Introduction
Lung cancer is one of the most common types of
malignancy (1). Worldwide, it remains
the leading cause of cancer-related mortality in males and females,
and was responsible for 1.56 million deaths annually in 2012
(2). Conventional treatments for lung
cancer include surgery, chemotherapy and radiotherapy (3). Although these treatments produce marked
benefits in patients with lung cancer, they have a range of
side-effects, such as hair loss, immunosuppression, and nausea and
vomiting. In addition, the prognosis of advanced lung cancer is
very poor, and the five-year survival is ~16.8% in the US and lower
still in the developing world (4,5). However,
gene therapy, which involves the delivery of therapeutic DNA into a
patient's cells, has been investigated as a novel treatment for
lung cancer in a number of clinical trials. For example, Morgan
et al (6) successfully treated
metastatic melanoma in two patients by using killer T cells that
had been genetically retargeted to attack cancer cells. Strategies
for gene therapy include induction of apoptosis, tumor suppressor
gene replacement, suicide gene expression, cytokine-based therapy,
vaccination-based approaches and adoptive transfer of modified
immune cells (7).
MCPH1, also termed BRIT1 (BRCT-repeat
inhibitor of hTERT expression), encodes the MCPH1 protein that
contains three BRCT domains: one in the N-terminus and two in the
C-terminus. Besides MCPH1, numerous other proteins that are
involved in tumor suppression and the DNA damage response, such as
BRCA1, BRCA2, 53BP1, XRCC1, Rad9, NBS1 and DNA polymerase λ, also
contain BRCT domains (8). Previous
studies have demonstrated that BRCT-containing MCPH1 may be
important in maintaining genome stability, which requires the
activation of cell cycle checkpoints and the repair of damaged DNA
(9,10). Indeed, MCPH1 knockdown reduces the
expression of BRCA1 and the checkpoint kinase, Chk1, in addition to
NBS1 phosphorylation, resulting in intra-S and G2/M checkpoint loss
(9).
Cancer development involves dysregulation of the
expression of oncogenes and tumor suppressors. A number of DNA
repair regulators have been associated with human cancer, such as
BRCA1 and BRCA2 (11). Therefore, it
is proposed that MCPH1, a key regulator of the DNA repair pathway
and cell cycle checkpoints, may be involved in cancer development
and progression. Indeed, recent studies have demonstrated that
MCPH1 is downregulated in a variety of types of human cancer,
including breast cancer (12,13), endometrial cancer (14), ovarian cancer (15), glioblastoma (16) and oral squamous cell carcinoma
(17). Furthermore, a recent
experiment using immunohistochemistry, conducted by our group,
demonstrated that MCPH1 expression was markedly suppressed in lung
cancer tissues (18). These results
support the hypothesis that MCPH1 is a tumor suppressor gene. Thus,
it was proposed that an increase in the expression of MCPH1 may be
an effective therapy for human lung cancer.
The present study examined MCPH1 mRNA expression in
human lung cancer tissues and normal lung tissues. The effect of
increased MCPH1 expression on cell apoptosis and proliferation in
the A549 non-small cell lung cancer cell line was subsequently
investigated, in addition to the molecular mechanisms underlying
this process.
Materials and methods
Lung cancer specimens
Lung Cancer specimens were obtained from 24 patients
with lung cancer, who underwent surgery in the First Affiliated
Hospital of Chongqing Medical University (Chongqing, China) from
July 2009 to June 2012. Normal adjacent tissues specimens were used
as controls. These patients received neither chemotherapy nor
radiotherapy prior to surgery. The acquisition and analysis of the
lung cancer specimens was approved by the ethics committee of the
hospital and the patients provided written informed consent.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from cell lines and tissue
samples using TRIzol™ reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA), according to the manufacturer's instructions.
Reverse transcription was performed at 42°C for 30 min followed by
inactivation at 94°C for 5 min. The resultant first-strand cDNA was
used as a template for PCR amplification. The cDNA was stored at
−20°C until use or immediately amplified by PCR in order to measure
the expression of the genes of interest. The following
oligonucleotide primers were used: Forward,
5′-CACCATCTTTCACTCACCTC-3′ and reverse, 5′-CTTTACTGAGGAACTCCTGG-3′
for MCPH1; and forward, 5′-ACCTGACCTGCCGTCTAGAA-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′ for GAPDH. Each amplification program
consisted of one cycle of 94°C for 5 min, followed by 30 cycles of
94°C for 10 sec, 60°C for 30 sec and 72°C for 30 sec. The PCR
products were separated by 1.5% agarose gel electrophoresis and
visualized by ethidium bromide staining (Sangon Biotech Co., Ltd.,
Shanghai, China). GAPDH was used as an internal control. Data
analysis was performed using the 2−∆∆Ct method.
Cell culture and transfection
A549 human lung cancer cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen Life
Technologies) supplemented with 10% heat-inactivated fetal bovine
serum (FBS; Invitrogen Life Technologies), penicillin (50 U/ml),
and streptomycin (50 µg/ml; Gibco BRL, Grand Island, NY, USA).
Cells were maintained at 37°C in a humidified atmosphere of 5%
CO2. In order to increase MCPH1 expression, a pcDNA3.1
(−)MCPH1 plasmid was constructed. A fragment of human MCPH1 was
amplified from the cDNA of HEK293T cells (Type Culture Collection
of the Chinese Academy of Sciences, Shanghai, China) using specific
primers (Forward, 5′-CACCATCTTTCACTCACCTC-3′ and reverse,
5′-CTTTACTGAGGAACTCCTGG-3′) with the HindIII and XhoI
restriction sites. PCR was performed using a total of 1.0 µl cDNA
and 0.25 µl Ex Taq™ Polymerase (Takara, Otsu, Japan) for 35 cycles
of 94°C for 1 min, 58°C for 180 sec and 72°C for 1 min, followed by
10 min at 72°C. The PCR product (2,508 bp) was purified and
digested with HindIII and XhoI, then cloned into a
mammalian expression vector pcDNA3.1(−) with the corresponding
restriction sites (Novagen, Darmstad, Germany). The recombinant
plasmid was confirmed by DNA sequencing using the HiSeq 2000
Sequencing System (Illumina Inc, San Diego, CA, USA). Transient
transfection was conducted using Lipofectamine 2000 (Invitrogen
Life Technologies) for A549 cells, according to the manufacturer's
instructions. Briefly, equal numbers of cells were plated in
24-well and 6-well plates and were grown to 80% confluence. Cells in
the 24-well plates were transfected with 0.5 µg of pcDNA3.1(−)MCPH1
vector or pcDNA3.1 empty vector. Cells in the 6-well plates were
tansfected with 1.0 µg of pcDNA3.1(−)MCPH1 vector or pcDNA3.1 empty
vector. The indicated quantities of vectors were combined in
Opti-MEM™ medium (Invitrogen Life Technologies) with Lipofectamine
2000. The solution was incubated for ~30 min at room temperature
and then added to the cultured cells. After 4–6 h, the medium was
changed for DMEM with 10% FBS.
MTT assay
A549 cells were transfected with pcDNA3.1 (−)MCPH1.
At 96 h following transfection, 20 ml of MTT (5 mg/ml) was added to
each well of a 96-well plate and the cells were cultured for 4 h.
Cells were observed using a phase contrast microscope (TE2000;
Nikon Corporation, Tokyo, Japan). The MTT was then discarded and
150 ml of dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA)
was added to each well. Absorbance was measured at 490 nm using a
multi-well spectrophotometer (Bio-Tek Instruments, Inc., Winooski,
VT, USA).
Flow cytometry analysis and apoptosis
assay
Cell cycle distribution was determined using flow
cytometry, following cell staining with propidium iodide
(Sigma-Aldrich). In brief, floating and adherent cells were
collected, washed with ice-cold phosphate-buffered saline and fixed
with 70% ethanol. Cells were then treated with 50 µg/ml of RNase A
(Sigma-Aldrich) and 50 µg/ml of propidium iodide for 30 min at room
temperature. The stained cells were analyzed using flow cytometry
(FACSCalibur™ flow cytometer; BD Biosciences, San Jose, CA,
USA).
Western blot analysis
Cells were lysed in a lysis buffer containing 25 mM
Tris-HCl, pH 7.5; 137 mM NaCl; 2.7 mM KCl; 1% Triton X-100; and
protease inhibitor cocktail (Sigma-Aldrich). The protein
concentration of cell lysates was determined using a Bio-Rad
Protein Assay Kit I (Bio-Rad Laboratories, Hercules, CA, USA),
according to the manufacturer's instructions. Bovine serum albumin
was used as a control. Equal quantities of cell lysates were
separated by 10% SDS-polyacrylamide gel electrophoresis,
electrotransferred onto Immobilon-P membrane filters (Millipore,
Billerica, MA, USA) and blocked with 0.5% non-fat milk in
Tris-buffered saline with 0.1% Tween-20 at 4°C. The membranes were
incubated with anti-human mouse monoclonal IgG1 antibody to Bcl-2
(cat no. sc-509; 1:500 dilution; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), rabbit monoclonal IgG antibody to MCPH1 (cat no.
ab123361; 1:200 dilution; Abcam, Cambridge, UK), rabbit monoclonal
IgG antibody to β-actin (cat no. A0483; 1:100 dilution;
Sigma-Aldrich), rabbit polyclonal IgG antibody to BAX (cat no.
B3428; 1:2,000 dilution; Sigma-Aldrich) and rabbit polyclonal IgG
antibody to caspase 3 (cat no. 9654; 1:500 dilution; Cell Signaling
Technology, Inc., Danvers, MA, USA) at room temperature for 1 h,
followed by incubation with horseradish peroxidase-conjugated
secondary antibodies (anti-rabbit IgG, cat no. 7074P2, 1:3,000
dilution; and anti-mouse IgG, cat no. 7072S; 1:3,000 dilution; Cell
Signaling Technology, Inc.) at room temperature for 1 h.
Immunoreactive bands were visualized using an ECL system (GE
Healthcare, Little Chalfont, UK), according to the manufacturer's
instructions.
Statistical analysis
Data are presented as the mean ± standard error of
the mean of ≥3 separate experiments. Multiple group comparisons
were analyzed using one-way analysis of variance and post hoc
Tukey's test, with plasmid treatment as the between-subjects
factor. Paired Student's t-test was used to analyze the results of
the RT-PCT assay. All statistical analyses were performed using the
SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
MCPH1 mRNA is downregulated in lung
cancer tissues
It has recently been reported that MCPH1 expression
is markedly decreased in lung cancer tissues (18). In order to confirm this results, the
present study measured MCPH1 mRNA expression in lung cancer tissues
and normal adjacent tissues, using RT-PCR. The clinicopathological
characteristics of the 24 patients with lung cancer are presented
in Table I. The results demonstrated
that MCPH1 mRNA expression was significantly reduced in lung cancer
tissues compared with that in normal adjacent tissues (P=0.008;
Fig. 1).
 | Table I.Clinicopathological characteristics of
patients with lung cancer in the present study. |
Table I.
Clinicopathological characteristics of
patients with lung cancer in the present study.
| Number | Gender | Age | Organ | Pathological
diagnosis | Classification | TNM | Type |
|---|
| 1 | M | 48 | Lung | Adenocarcinoma | 1 | T2N0M0 | Malignant |
| 2 | M | 41 | Lung | Adenocarcinoma | 1 | T2N0M0 | Malignant |
| 3 | F | 55 | Lung | Adenocarcinoma | 1 | T2N1M0 | Malignant |
| 4 | M | 56 | Lung | Adenocarcinoma | 1 | T1N0M0 | Malignant |
| 5 | F | 81 | Lung | Adenocarcinoma | 1 | T2N1M0 | Malignant |
| 6 | F | 58 | Lung | Papillary
adenocarcinoma | 1 | T2N1M0 | Malignant |
| 7 | M | 44 | Lung | Mucinous
adenocarcinoma | 2 | T2NxM0 | Malignant |
| 8 | M | 32 | Lung | Adenocarcinoma | 1 | T2N0M0 | Malignant |
| 9 | F | 35 | Lung | Mucinous
adenocarcinoma | 2 | T2N1M0 | Malignant |
| 10 | M | 42 | Lung | Adenocarcinoma | 2 | T2N1M0 | Malignant |
| 11 | F | 63 | Lung | Adenocarcinoma | 1 | T2N1M0 | Malignant |
| 12 | F | 31 | Lung | Adenocarcinoma | 3 | T2N1M0 | Malignant |
| 13 | F | 55 | Lung | Adenocarcinoma | 3 | T2N0M0 | Malignant |
| 14 | M | 64 | Lung | Adenocarcinoma
(sparse carcinoma infiltrating lung tissue) | 3 | T2N1M0 | Malignant |
| 15 | M | 64 | Lung | Mucinous
adenocarcinoma | 3 | T2N0M0 | Malignant |
| 16 | M | 52 | Lung | Adenocarcinoma | 3 | T2NxM0 | Malignant |
| 17 | M | 71 | Lung | Adenocarcinoma | 3 | T2N2M0 | Malignant |
| 18 | M | 70 | Lung | Adenocarcinoma | 3 | T2NxM0 | Malignant |
| 19 | F | 66 | Lung | Adenocarcinoma | 3 | T2N2M0 | Malignant |
| 20 | M | 54 | Lung | Adenocarcinoma | 3 | T2N1M0 | Malignant |
| 21 | F | 35 | Lung | Adenocarcinoma | 3 | T2N0M0 | Malignant |
| 22 | M | 53 | Lung | Adenosquamous
carcinoma | 3 | T2N1M0 | Malignant |
| 23 | M | 75 | Lung | Squamous cell
carcinoma | 1 | T2N1M0 | Malignant |
| 24 | M | 56 | Lung | Squamous cell
carcinoma | 1 | T2N2M0 | Malignant |
MCPH1 overexpression inhibits cell
proliferation in A549 lung cancer cells
As MCPH1 has been shown to be involved in the
activation of cell cycle checkpoints (18) and its expression is markedly reduced
in lung cancer (Fig. 1), the present
study aimed to determine whether overexpression of MCPH1 in the
A549 cell line may inhibit uncontrolled cell growth. In order to
increase MCPH1 expression, a pcDNA3.1 (−)MCPH1 plasmid was
constructed. It was observed that A549 cells transfected with the
pcDNA3.1 (−)MCPH1 plasmid exhibited significantly increased MCPH1
expression, compared with those transfected with the empty vector
pcDNA3.1 (P=0.589; Fig. 2A and B) or
with the untreated control.
Subsequently, the effect of MCPH1 overexpression on
the proliferation of the A549 cell line was examined. The results
from the MTT assay demonstrated that A549 cells transfected with
the pcDNA3.1 (−)MCPH1 plasmid exhibited significantly reduced cell
proliferation compared with cells transfected with the empty vector
(P=0.145) or with cells not transfected with pcDNA3.1(−) MCPH1 or
the empty vector (P<0.001; Fig. 3A and
B).
A previous study has demonstrated that MCPH1 may
regulate the S phase and G2/M cell cycle checkpoints in breast
cancer (19). Therefore, the present
study sought to determine whether MCPH1 overexpression results in S
phase and G2 phase arrest, and subsequently inhibits uncontrolled
cell growth in A549 cells. The results of the flow cytometry assay
demonstrated that transfection with pcDNA3.1 (−)MCPH1 resulted in a
significant increase in the proportion of A549 cells in S phase
(11.6700±0.352%) compared with untreated control cells
(9.8567±0.06936%; P=0.006) and cells transfected with pcDNA3.1
(10.287±0.388%; P=0.018 Fig. 4A–C).
In addition, transfection with pcDNA3.1 (−)MCPH1, resulted in a
significant increase in the proportion of cells in the G2/M phase
(11.817±0.2980%) compared with untreated control cells
(6.133±0.227%; P<.0001) and with cells transfected with pcDNA3.1
(6.953±0.2773%; P<0.001; Fig. 4A, B
and D).
These results suggested that overexpression of MCPH1
leads to G2/M and S phase cell cycle arrest, and subsequently
inhibits cell proliferation in the A549 lung cancer cell line.
MCPH1 overexpression promotes cell
apoptosis in the A549 cell line
In addition to uncontrolled cell growth, a further
characteristic of cancerous cells is the evasion of apoptosis
(20,21). Therefore, the present study sought to
determine whether MCPH1 overexpression may promote cell apoptosis
in addition to inhibiting cell proliferation.
In order to evaluate the effect of MCPH1
overexpression on cell apoptosis, flow cytometry analysis was
conducted. The results of this analysis demonstrated that A549
cells transfected with the pcDNA3.1 (−)MCPH1 plasmid exhibited a
significant increase in the rate of apoptosis compared with
untreated control cells and cells transfected with pcDNA3.1 (both
P<0.001; Fig. 5A and B).
Cell apoptosis is known to be associated with
alterations in the expression of certain genes, such as caspase-3,
Bax and Bcl-2. Therefore, the present study measured changes in the
expression of these proteins, following MCPH1 overexpression in
A549 cells. The results demonstrated that the expression of active
caspase-3 was significantly increased in cells transfected with
pcDNA3.1 (−)MCPH1 compared with untreated control cells and cells
transfected with pDNA3.1 (P=0.012 and P=0.016, respectively;
Fig. 6A and B), while the expression
of inactive caspase-3 was significantly decreased in A549 cells
transfected with the pcDNA3.1 (−)MCPH1 plasmid, compared with
untreated control cells and cells treated with pcDNA3.1 (P=0.001
and P<0.001, respectively; Fig. 6A and
C). A549 cells transfected with the empty vector, pcDNA3.1,
exhibited an increase in the expression of active caspase-3
compared with untreated control cells (P=0.021; Fig. 6A and C). However, there was no
significant difference in the expression of active caspase-3 in
this group compared with the untreated control cells (P=0.970;
Fig. 6A and B). Furthermore, the
expression of Bax was significantly increased in A549 cells that
had been transfected with the pcDNA3.1 (−)MCPH1 plasmid compared
with untreated control cells and cells transfected with the empty
vector (both P=0.001; Fig. 6A and D).
There was no significant difference in Bax expression between the
untreated control cells and the cells transfected with the empty
vector (P=0.932; Fig. 6A and D). By
contrast, the expression of Bcl-2, an antiapoptotic protein, was
significantly reduced in A549 cells that were transfected with the
pcDNA3.1 (−)MCPH1 plasmid, compared with that in untreated control
cells and cells transfected with pcDNA3.1 (P=0.004 and P=0.002,
respectively; Fig. 6A and E). The
expression of Bcl-2 in cells transfected with pcDNA3.1 was not
significantly different from that in the untreated control cells
(P=0.741; Fig. 6A and E). These
results indicated that overexpression of MCPH1 may suppress
tumorigenesis via the promotion of cell apoptosis.
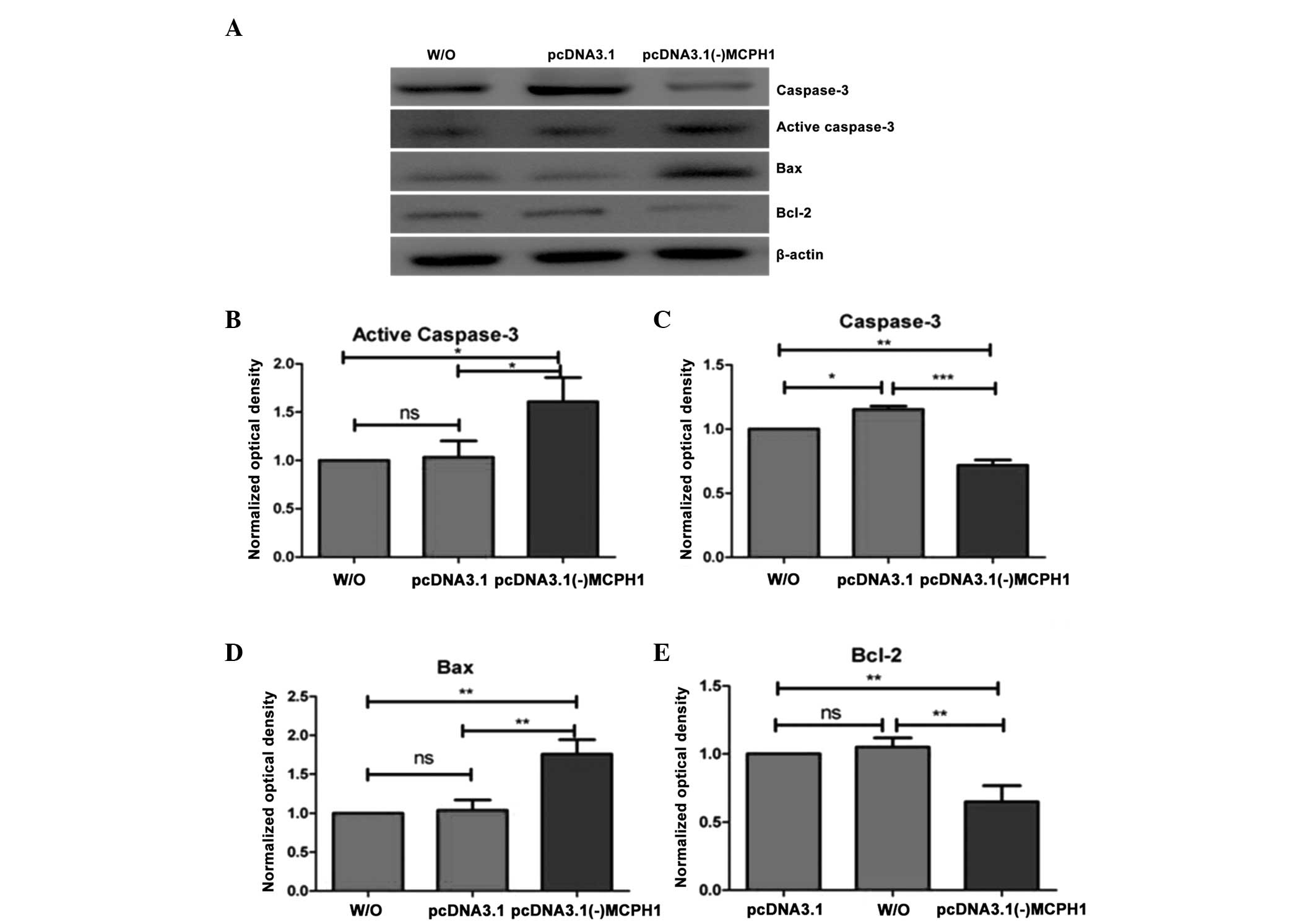 | Figure 6.MCPH1 overexpression reduced the
Bcl-2/Bax ratio and increased active caspase-3 expression in A549
cells. (A) Western blot analysis of the effects of MCPH1
overexpression on caspase-3, Bax and Bcl-2 expression. (B) and (C)
A549 cells transfected with pcDNA3.1 (−)MCPH1 exhibited
significantly increased expression of active caspase-3 (B) and
decreased expression of inactive caspase-3 (C), compared with those
transfected with pcDNA3.1 and with the W/O control group (n=3). (D)
and (E) A549 cells transfected with pcDNA3.1 (−)MCPH1 exhibited
significantly increased expression of Bax (D) and decreased
expression of Bcl-2 (E) compared with those transfected pcDNA3.1
and with the W/O control group (n=3). One-way analysis of variance
was used: F (2,6)=11.52, P=0.009 for active caspase-3; F
(2,6)=60.278, P<0.001 for caspase-3; F
(2,6)=30.979, P=0.001 for Bax; and F (2,6)=22.922,
P=0.001 for Bcl-2. *P<0.05, **P<0.01 and ***P<0.001.
MCPH1, microcephalin; W/O, cells not transfected with pcDNA3.1(−)
MCPH1 or the empty vector; ns, no significant difference. |
Discussion
The present study confirmed that MCPH1 expression is
downregulated in human lung cancer, and demonstrated that an
increase in MCPH1 expression in vitro suppresses
uncontrolled cell proliferation and promotes cell apoptosis. MCPH1
may therefore be involved in the pathogenesis of lung cancer.
Increasing evidence has suggested that MCPH1
expression is associated with the development of a number of types
of cancer, such as breast cancer (12,13),
endometrial cancer (14), ovarian
cancer (15), glioblastoma (16) and oral squamous cell carcinoma
(17). A more recent study reported
that knockdown of MCPH1 in mice leads to genomic instability and
may enhance cancer susceptibility (10). In accordance with the results of a
recent study conducted by our group (18), the present study demonstrated that the
expression of MCPH1 mRNA was significantly downregulated in human
lung cancer tissues (Fig. 1).
Furthermore, induced overexpression of MCPH1 in A549 non-small cell
lung cancer cells resulted in the suppression of cell proliferation
(Fig. 3) and an increase in cell
apoptosis (Fig. 5).
Previous studies have reported that MCPH1 is
required for DNA damage-induced intra-S and G2/M checkpoints, and
that this requirement may in part result from its regulation of the
expression of BRCA1 and Chk1 (9,19).
Therefore, MCPH1 downregulation in lung cancer tissues may lead to
loss of the intra-S phase and G2/M phase checkpoints. In the
current study, MCPH1 overexpression in A549 cells arrested the cell
cycle in the S and G2/M phases (Fig.
4), and subsequently inhibited cell proliferation. The precise
mechanisms underlying this effect remain to be elucidated, although
they may involve the inhibitory effects of MCPH1 on cyclins and
related cyclin-dependent kinase (CDK) enzymes, as a recent study
reported that overexpression of MCPH1 decreases the expression of
cyclin A2 and cyclin B1 in cervical cancer cells (22). Thus, further investigation of the
effects of cyclin and CDK in lung cancer cells, with or without
MCPH1 overexpression, may help to determine whether the inhibitory
effect of MCPH1 on lung cancer cell proliferation is a result of
its inhibitory effect on cyclin and CDK expression.
In addition to cell proliferation, cell apoptosis
may also contribute to the inhibition of uncontrolled cell growth
in lung cancer cells that are overexpressing MCPH1. A recent study
reported that MCPH1 is involved in E2F1-mediated apoptosis;
knockdown of MCPH1 in HEK293 cells resulted in decreased expression
of E2F1 target genes, such as caspase-3, caspase-7, BRCA1 and Chk1
(23), which are important in cell
apoptosis. Mitochondria participate in the intrinsic apoptotic
pathway and tumors arise more frequently through the intrinsic
pathway than the extrinsic pathway as a result of the sensitivity
of the intrinsic pathway to apoptosis (24). In the intrinsic pathway, apoptosis is
mediated by proteins in the Bcl-2 family. Alterations in the
equilibrium between Bcl-2 and Bax lead to increased
permeabilization of the mitochondrial outer membrane, with
consequent cytochrome c release and ultimately activation of
the caspase cascades (25,26). In the present study, MCPH1
overexpression was shown to induce cell apoptosis in human lung
cancer cells by flow cytometric analysis (Fig. 5). Furthermore, MCPH1 overexpression
reduced the Bcl-2/Bax ratio, as reflected by an increase in the
expression of Bax and a decrease in that of Bcl-2 (Fig. 6), indicating that
mitochondrial-mediated apoptosis had been initiated. In addition,
caspase-3 was activated by MCPH1 overexpression (Fig. 6), which suggested that MCPH1 induces
caspase-associated cell apoptosis. Therefore, overexpression of
MCPH1 may induce cell apoptosis in lung cancer cells via caspase
activation-dependent induction of the intrinsic apoptotic
pathway.
In conclusion, MCPH1 expression was reduced in human
lung cancer tissue in comparison with normal adjacent tissues, and
overexpression of MCPH1 inhibited uncontrolled lung cancer cell
growth via induction of cell cycle arrest at S phase and G2/M
phase, and promotion of mitochondrial apoptosis. These findings
suggest that MCPH1 may function as a tumor suppressor gene, and
that it may be involved in the development and progression of human
lung cancer. Furthermore, inducing an increase in MCPH1 expression
may have potential as a therapeutic approach in lung cancer.
Acknowledgements
This study was supported by the 973 Program of the
Ministry of Science and Technology of China (grant no.
2014CB548100). We thank Dr. Xueshuang Gu from the First Affiliated
Hospital of Chongqing Medical University (Chongqing, China) for
providing the patient specimens.
References
|
1
|
Klein JS and Webb WR: The radiologic
staging of lung cancer. J Thorac Imaging. 7:29–47. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stewart BW and Wild CP: World cancer
report 2014. World Health Organization. (Chapter 1.1.). 2014.
|
|
3
|
Zhang Y and He J: The development of
targeted therapy in small cell lung cancer. J Thorac Dis.
5:538–548. 2013.PubMed/NCBI
|
|
4
|
SEER Stat Fact Sheets: Statistics at a
glance. Lung and Bronchus Cancer (Bethesda, MD). National Cancer
Institute. 2014.PubMed/NCBI
|
|
5
|
Ridge CA, McErlean AM and Ginsberg MS:
Epidemiology of lung cancer. Semin Intervent Radiol. 30:93–98.
2014. View Article : Google Scholar
|
|
6
|
Morgan RA, Dudley ME, Wunderlich JR,
Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US,
Restifo NP, et al: Cancer regression in patients after transfer of
genetically engineered lymphocytes. Science. 314:126–129. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vachani A, Moon E, Wakeam E, Haas AR,
Sterman DH and Albelda SM: Gene therapy for lung neoplasms. Clin
Chest Med. 32:865–885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leung CC and Glover JN: BRCT domains: Easy
as one, two, three. Cell Cycle. 10:2461–2470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin SY, Rai R, Li K, Xu ZX and Elledge SJ:
BRIT1/MCPH1 is a DNA damage responsive protein that regulates the
Brca1-Chk1 pathway, implicating checkpoint dysfunction in
microcephaly. Proc Natl Acad Sci USA. 102:15105–15109. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang Y, Gao H, Lin SY, Peng G, Huang X,
Zhang P, Goss JA, Brunicardi FC, Multani AS, Chang S and Li K:
BRIT1/MCPH1 is essential for mitotic and meiotic recombination DNA
repair and maintaining genomic stability in mice. PLoS Genet.
6:e10008262010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Domchek SM, Friebel TM, Singer CF, Evans
DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles
R, et al: Association of risk-reducing surgery in BRCA1 or BRCA2
mutation carriers with cancer risk and mortality. JAMA.
304:967–975. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rai R, Phadnis A, Haralkar S, Badwe RA,
Dai H, Li K and Lin SY: Differential regulation of centrosome
integrity by DNA damage response proteins. Cell Cycle. 7:2225–2233.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Richardson J, Shaaban AM, Kamal M, Alisary
R, Walker C, Ellis IO, Speirs V, Green AR and Bell SM:
Microcephalin is a new novel prognostic indicator in breast cancer
associated with BRCA1 inactivation. Breast Cancer Res Treat.
127:639–648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bilbao C, Ramírez R, Rodríguez G, Falcón
O, León L, Díaz-Chico N, Perucho M and Díaz-Chico JC: Double strand
break repair components are frequent targets of microsatellite
instability in endometrial cancer. Eur J Cancer. 46:2821–2827.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rai R, Dai H, Multani AS, Li K, Chin K,
Gray J, Lahad JP, Liang J, Mills GB, Meric-Bernstam F and Lin SY:
BRIT1 regulates early DNA damage response, chromosomal integrity
and cancer. Cancer Cell. 10:145–157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hagemann C, Anacker J, Gerngras S, Kühnel
S, Said HM, Patel R, Kämmerer U, Vordermark D, Roosen K and Vince
GH: Expression analysis of the autosomal recessive primary
microcephaly genes MCPH1 (microcephalin) and MCPH5 (ASPM, abnormal
spindle-like, microcephaly associated) in human malignant gliomas.
Oncol Rep. 20:301–308. 2008.PubMed/NCBI
|
|
17
|
Venkatesh T, Nagashri MN, Swamy SS,
Mohiyuddin SM, Gopinath KS and Kumar A: Primary microcephaly gene
MCPH1 shows signatures of tumor suppressors and is regulated by
miR-27a in oral squamous cell carcinoma. PLoS One. 8:e546432013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Wu XB, Fan JJ, Mai L, Cai W, Li
D, Yuan CF, Bu YQ and Song FZ: MCPH1 Protein expression in normal
and neoplastic lung tissues. Asian Pac J Cancer Prev. 14:7295–7300.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu X, Lee J and Stern DF: Microcephalin is
a DNA damage response protein involved in regulation of CHK1 and
BRCA1. J Biol Chem. 279:34091–34094. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mai L, Yi F, Gou X, Zhang J, Wang C, Liu
G, Bu Y, Yuan C, Deng L and Song F: The overexpression of MCPH1
inhibits cell growth through regulating cell cycle-related proteins
and activating cytochrome c-caspase 3 signaling in cervical
cancer. Mol Cell Biochem. 392:95–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang SZ, Lin FT and Lin WC: MCPH1/BRIT1
cooperates with E2F1 in the activation of checkpoint, DNA repair
and apoptosis. EMBO Rep. 9:907–915. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohan S, Abdul AB, Abdelwahab SI,
Al-Zubairi AS, Sukari MA, Abdullah R, Elhassan Taha MM, Ibrahim MY
and Syam S: Typhonium flagelliforme induces apoptosis in CEMss
cells via activation of caspase-9, PARP cleavage and cytochrome
c release: Its activation coupled with G0/G1 phase cell
cycle arrest. J Ethnopharmacol. 131:592–600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C and Youle RJ: The role of
mitochondria in apoptosis. Annu Rev Genet. 43:95–118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang F, Kong DS, Zhang ZL, Lei N, Zhu XJ,
Zhang XP, Chen L, Lu Y and Zheng SZ: Tetramethylpyrazine induces
G0/G1 cell cycle arrest and stimulates mitochondrial-mediated and
caspase-dependent apoptosis through modulating ERK/p53 signaling in
hepatic stellate cells in vitro. Apoptosis. 18:135–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|




















