Introduction
Peripheral primitive neuroectodermal tumor (PNET) is
a type of soft tissue sarcoma, described as arising intracranially
(1,2).
PNETs of the chest wall were originally reported by Askin et
al in 1979 (3); since then, a
PNET that occurs within the thoracopulmonary region is named an
Askin's tumor. Askin's tumor has been reported to primarily occur
in children and young adults (3). In
addition, it is a highly misdiagnosed and rare disease with a lack
of clinical and pathological morphology in nature, which is easily
confused with other small round-cell tumors (3). Given the rarity of this disease, no
regimen has been validated for its treatment. The present study
aimed to evaluate the clinical characteristics, prognostic factors
and treatment outcomes of 11 cases of Askin's tumor, using clinical
data as well as histopathological and immunohistochemical
analysis.
Materials and methods
Patient's description
In the present study, a retrospective analysis was
performed of 11 cases of pathological Askin's tumor. Patients were
treated at the First Affiliated Hospital of Zhengzhou University
between April 2010 and June 2013. These patients included three
males and eight females, with a mean age of 14.5 years old and a
range of 8 to 22 years old. All the patients were pathologically
confirmed and the stages were recorded as follows: Stage I–III, six
cases; stage IV, two cases; right pleural huge Askin's tumor (stage
III), one case, and left side of the chest wall Askin's tumor stage
II, two cases. This classification adopted the staging of soft
tissue sarcoma established by American Joint Committee on Cancer in
2010 (4). The present study was
approved by the ethnics committee of the First Affiliated Hospital
of Zhengzhou University, Henan, China.
Diagnostics
All patients were admitted to the First Affiliated
Hospital of Zhengzhou University with different degrees of
symptoms, including fever, cough with progressive increase of chest
pain, chest tightness and shortness of breath, certain patients
presented with chest pain and chest wall tumor progressive
enlargement, among other symptoms. Following admission, patients
underwent chest computed tomography (CT) examination. Postoperative
specimens obtained by biopsy embedded in paraffin were sliced into
sections and underwent hematoxylin-eosin (HE) staining, periodic
acid-Schiff staining and immunohistochemistry with antibodies for
CD99, vimentin, neuron-specific enolase (NSE), S-100, creatine
kinase (CK), leucocyte common antigen (LCA), myoglobin were
performed. Lactate dehydrogenase (LDH) levels were also
detected.
Treatment and follow-up
Eight patients underwent treatment with combined
therapy, of which five underwent radical surgery with
chemotherapy-radiotherapy without recurrence and three cases
suffered local recurrence at 3–6 months and underwent a second
surgery and post-operative adjuvant chemotherapy. These three
patients underwent a second surgery and postoperative adjuvant
chemotherapy. One patient only received radiotherapy, one received
chemotherapy and one patient with chemotherapy-radiotherapy.
Radiotherapy was performed using a 6 MV-X-ray, 3
dimensional-intensity-modulated radiation therapy dose of tumor
(DT) at 40–50 Gy/20-25 fractions. Cyclophosphamide (300
mg/m2), adriamycin (10 mg/m2) and vincristine
(2 mg/m2; CAV) combination chemotherapy was used for
initial chemotherapy and topotecan and cisplatin (TP) combination
was used for the secondary chemotherapy following recurrence. All
patients were followed up for 6–24 months following treatment. The
Response Evaluation Criteria in Solid Tumors (1.1) assessment
criteria for physical tumor efficacy of 2009 was used to evaluate
patients (5).
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for all statistical analysis. The Kaplan-Meier method was
applied to calculate survival, Log-rank inspection. P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
Clinical features
In the present study, the common clinical symptoms
of Askin's tumor include fever, cough, chest pain and suffocation,
although it may present as chest lumps, swelling and pain. Certain
other signs, such as short-term sickness, rapid development and
plethora metastasis as well as bone and lung metastasis, were also
observed. Radiographic examinations revealed that the lung, pleural
or chest wall do not only have larger inhomogeneous, lobulated and
soft tissue masses that occasionally presented with with
hemorrhage, necrosis, cystic degeneration and calcification of
small needle-like tissue masses, but also have overspreading,
proliferating and invasive mediastinal tissues, vertebrae or
ribs.
In the present study, nine cases presented with the
symptoms of fever, cough with chest pain and suffocation, while two
cases presented with a mass in the chest and throat. Short-term
symptoms progressed rapidly, with an average of 10–20 days. The
range of Askin's tumor diameters of the seven cases was 5–12.5 cm,
as determined through CT prior to surgery. For the nine patients in
which metastasis occurred, one case had pulmonary infiltrates or
pulmonary metastasis and one case demonstrated clavicle and
mediastinal lymph node metastasis. A further two cases were
accompanied by pathological damage to the ribs and another two
cases had pleural effusion. As shown in Fig. 1A–C, the patient with clavicle and
mediastinal lymph node metastasis in the right upper lobe showed a
lobulated mass of 5.64×5.69 cm with no enhancement (Fig. 1A–C). In addition, surrounding the
tumor were multiple heterogeneous enhancements of irregular-shaped
nodular soft tissue in the right upper lung field (Fig. 1A–C). Furthermore, CT scans from
another case demonstrated a soft-tissue density mass of
12.5×9.5×8.3 cm in the right rib cage with partial destruction of
the seventh rib and pleural effusion (Fig. 1D–F).
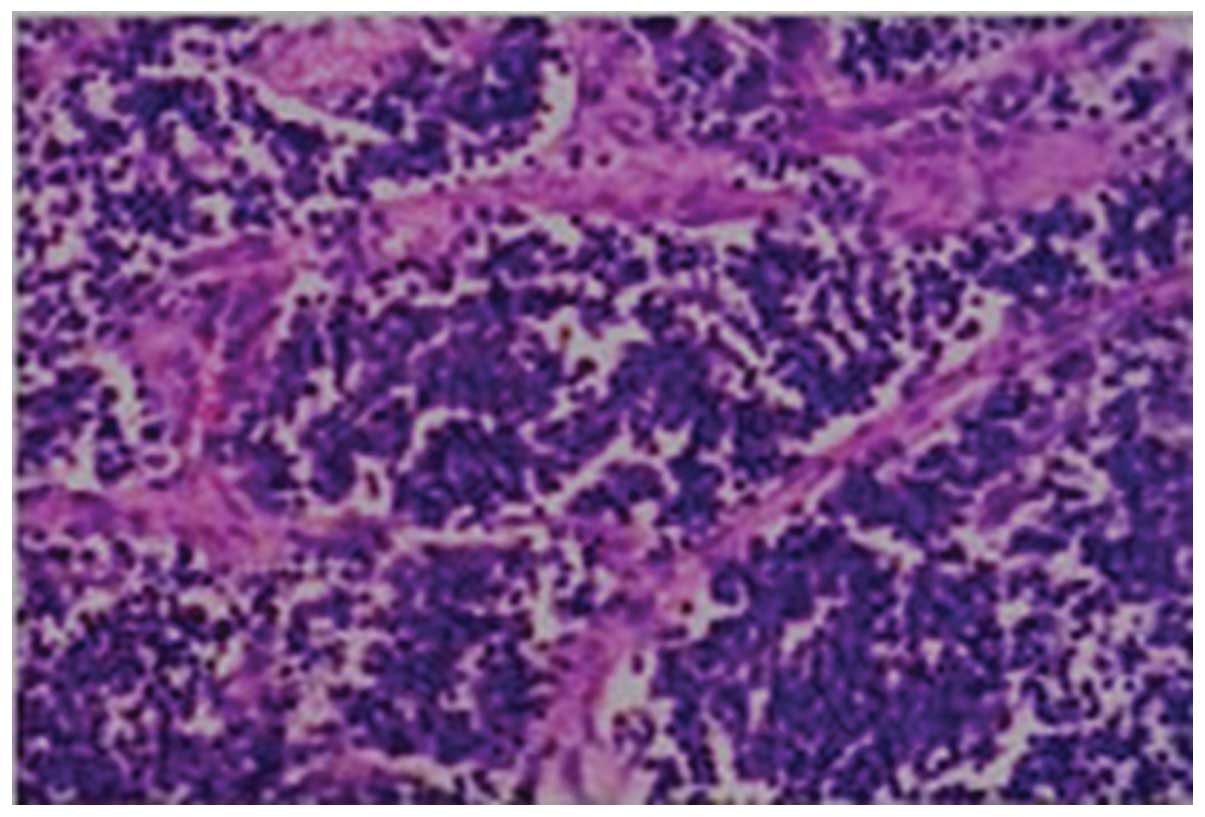
Through HE staining, the morphology of biopsy
specimens was observed under a microscope; the representative
specimen shown in Fig. 2 was
diagnosed as a single, small round-cell tumor, with neural or oval
cell differentiation tendencies (Fig.
2). Of note, mitosis was common among tumor cells, fibrous
stroma and blood vessels. Staining was present to a lesser degree
in the cytoplasm, whereas the nucleus exhibited intense staining.
The results of the immunohistochemical analysis showed that of the
11 cases, 10 (90.9%) cases were positive for CD99 and NSE
expression, while the other case was positive for NSE and S-100
expression. In addition, seven cases showed vimentin-positive
expression, with negative expression for CK and LCA. These results
were in accordance with the features previously reported for
Askin's tumor (3).
Treatment outcomes
During the follow-up period (6–24 months), the
median survival time for the combined treatment group
(surgery-chemotherapy-radiotherapy) and the single treatment group
(chemotherapy or radiotherapy) were 15 and 7 months, respectively.
Therefore, the survival ratio was significantly improved in the
combined treatment group compared with that of the single treatment
group (P<0.05).
Univariate analysis revealed that poor prognosis was
associated with a tumor diameter >5 cm, LDH levels >240 U/l
and late stage (stage III or IV) tumors. By contrast, the patient's
age, gender and tumor location were not correlated with prognosis
(Table I).
 | Table I.Patients with general information and
analysis of prognostic factors. |
Table I.
Patients with general information and
analysis of prognostic factors.
| Classification | No. of cases | Proportion, % | Median survival,
months | P-value |
|---|
| Gender |
|
|
| 0.306 |
| Male | 3 | 27.3 | 11 |
|
|
Female | 8 | 72.7 | 9 |
|
| Age, years |
|
|
| 0.412 |
| ≤10 | 5 | 45.5 | 8 |
|
|
>10 | 6 | 55.5 | 7 |
|
| Tumor size, cm |
|
|
| 0.793 |
| ≤5 | 7 | 63.6 | 5 |
|
|
>5 | 4 | 36.4 | 15 |
|
| Lactate
dehydrogenase, U/l |
|
|
| 0.032 |
|
80–240 | 8 | 72.7 | 12 |
|
| ≥240 | 3 | 27.3 | 8 |
|
| Metastasis |
|
|
| 0.000 |
| With
metastasis | 9 | 81.8 | 14 |
|
| Without
metastasis | 2 | 18.2 | 9 |
|
| Therapy |
|
|
| 0.000 |
|
Combined | 8 | 81.8 | 15 |
|
|
Mono-therapy | 2 | 18.2 | 7 |
|
Discussion
Askin's tumor was firstly reported by Askin et
al in 1979 (3). It was revealed
that Askin's tumor shares the characteristic chromosomal
translocations and has a comparable morphology,
immunohistochemistry and ultrastructure with classical peripheral
PNET and Ewing's tumor (1,2). According to classification of tumors of
the nervous system, Askin's tumor is a member of the Ewing's
sarcoma family of tumors/peripheral PNETs. The clinical occurrence
of Askin's tumor is rare and only a limited number of cases have
been documented (6–11). The present study reported 11 cases of
Askin's tumor, a relatively large sample size in terms of its
incidence rate. The characteristics and treatment strategies used
in the present study may therefore enhance current understanding
and aid future diagnosis and treatment of Askin's tumor.
Askin's tumor may occur at any age, although it is
more commonly found in children and young adults. This disease
primarily occurs in the soft tissue of the chest wall, rib
periosteum, chest and lung. Clinically, Askin's tumor commonly
presents with the symptoms of fever, cough, chest pain, chest
tightness and shortness of breath. However, symptoms may also
include a chest wall mass, pleural effusion, superficial lymph
nodes and rib fractures. Askin's tumor has the characteristics of a
high degree of malignancy, poor efficacy and easy local recurrence.
Vessel metastasis exists in the majority of cases; in addition,
bone and lung metastases often occur, although metastases may also
be transferred to brain, mediastinum, neck, mouth, liver and
adrenal gland. Imaging features have been demonstrated to aid the
preoperative stage diagnosis, the determination of the surgical
procedure and the evaluation of treatment efficacy; however,
imaging features lack specificity. Multiple magnetic resonance
imaging was reported to be able to determine the source of the
tumor and the scope of the chest wall invasion (12). However, CT scans appear to be more
useful for the diagnosis of Askin's tumor. Typical CT scan
observations for Askin's tumor include a large soft tissue mass
based on the signal chest wall with or without ipsilateral pleural
effusion and rib damage (9). Thus, in
the present study, CT scans were performed for all patients in
order to aid diagnosis.
Histopathology and immunohistochemical analysis are
the basis for Askin's tumor diagnosis. Cytologic smears of the
tumor revealed small round malignant cells with the typical feature
of the Homer-Wright rosettes, with various layers of cells with
fibrillary material (13,14). For immunohistochemical analysis, the
tumor was previously reported to be positive for several neural
markers including NSE, CD99 and vimentin (13,15).
Cellular and molecular genetics studies have suggested that PNET
has a characteristic chromosomal translocation t (11:22)(q24; q12),
which provides an additional firm diagnostic criterion for Askin's
tumor (16,17). In addition, the characteristics of the
disease phenotype were identified using immunohistochemical
analysis, which demonstrated positive expression of CD99, NSE,
S-100 and vimentin protein, while tests for CK, CgA, Syn, LCA,
TTF-1 provided negative results. These results are in accordance
with previous reports (3,16,17).
At present, combined treatment for Askin's tumor is
advised. The common procedure involves extensive resection of the
tumor and surrounding tissue invading into the ribs or lungs, as
well as regional lymph node dissection, followed by postoperative
radiotherapy and chemotherapy. Surgical resection of the entire
tumor structure and chest wall reconstruction may result in a good
local control rate for thoracic lung cancer. A common regimen for
radiotherapy is 40–60 Gy over 4–5 weeks and chemotherapy may
include CAV, cisplatin plus etoposide or ifosfamide, etoposide plus
cisplatin. However, no previous studies have reported which
treatment solution is the most effective strategy for Askin's
tumor. Christiansen et al (17) recommended that the most appropriate
treatment plan should include preoperative and postoperative
chemotherapy combined with complete surgical resection. Takanami
et al (18) reported one case
of a 16-year-old male patient, who suffered local recurrence and
underwent six surgeries and postoperative chemotherapy; this
patient was still alive at 7 years post surgery, therefore showing
the importance of integrated treatment. In the present study, the
combination of surgery, chemotherapy and radiotherapy resulted in
optimal outcome. However, due to the number of cases and the
follow-up duration, it cannot be confirmed as the most effective
regimen for Askin's tumor. These results require verification from
a larger number of cases in multi-center clinical trials. In
conclusion, chemotherapy, treatment modalities, tumor size, tumor
stage and LDH levels, among other factors may affect the survival
and prognosis of patients. The development of rational and more
effective strategies, such as gene therapy, based on
multidisciplinary discussion may be used to improve treatment
efficacy and patient prognosis.
References
|
1
|
Bunyaratavej K, Khaoroptham S, Phonprasert
C, Tanboon J and Shuangshoti S: Primary intracranial peripheral
primitive neuroectodermal tumor/Ewing's sarcoma presenting with
acute intracerebral hemorrhage. Clin Neuropathol. 24:184–190.
2005.PubMed/NCBI
|
|
2
|
Mobley BC, Roulston D, Shah GV, Bijwaard
KE and McKeever PE: Peripheral primitive neuroectodermal
tumor/Ewing's sarcoma of the craniospinal vault: Case reports and
review. Hum Pathol. 37:845–853. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Askin FB, Rosai J, Sibley RK, Dehner LP
and McAlister WH: Malignant small cell tumor of the
thoracopulmonary region in childhood: A distinctive
clinicopathologic entity of uncertain histogenesis. Cancer.
43:2438–2451. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Therasse PI, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shrestha B, Kapur BN, Karmacharya K,
Kakkar S and Ghuliani R: Askin's tumor: A dual case study. Int J
Pediatr. 2011:2521962011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sikri V and Sobti S: Askin tumour: a rare
thoracopulmonary tumour in adults. Indian J Chest Dis Allied Sci.
55:233–235. 2013.PubMed/NCBI
|
|
8
|
Benbrahim Z, Arifi S, Daoudi K, Serraj M,
Amara B, Benjelloun MC, Mellas N and El Mesbahi O: Askin's tumor: a
case report and literature review. World J Surg Oncol. 11:102013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu Q, Xu K, Yang C, Zhang X, Meng Y and
Quan Q: Askin tumor: Four case reports and a review of the
literature. Cancer Imaging. 11:184–188. 2011.PubMed/NCBI
|
|
10
|
Gunay E, Ucar N, Aksu F, Gunay S, Orsel O,
Kurt B, Taştepe I and Memiş L: A case of Askin's tumor presenting
with pleural effusion and high level of adenosine deaminase.
Hippokratia. 15:189–190. 2011.PubMed/NCBI
|
|
11
|
Katsenos S, Nikopoloulou M, Kokkonouzis I
and Archondakis S: Askin's tumor: A rare chest wall neoplasm. Case
report and short review. Thorac Cardiovasc Surg. 56:308–310. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Winer-Muram HT, Kauffman WM, Gronemeyer SA
and Jennings SG: Primitive neuroectodermal tumors of the chest wall
(Askin tumors): CT and MR findings. AJR Am J Roentgenol.
161:265–268. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar PV: Fine needle aspiration cytologic
findings in malignant small cell tumor of the thoracopulmonary
region (Askin tumor). Acta Cytol. 38:702–706. 1994.PubMed/NCBI
|
|
14
|
Schmidt D, Herrmann C, Jurgens H and Harms
D: Malignant peripheral neuroectodermal tumor and its necessary
distinction from Ewing's sarcoma. A report from the Kiel Pediatric
Tumor Registry. Cancer. 68:2251–2259. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khoury V and Mitchell MJ: Residents'
corner. Answer to case of the month #83. Askin tumour of the chest
wall. Can Assoc Radiol J. 52:269–271. 2001.PubMed/NCBI
|
|
16
|
Downing JR, Head DR, Parham DM, Douglass
EC, Hulshof MG, Link MP, Motroni TA, Grier HE, Curcio-Brint AM and
Shapiro DN: Detection of the (11;22) (q24;q12) translocation of
Ewing's sarcoma and peripheral neuroectodermal tumor by reverse
transcription polymerase chain reaction. Am J Pathol.
143:1294–1300. 1993.PubMed/NCBI
|
|
17
|
Christiansen S, Semik M,
Dockhorn-Dworniczak B, Rötker J, Thomas M, Schmidt C, Jürgens H,
Winkelmann W and Scheld HH: Diagnosis, treatment and outcome of
patients with Askin-tumors. Thorac Cardiovasc Surg. 48:311–315.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takanami I and Imamura T: The treatment of
Askin tumor: Results of two cases. J Thorac Cardiovasc Surg.
123:391–392. 2002. View Article : Google Scholar : PubMed/NCBI
|