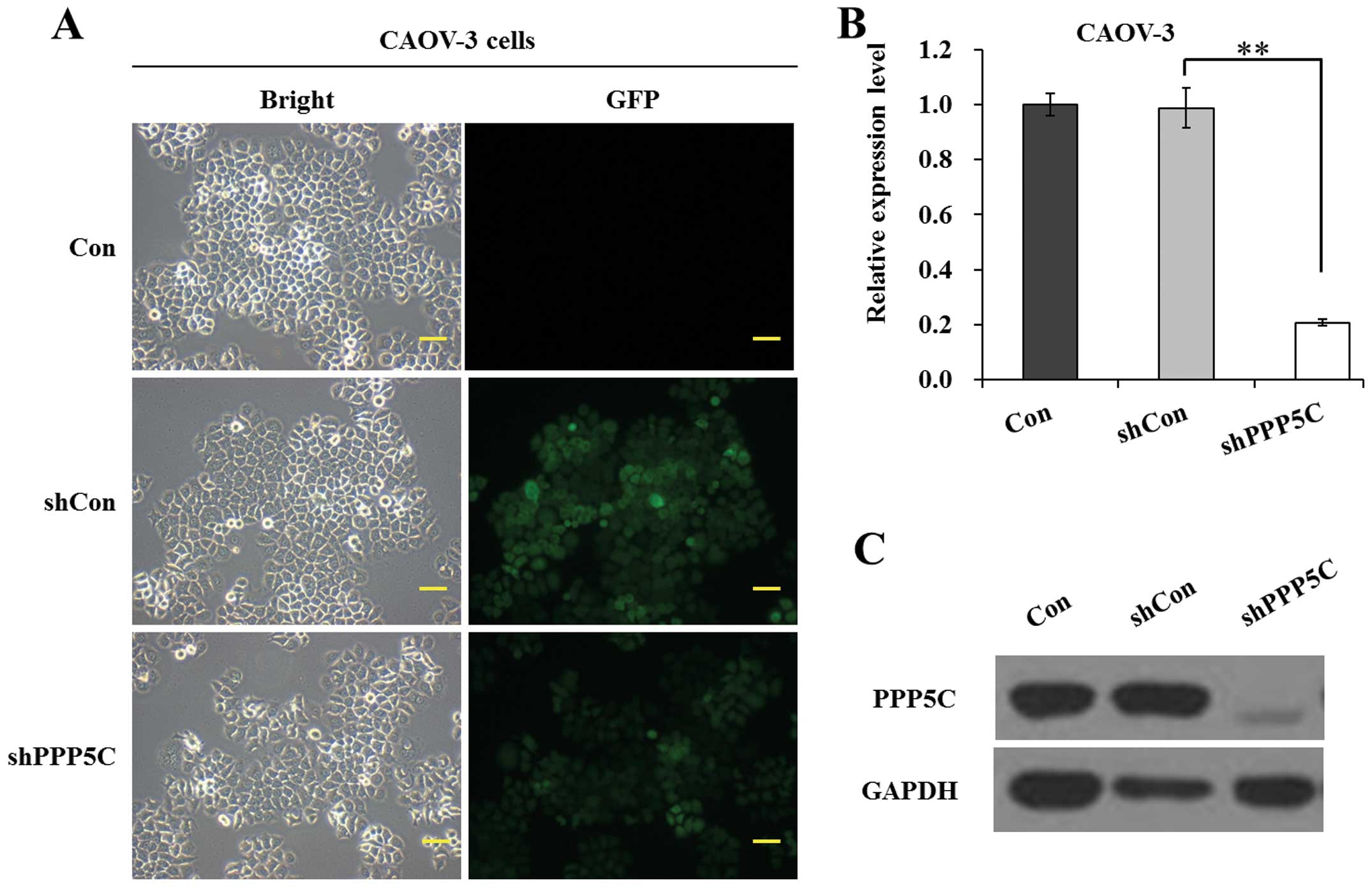
shown in Fig. 1A, the ratio of cells
with green fluorescence protein (GFP) expression to shRNA-treated
cells was >80%, indicating a satisfactory infection efficiency.
As shown in Fig. 1B, the mRNA level
of PPP5C was significantly (P<0.01) reduced in shPPP5C-treated
cells compared with non-treated and shCon-treated cells. The
knockdown efficiency of PP5 was calculated as 79.0% in CAOV-3
cells. Moreover, the protein level of PP5 was significantly
downregulated in shPPP5C-treated cells compared with non-treated
and shCon-treated cells (Fig. 1C).
The data indicated that the lentivirus-mediated shRNA silencing
efficiently suppressed the expression of endogenous PP5 in CAOV-3
ovarian cancer cells.
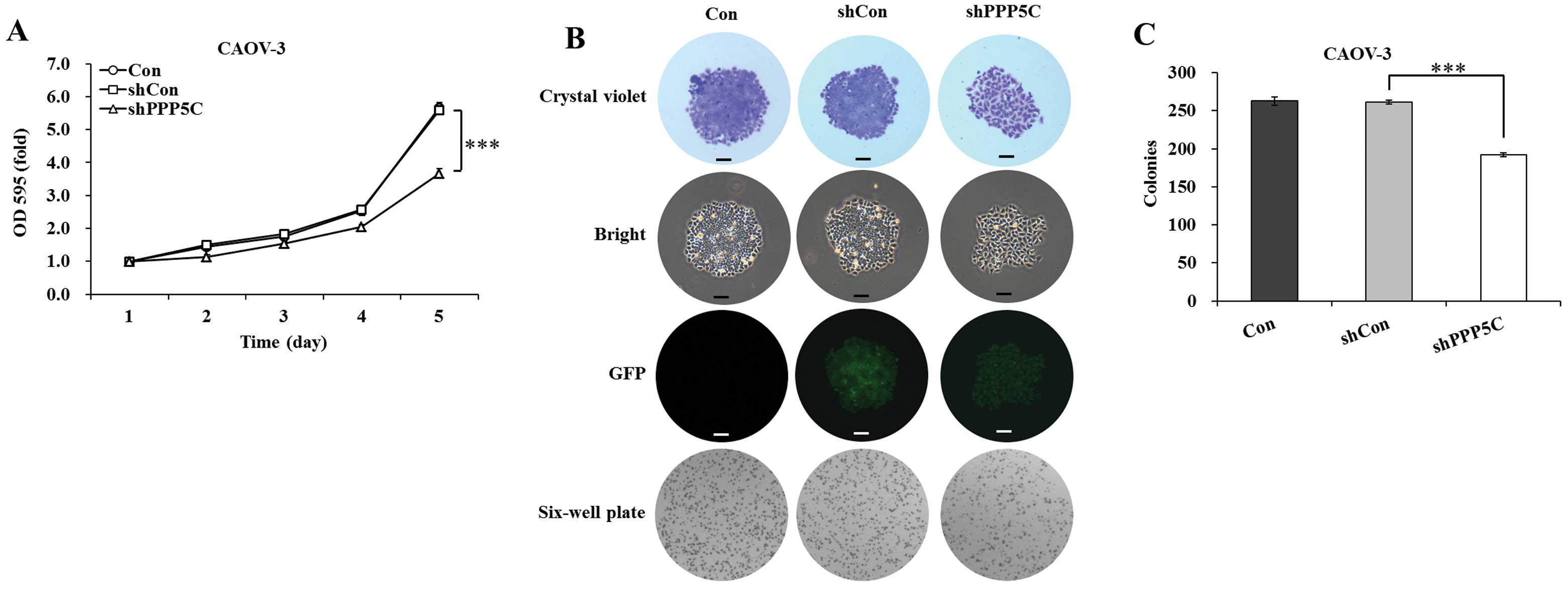
PP5 knockdown inhibits the proliferation and colony
formation of CAOV-3 ovarian cancer cells. The effect of PP5
silencing on the proliferation of CAOV-3 cells was determined by
MTT assay. The cell viability was observed for 5 days in
non-treated, shCon-treated and shPPP5C-treated cells. As shown in
Fig. 2A, the growth curve of
shPPP5C-treated cells started to drop from the second day, compared
with that of non-treated and shCon-treated cells. The decline
reached 34.4% (P<0.001) on the fifth day, compared with
shCon-treated cells. There was no difference with regard to cell
viability between non-treated and shCon-treated cells. The data
indicated that PP5 knockdown significantly inhibited the
proliferation of CAOV-3 ovarian cancer cells.
The long-term effect of PP5 silencing on the colony
forming ability of CAOV-3 cells was determined by colony formation
assay. As shown in Fig. 2B, the size
of independent colonies was much smaller in shPPP5C-treated cells
compared with non-treated and shCon-treated cells. Moreover, the
number of colonies formed in CAOV-3 cells was significantly
(P<0.001) decreased following PP5 knockdown (Fig. 2C). The data indicated that PP5
knockdown also significantly inhibited the colony formation of
CAOV-3 ovarian cancer cells.
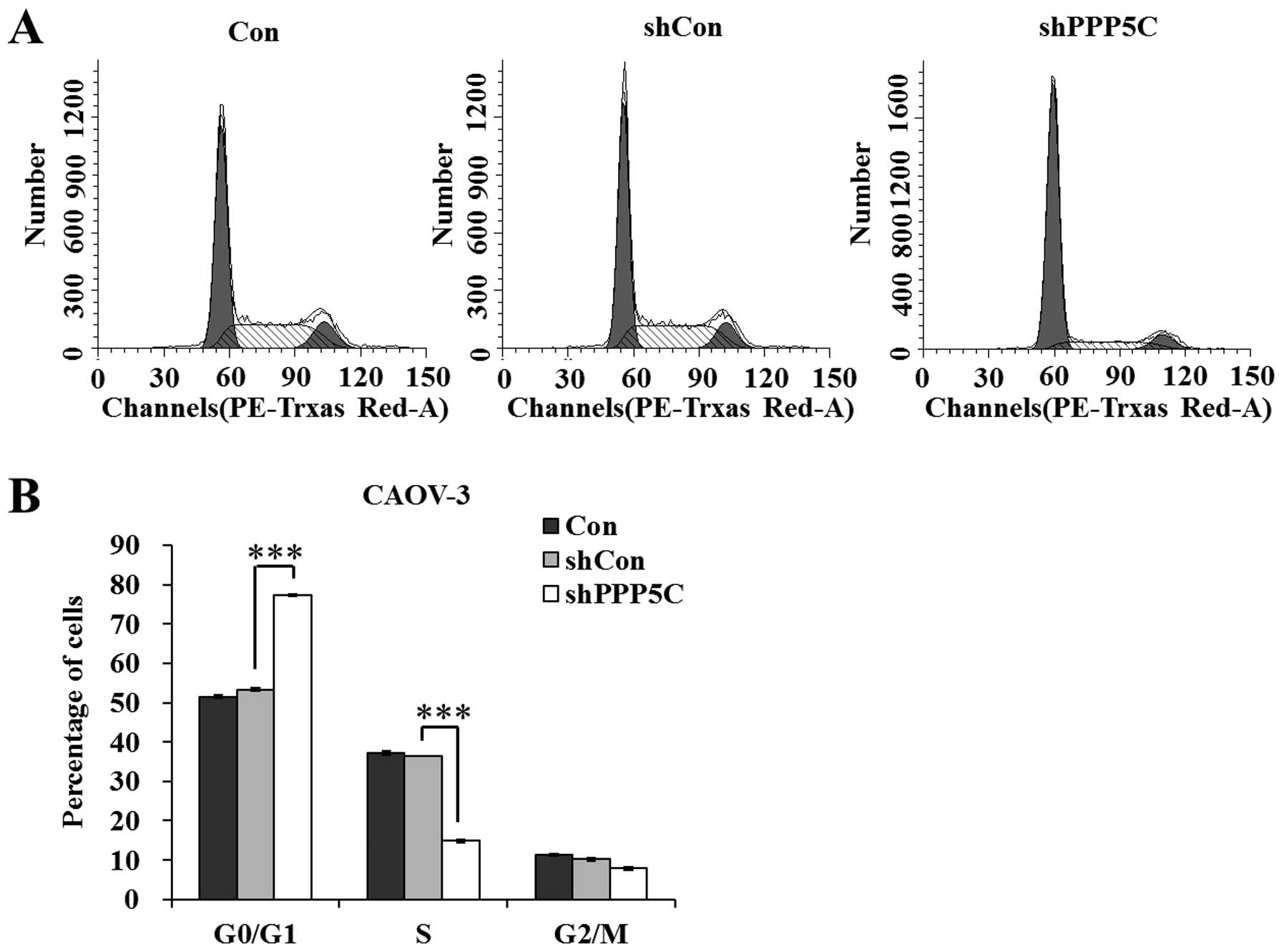
PP5 knockdown arrests CAOV-3 cells at
the G0/G1 phase
To investigate the mechanisms underlying the growth
suppression effect of PP5 knockdown, the cell cycle distribution of
CAOV-3 cells was analyzed using a flow cytometer. The results shown
in Fig. 3 indicate that
shPPP5C-treated cells presented an increased G0/G1-phase population
and a decreased S-phase population (P<0.001), compared with
non-treated and shCon-treated cells. The data revealed that PP5
knockdown arrested the cell cycle at the G0/G1 phase. Taken
together, we suggest that PP5 knockdown suppresses ovarian cancer
cell growth via the blockade of cell cycle progression.
Discussion
Ovarian cancer is the leading cause of mortality
from gynecological cancers and the fifth most common cause of
cancer mortality among females (20).
An increased understanding of the molecular and genetic changes
causing ovarian cancer progression is likely to produce strategies
to predict and prevent the occurrence of refractory disease.
Targeted therapy is a type of medication that blocks the growth of
cancer cells by interfering with the specific molecules needed for
carcinogenesis and tumor growth, rather than interfering with all
rapidly dividing cells. In this study, PP5 was identified as a
specific molecule that drives ovarian cancer progression. Using
lentivirus-mediated shRNA silencing, we potently suppressed the
expression of PPP5C at the mRNA and protein levels in CAOV-3
ovarian cancer cells. PP5 knockdown significantly inhibited the
proliferation and colony formation of CAOV-3 cells.
A previous study has reported that treatment of
cells with PP5 antisense RNA leads to hyperphosphorylation of p53
and subsequent G1 growth arrest (21). Herein, PI staining combined with flow
cytometry analysis was performed to determine whether PP5 knockdown
using lentivirus-mediated RNAi blocks cell cycle progression in
CAOV-3 cells. As expected, PP5 knockdown significantly arrested
CAOV-3 cells at the G0/G1 phase, which was in accordance with the
growth suppression effect of PP5 knockdown.
Finally, it is known that PP5 plays a crucial role
in DNA damage repair and cell cycle arrest by attenuating the
activities of ATM and ATR, two closely related checkpoint kinases.
A more recent study utilizing cells from PP5-deficient mice has
confirmed the role of PP5 in ATM signaling (22). Thus, further study is desired to
examine whether PP5 attenuates the checkpoint kinases ATM and ATR
in ovarian cancer cells. Furthermore, PP5 has been observed to act
as a suppressor of apoptosis signal-regulating kinase 1 (23,24), p53
(25) and DNA-dependent protein
kinase catalytic subunits (26). It
is likely that PP5 promotes ovarian cancer cell growth by inducing
cell cycle arrest, as well as cell apoptosis. Therefore, the effect
of PP5 knockdown on the apoptosis of ovarian cancer cells should
also be determined in a subsequent study.
In conclusion, this study highlights the crucial
role of PP5 in promoting ovarian cancer cell proliferation and
demonstrates that silencing PP5 with targeted shRNA delivered to
tumor cells via lentivirus is an effective method to reduce PP5
activity in the long term. These results provide a foundation for
further study into the clinical potential of lentiviral-mediated
delivery of PP5 RNAi therapy for the treatment of ovarian
cancer.
Acknowledgements
The authors are grateful for the support of the
Natural Science Foundation of Ning Bo (201301A6110080 and
201301A6110087).
Glossary
Abbreviations
Abbreviations:
|
PP5
|
protein phosphatase 5
|
|
RNAi
|
RNA interference
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
PI
|
propidium iodide
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu Y, Zhang M, Zhang X, Cai Q, Hong S,
Jiang W and Xu C: Synergistic effects of combined
platelet-activating factor receptor and epidermal growth factor
receptor targeting in ovarian cancer cells. J Hematol Oncol.
7:392014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chalmers MJ, Kolch W, Emmett MR, Marshall
AG and Mischak H: Identification and analysis of phosphopeptides. J
Chromatogr B Analyt Technol Biomed Life Sci. 803:111–120. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olsen JV, Blagoev B, Gnad F, Macek B,
Kumar C, Mortensen P and Mann M: Global, in vivo and site-specific
phosphorylation dynamics in signaling networks. Cell. 127:635–648.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ballif BA, Roux PP, Gerber SA, MacKeigan
JP, Blenis J and Gygi SP: Quantitative phosphorylation profiling of
the ERK/p90 ribosomal S6 kinase-signaling cassette and its targets,
the tuberous sclerosis tumor suppressors. Proc Natl Acad Sci USA.
102:667–672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoffert JD, Pisitkun T, Wang G, Shen RF
and Knepper MA: Quantitative phosphoproteomics of
vasopressin-sensitive renal cells: Regulation of aquaporin-2
phosphorylation at two sites. Proc Natl Acad Sci USA.
103:7159–7164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang F, Stenoien DL, Strittmatter EF, Wang
J, Ding L, Lipton MS, Monroe ME, Nicora CD, Gristenko MA, Tang K,
et al: Phosphoproteome profiling of human skin fibroblast cells in
response to low- and high-dose irradiation. J Proteome Res.
5:1252–1260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ham BM, Jayachandran H, Yang F, Jaitly N,
Polpitiya AD, Monroe ME, Wang L, Zhao R, Purvine SO, Livesay EA, et
al: Novel Ser/Thr protein phosphatase 5 (PP5) regulated targets
during DNA damage identified by proteomics analysis. J Proteome
Res. 9:945–953. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamaguchi F, Umeda Y, Shimamoto S,
Tsuchiya M, Tokumitsu H, Tokuda M and Kobayashi R: S100 proteins
modulate protein phosphatase 5 function: a link between CA2+ signal
transduction and protein dephosphorylation. J Biol Chem.
287:13787–13798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen MX, McPartlin AE, Brown L, Chen YH,
Barker HM and Cohen PT: A novel human protein serine/threonine
phosphatase, which possesses four tetratricopeptide repeat motifs
and localizes to the nucleus. EMBO J. 13:4278–4290. 1994.PubMed/NCBI
|
|
11
|
Connarn JN, Assimon VA, Reed RA, Tse E,
Southworth DR, Zuiderweg ER, Gestwicki JE and Sun D: The molecular
chaperone Hsp70 activates protein phosphatase 5 (PP5) by binding
the tetratricopeptide repeat (TPR) domain. J Biol Chem.
289:2908–2917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
von Kriegsheim A, Pitt A, Grindlay GJ,
Kolch W and Dhillon AS: Regulation of the Raf-MEK-ERK pathway by
protein phosphatase 5. Nat Cell Biol. 8:1011–1016. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zuo Z, Urban G, Scammell JG, Dean NM,
McLean TK, Aragon I and Honkanen RE: Ser/Thr protein phosphatase
type 5 (PP5) is a negative regulator of glucocorticoid
receptor-mediated growth arrest. Biochemistry. 38:8849–8857. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zuo Z, Dean NM and Honkanen RE:
Serine/threonine protein phosphatase type 5 acts upstream of p53 to
regulate the induction of p21(WAF1/Cip1) and mediate growth arrest.
J Biol Chem. 273:12250–12258. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chinkers M: Targeting of a distinctive
protein-serine phosphatase to the protein kinase-like domain of the
atrial natriuretic peptide receptor. Proc Natl Acad Sci USA.
91:11075–11079. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Silverstein AM, Galigniana MD, Chen MS,
Owens-Grillo JK, Chinkers M and Pratt WB: Protein phosphatase 5 is
a major component of glucocorticoid receptor hsp90 complexes with
properties of an FK506-binding immunophilin. J Biol Chem.
272:16224–16230. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Davies TH, Ning YM and Sánchez ER:
Differential control of glucocorticoid receptor hormone-binding
function by tetratricopeptide repeat (TPR) proteins and the
immunosuppressive ligand FK506. Biochemistry. 44:2030–2038. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Urban G, Golden T, Aragon IV, Scammell JG,
Dean NM and Honkanen RE: Identification of an estrogen-inducible
phosphatase (PP5) that converts MCF-7 human breast carcinoma cells
into an estrogen-independent phenotype when expressed
constitutively. J Biol Chem. 276:27638–27646. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Golden T, Aragon IV, Zhou G, Cooper SR,
Dean NM and Honkanen RE: Constitutive over expression of
serine/threonine protein phosphatase 5 (PP5) augments
estrogen-dependent tumor growth in mice. Cancer Lett. 215:95–100.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ali MW, Cacan E, Liu Y, Pierce JY,
Creasman WT, Murph MM, Govindarajan R, Eblen ST, Greer SF and Hooks
SB: Transcriptional suppression, DNA methylation and histone
deacetylation of the regulator of G-protein signaling 10 (RGS10)
gene in ovarian cancer cells. PloS one. 8:e601852013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chinkers M: Protein phosphatase 5 in
signal transduction. Trends Endocrinol Metab. 12:28–32. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yong W, Bao S, Chen H, Li D, Sánchez ER
and Shou W: Mice lacking protein phosphatase 5 are defective in
ataxia telangiectasia mutated (ATM)-mediated cell cycle arrest. J
Biol Chem. 282:14690–14694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou G, Golden T, Aragon IV and Honkanen
RE: Ser/Thr protein phosphatase 5 inactivates hypoxia-induced
activation of an apoptosis signal-regulating kinase 1/MKK-4/JNK
signaling cascade. J Biol Chem. 279:46595–46605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang S, Shu L, Easton J, Harwood FC,
Germain GS, Ichijo H and Houghton PJ: Inhibition of mammalian
target of rapamycin activates apoptosis signal-regulating kinase 1
signaling by suppressing protein phosphatase 5 activity. J Biol
Chem. 279:36490–36496. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Urban G, Golden T, Aragon IV, Cowsert L,
Cooper SR, Dean NM and Honkanen RE: Identification of a functional
link for the p53 tumor suppressor protein in dexamethasone-induced
growth suppression. J Biol Chem. 278:9747–9753. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wechsler T, Chen BP, Harper R,
Morotomi-Yano K, Huang BC, Meek K, Cleaver JE, Chen DJ and Wabl M:
DNA-PKcs function regulated specifically by protein phosphatase 5.
Proc Natl Acad Sci USA. 101:1247–1252. 2004. View Article : Google Scholar : PubMed/NCBI
|