Introduction
Fibroblast growth factor (FGF) was one of the first
confirmed angiogenesis-associated growth factors (1) that play a critical role in the
neovascularization of various solid tumors (2–5). By
binding to FGF receptors, FGF2 activates the mitogen-activated
protein kinase (MAPK) pathway (6,7). As a
characteristic MAPK, extracellular signal-regulated kinase 1/2
(ERK1/2) plays a central role in mitogenic signaling, and activated
ERK1/2 triggers a series of responses in target cells, including
the proliferation and migration of cells (8).
Similar expression to FGF (hSef) is a negative
feedback regulator of FGF-mediated MAPK/ERK1/2 signaling (9–11) and
exerts an inhibitory function by affecting the FGF signaling
cascade at multiple levels (9).
Sprouty genes have been reported to act as an additional negative
feedback regulator of FGF signaling (12) by interrupting the interaction of the
growth factor receptor-bound protein 2/son of sevenless complex
with fibroblast growth factor receptor substrate 2 and Src homology
2 domain-containing tyrosine phosphatase (13,14),
preventing Raf activation (15,16).
Aberrant FGF signaling has been identified in human
endometrial carcinoma, and this aberrant expression facilitates the
growth and invasion of cancer cells (17). In a previous study, hSef was
identified as a negative feedback regulator of FGF-mediated
MAPK/ERK1/2 signaling in endometrial cancer cells (11). Plasmid-driven hSef expression has also
been revealed to significantly downregulate the growth of
endometrial carcinoma Ishikawa cells (11). However, the expression pattern of
these negative regulators in endometrial cancers has not yet been
investigated. In the current study, the aberrant expression of hSef
and Sprouty4 was explored in endometrial adenocarcinoma.
Materials and methods
Tissue collection and
immunohistochemical analysis
In the present study, immunohistochemical analysis
was performed on the normal tissue samples obtained from
endometrial biopsies performed on 27 women of reproductive age at
Shandong Provincial Hospital Affiliated to Shandong University
(Jinan, China), with 15 tissues excised in the proliferative phase
and 13 in the secretory phase, and cancer tissues obtained from 28
patients with endometrial adenocarcinoma. The diagnosis of
endometrial adenocarcinoma was confirmed by histological
examination. None of the participants received any hormonal therapy
throughout the 3 months prior to the surgical procedure. The
present study was approved by the Institutional Review Board of
Shandong Provincial Hospital Affiliated to Shandong University and
written informed consent was obtained from all participants.
The immunohistochemical analysis was performed as
previously described (18). Briefly,
the fresh tissues were washed with phosphate-buffered saline (PBS)
and then fixed in 4% paraformaldehyde. Subsequent to dehydration
and paraffin-embedding, the samples were cut into 5-µm sections and
mounted onto glass slides. Deparaffinized and rehydrated sections
were incubated with 100 µl 3% H2O2 for 10 min
at room temperature and antigen retrieval was performed. Subsequent
to blocking, the sections were incubated overnight with goat
anti-human hSef primary polyclonal antibody diluted in
phosphate-buffered saline (PBS; dilution, 1:100; cat no. AF2275;
R&D Systems, Inc., Minneapolis, MN, USA) or rabbit anti-human
Sprouty4 primary monoclonal antibody diluted in PBS (dilution,
1:100; cat no. ab103114; Abcam, Cambridge, UK) overnight in a wet
chamber at 4°C. Horseradish peroxidase-conjugated rabbit anti-goat
or goat anti-rabbit IgG was used as secondary antibody. Tissue
sections incubated with non-immune serum instead of primary
antibody were used as a negative control. The experiments were
repeated in duplicate or triplicate.
The immunohistochemical score was evaluated as
previously described (18). Two
sections per sample were evaluated for immunohistochemistry in a
blind manner, without any knowledge of the clinical or pathological
data.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 11.5 (SPSS, Inc., Chicago, IL, USA). The data
were expressed as the mean ± standard deviation. Differences
between two and multiple groups were determined by one-way analysis
of variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of hSef is upregulated in
human endometrial adenocarcinoma
The endothelial expression of hSef in human
endometrial adenocarcinoma was determined using immunohistochemical
analysis. The immunostaining of hSef in endometrial adenocarcinoma
tissues was strong and confined to the cytoplasm of the cancer
cells (Fig. 1A–C and F–H). As shown
in Fig. 1, in the normal endometrium,
the immunostaining of hSef was present or null and mostly confined
to the cytoplasm of epithelial cells (Fig. 1D, E, I and J). However, no significant
difference was identified between the endometrial tissue samples in
the proliferative and secretory phases (Fig. 2B; P>0.05). Compared with normal
endometrial tissue, endometrial carcinoma demonstrated increased
hSef expression (Fig. 2A, C and D;
P<0.05). These data suggest the possible role of hSef
overexpression in the pathogenesis and development of human
endometrial adenocarcinoma.
Expression of hSef is downregulated in
the blood vessels of human endometrial adenocarcinoma tissues
During the immunohistochemical analysis of hSef
expression in endometrial carcinoma, the blood vessels in
endometrial carcinoma tissues (Fig.
1D–F) were found to exhibit decreased hSef expression compared
with the expression of hSef in normal endometrial tissue (Figs. 1A–C and 3; P<0.05). No significant difference was
identified between the hSef expression in endometrial tissue in the
proliferative phase and endometrial tissue in the secretory phase
(Fig. 3B; P>0.05). These data
indicate the possible role of the downregulation of hSef expression
in blood vessels in the pathogenesis and development of human
endometrial adenocarcinoma.
Loss of Sprouty4 expression occurs in
human endometrial adenocarcinoma
The expression of Sprouty4, another negative
regulator of FGF signaling, was also detected in human endometrial
carcinoma tissue samples. To determine the expression of Sprouty4
in human endometrial adenocarcinoma, immunohistochemical analysis
was performed. Endometrial cancer tissue samples obtained from 31
patients with endometrial adenocarcinoma were used in the analysis.
Normal endometrial tissue samples obtained from 30 women of
reproductive age were used as a control. As demonstrated by the
immunohistochemical analysis results, the immunostaining for
Sprouty4 was strong and constricted to the cytoplasm of glandular
cells in normal endometrial tissues (Fig.
4A, B, E and F). In endometrial adenocarcinoma tissues, the
staining for Sprouty4 revealed absent or weak expression, either in
the cancer tissues or the stroma (Fig.
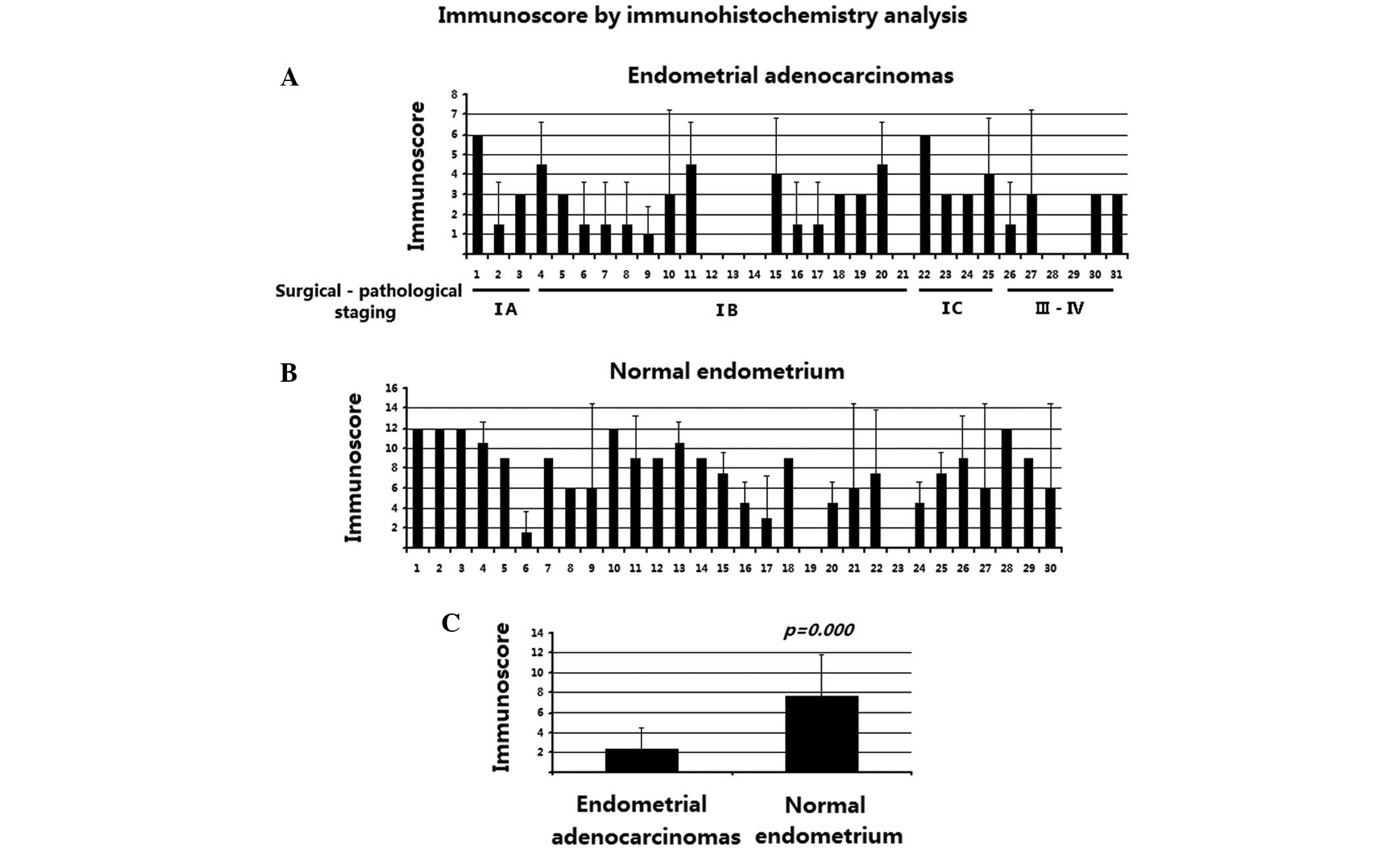
4C, D, G and H). The detailed immunostaining scores are
reported in Fig. 5. As shown in
Fig. 5, the Sprouty4 immunostaining
was significantly decreased in endometrial adenocarcinoma tissues
(P<0.01). These data suggest that the loss of Sprouty4
expression is involved in the pathogenesis and development of human
endometrial adenocarcinoma.
Discussion
Abnormal angiogenesis has been reported to
participate in the pathogenesis of human endometrial carcinoma,
which is one of the most common malignancies of the female genital
tract (19). FGF signaling is closely
associated with neovascularization in numerous human carcinomas
(2–5).
In the present study, hSef expression was observed to be
upregulated in endometrial carcinoma tissues, while the expression
of Sprouty4 was downregulated in these tissues. However, the blood
vessels in these tissue specimens exhibited decreased hSef
expression.
As a negative feedback regulator of FGF/MAPK
signaling, hSef exerts an inhibitory effect by acting on multiple
points of the FGF/RAS/MAPK cascade. hSef has been found to inhibit
RAS (20) and FGF-induced
phosphorylation of FGF receptors (20), and block the nuclear translocation of
activated ERK1/2 (21). hSef
expression is induced by FGF signaling (11), and therefore, hSef is a negative
feedback mechanism and self-restricting element of FGF signaling.
Activated MAPK/ERK1/2 signaling is involved in caryomitosis, gene
expression, and cell proliferation and survival, which indicates
that FGF/MAPK signaling is significant in the genesis and
development of tumors.
FGF-mediated signaling is involved in the
pathogenesis and development of endometrial carcinoma (17). Elevated FGF expression and secretion
have been reported to be present in endometrial cancers (17). Plasmid-driven overexpression of FGF in
endometrial adenocarcinoma cells has been revealed to promote the
formation and growth of tumors in nude mice (22), while administration of the FGF
antibody attenuates this process (23). Thus, as a negative feedback regulator
of FGF signaling, hSef plays an important role in the pathogenesis
of carcinoma. The loss of hSef expression may lead to
over-activated FGF signaling, and therefore, to the development of
endometrial carcinoma.
Loss of hSef expression is considered to be a common
mechanism in epithelial neoplasia. Decreased hSef expression was
identified in prostate cancer cells and advanced prostate cancer,
and siRNA-mediated hSef downregulation enhanced FGF-induced cell
migration and invasion in prostate cancer cells (24,25). In
in vitro studies, hSef overexpression was found to inhibit
cell proliferation, migration and invasive potential in prostate
cancer cells (26). Loss of hSef
expression was also observed in breast, thyroid and ovarian
carcinomas. The downregulation of hSef expression was found to be
associated with tumor progression (27). In addition, ectopic hSef expression
reduced the growth of breast cancer cells, while inhibition of hSef
expression accelerated FGF-induced growth in cervical cancer cells
(27).
In the current study, an unexpected increase in hSef
expression was observed in endometrial adenocarcinomas. As hSef is
inducible by FGF-mediated MAPK signaling, the upregulation of hSef
expression may be due to the elevated activity of MAPK. In previous
studies, plasmid-driven hSef expression has been demonstrated to
reduce FGF2-mediated MAPK/ERK signaling and cell growth in Ishikawa
cells, a well-differentiated endometrial adenocarcinoma cell line
(11). This indicates that hSef may
function as a tumor suppresser in endometrial cancer cells.
However, considering the current study, it is hypothesized that
hSef may be also involved in other mechanisms. These data indicate
the potential roles of hSef in the pathogenesis of endometrial
carcinomas.
In the present study, the blood vessels in
endometrial cancer tissues were also found to demonstrate decreased
hSef expression. The human endometrium undergoes cyclic changes of
degradation and reestablishment, which results in angiogenesis
being of considerable significance in the physiology and
carcinogenesis of the endometrium. Considering the critical role of
FGF signaling in the neovascularization of various solid tumors
(2–5),
the loss of hSef expression may facilitate the activity of FGF
signaling, subsequently promoting the neovascularization in
endometrial cancers. The potential role of hSef in the
neovascularization of tumors requires additional investigation.
The loss of Sprouty expression has been identified
in a series of human carcinomas, including prostate cancer
(28–30), breast carcinoma (31,32),
hepatocellular cancer (33,34), lung carcinoma (35), melanoma (36), colon cancer (37) and liver cancer (38). In addition, the downregulation of
Sprouty2 has been identified in endometrial carcinoma tissue
(39). In the present study, the loss
of Sprouty4 expression was also identified in human endometrial
adenocarcinoma tissue samples, indicating the possible role of
Sprouty4 in endometrial carcinogenesis.
Overall, to the best of our knowledge, the present
study demonstrated that increased hSef expression and decreased
Sprouty4 are present in endometrial carcinoma tissue, while
decreased hSef expression is observed in the blood vessels of
endometrial carcinoma. These data indicate the various roles of
negative regulators of FGF signaling in the pathology and genesis
of human endometrial carcinoma tissues. The present study provides
novel insight into the pathogenesis of endometrial cancer, and
suggests a potential therapeutic target for gene-target therapy of
the disease.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81170549 and 81300468), the
Shandong Province Excellent Youth Scientist Foundation (grant nos.
BS2013YY008 and 2009BSB14147) and the Shandong Natural Science
Foundation (grant no. ZR2011HM045).
References
|
1
|
Shing Y, Folkman J, Sullivan R,
Butterfield C, Murray J and Klagsbrun M: Heparin-affinity:
Purification of a tumor-derived capillary endothelial cell growth
factor. Science. 223:1296–1298. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soulitzis N, Karyotis I, Delakas D and
Spandidos DA: Expression analysis of peptide growth factors VEGF,
FGF2, TGFB1, EGF and IGF1 in prostate cancer and benign prostatic
hyperplasia. Int J Oncol. 29:305–314. 2006.PubMed/NCBI
|
|
3
|
Straume O and Akslen LA: Importance of
vascular phenotype by basic fibroblast growth factor and influence
of the angiogenic factors basic fibroblast growth factor/fibroblast
growth factor receptor-1 and EphA1/EphA2 on melanoma progression.
Am J Pathol. 160:1009–1019. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Compagni A, Wilgenbus P, Impagnatiello MA,
Cotten M and Christofori G: Fibroblast growth factors are required
for efficient tumor angiogenesis. Cancer Res. 60:7163–7169.
2000.PubMed/NCBI
|
|
5
|
Soufla G, Sifakis S, Baritaki S,
Zafiropoulos A, Koumantakis E and Spandidos DA: VEGF, FGF2, TGFB1
and TGFBR1 mRNA expression levels correlate with the malignant
transformation of the uterine cervix. Cancer Lett. 221:105–118.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Javerzat S, Auguste P and Bikfalvi A: The
role of fibroblast growth factors in vascular development. Trends
Mol Med. 8:483–489. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schlessinger J, Plotnikov AN, Ibrahimi OA,
Eliseenkova AV, Yeh BK, Yayon A, Linhardt RJ and Mohammadi M:
Crystal structure of a ternary FGF-FGFR-heparin complex reveals a
dual role for heparin in FGFR binding and dimerization. Mol Cell.
6:743–750. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thisse B and Thisse C: Functions and
regulations of fibroblast growth factor signaling during embryonic
development. Dev Biol. 287:390–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsang M and Dawid IB: Promotion and
attenuation of FGF signaling through the Ras-MAPK pathway. Sci
STKE. 2004:pe172004.PubMed/NCBI
|
|
11
|
Zhang H, Zhao X, Yan L and Li M: Similar
expression to FGF (Sef) reduces endometrial adenocarcinoma cells
proliferation via inhibiting fibroblast growth factor 2-mediated
MAPK/ERK signaling pathway. Gynecol Oncol. 122:669–674. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hacohen N, Kramer S, Sutherland D, Hiromi
Y and Krasnow MA: sprouty encodes a novel antagonist of FGF
signaling that patterns apical branching of the Drosophila airways.
Cell. 92:253–263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gross I, Bassit B, Benezra M and Licht JD:
Mammalian sprouty proteins inhibit cell growth and differentiation
by preventing ras activation. J Biol Chem. 276:46460–46468. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hanafusa H, Torii S, Yasunaga T, Matsumoto
K and Nishida E: Shp2, an SH2-containing protein-tyrosine
phosphatase, positively regulates receptor tyrosine kinase
signaling by dephosphorylating and inactivating the inhibitor
Sprouty. J Biol Chem. 279:22992–22995. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sasaki A, Taketomi T, Kato R, Saeki K,
Nonami A, Sasaki M, Kuriyama M, Saito N, Shibuya M and Yoshimura A:
Mammalian SPRY4 suppresses Ras-independent ERK activation by
binding to Raf1. Nat Cell Biol. 5:427–432. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yusoff P, Lao DH, Ong SH, Wong ES, Lim J,
Lo TL, Leong HF, Fong CW and Guy GR: Sprouty2 inhibits the Ras/MAP
kinase pathway by inhibiting the activation of Raf. J Biol Chem.
277:3195–3201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Billottet C, Elkhatib N, Thiery JP and
Jouanneau J: Targets of fibroblast growth factor 1 (FGF-1) and
FGF-2 signaling involved in the invasive and tumourigenic behavior
of carcinoma cells. Mol Biol Cell. 15:4725–4734. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Li M, Zheng X, Sun Y, Wen Z and
Zhao X: Endometriotic stromal cells lose the ability to regulate
cell-survival signaling in endometrial epithelial cells in vitro.
Mol Hum Reprod. 15:653–663. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abulafia O, Triest WE and Sherer DM:
Angiogenesis in malignancies of the female genital
tract. Gynecol Oncol. 72:220–231. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Macdonald SG, Crews CM, Wu L, Driller J,
Clark R, Erikson RL and McCormick F: Reconstitution of the
Raf-1-MEK-ERK signal transduction pathway in vitro. Mol Cell Biol.
13:6615–6620. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ballif BA and Blenis J: Molecular
mechanisms mediating mammalian mitogen-activated protein kinase
(MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ.
12:397–408. 2001.PubMed/NCBI
|
|
22
|
Giavazzi R, Sennino B, Coltrini D,
Garofalo A, Dossi R, Ronca R, Tosatti MP and Presta M: Distinct
role of fibroblast growth factor-2 and vascular endothelial growth
factor on tumour growth and angiogenesis. Am J Pathol.
162:1913–1926. 2013. View Article : Google Scholar
|
|
23
|
Hori A, Sasada R, Matsutani E, Naito K,
Sakura Y, Fujita T and Kozai Y: Suppression of solid tumor growth
by immunoneutralizing monoclonal antibody against human basic
fibroblast growth factor. Cancer Res. 51:6180–6184. 1991.PubMed/NCBI
|
|
24
|
Darby S, Sahadevan K, Khan MM, Robson CN,
Leung HY and Gnanapragasam VJ: Loss of Sef (similar expression to
FGF) expression is associated with high grade and metastatic
prostate cancer. Oncogene. 25:4122–4127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murphy T, Darby S, Mathers ME and
Gnanapragasam VJ: Evidence for distinct alterations in the FGF axis
in prostate cancer progression to an aggressive clinical phenotype.
J Pathol. 220:452–460. 2010.PubMed/NCBI
|
|
26
|
Darby S, Murphy T, Thomas H, Robson CN,
Leung HY, Mathers ME and Gnanapragasam VJ: Similar expression to
FGF (Sef) inhibits fibroblast growth factor-induced tumourigenic
behaviour in prostate cancer cells and is downregulated in
aggressive clinical disease. Br J Cancer. 101:1891–1899. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zisman-Rozen S, Fink D, Ben-Izhak O, Fuchs
Y, Brodski A, Kraus MH, Bejar J and Ron D: Downregulation of Sef,
an inhibitor of receptor tyrosine kinase signaling, is common to a
variety of human carcinomas. Oncogene. 26:6093–6098. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Thompson B, Ren C, Ittmann M and
Kwabi-Addo B: Sprouty4, a suppressor of tumor cell motility, is
down regulated by DNA methylation in human prostate cancer.
Prostate. 66:613–624. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kwabi-Addo B, Wang J, Erdem H, Vaid A,
Castro P, Ayala G and Ittmann M: The expression of Sprouty1, an
inhibitor of fibroblast growth factor signal transduction, is
decreased in human prostate cancer. Cancer Res. 64:4728–4735. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fritzsche S, Kenzelmann M, Hoffmann MJ,
Müller M, Engers R, Gröne HJ and Schulz WA: Concomitant
down-regulation of SPRY1 and SPRY2 in prostate carcinoma. Endocr
Relat Cancer. 13:839–849. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lo TL, Yusoff P, Fong CW, McCaw BJ,
Phillips WA, Yang H, Wong ES, Leong HF, Zeng Q, Putti TC, et al:
The ras/mitogen-activated protein kinase pathway inhibitor and
likely tumor suppressor proteins, sprouty 1 and sprouty 2 are
deregulated in breast cancer. Cancer Res. 64:6127–6136. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Faratian D, Sims AH, Mullen P, Kay C, Um
I, Langdon SP and Harrison DJ: Sprouty 2 is an independent
prognostic factor in breast cancer and may be useful in stratifying
patients for trastuzumab therapy. PLoS One. 6:e237722011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fong CW, Chua MS, McKie AB, Ling SH, Mason
V, Li R, Yusoff P, Lo TL, Leung HY, So SK, et al: Sprouty 2, an
inhibitor of mitogen-activated protein kinase signaling, is
down-regulated in hepatocellular carcinoma. Cancer Res.
66:2048–2058. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sirivatanauksorn Y, Sirivatanauksorn V,
Srisawat C, Khongmanee A and Tongkham C: Differential expression of
sprouty genes in hepatocellular carcinoma. J Surg Oncol.
105:273–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sutterlüty H, Mayer CE, Setinek U, Attems
J, Ovtcharov S, Mikula M, Mikulits W, Micksche M and Berger W:
Down-regulation of Sprouty2 in non-small cell lung cancer
contributes to tumor malignancy via extracellular signal-regulated
kinase pathway-dependent and-independent mechanisms. Mol Cancer
Res. 5:509–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qi J, Nakayama K, Gaitonde S, Krajewski S,
Eroshkin A, Bar-Sagi D, Bowtell D and Ronai Z: The ubiquitin ligase
Siah2 regulates tumorigenesis and metastasis by HIF-dependent and
-independent pathways. Proc Natl Acad Sci USA. 105:16713–16781.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng YH, Wu CL, Tsao CJ, Chang JG, Lu PJ,
Yeh KT, Uen YH, Lee JC and Shiau AL: Deregulated expression of
sprouty2 and microRNA-21 in human colon cancer: Correlation with
the clinical stage of the disease. Cancer Biol Ther. 11:111–121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee SA, Ho C, Roy R, Kosinski C, Patil MA,
Tward AD, Fridlyand J and Chen X: Integration of genomic analysis
and in vivo transfection to identify sprouty2 as a candidate tumor
suppressor in liver cancer. Hepatology. 47:1200–1210. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Velasco A, Pallares J, Santacana M, Gatius
S, Fernandez M, Domingo M, Valls J, Yeramian A, Encinas M, Dolcet
X, et al: Promoter hypermethylation and expression of sprouty 2 in
endometrial carcinoma. Hum Pathol. 42:185–193. 2011. View Article : Google Scholar : PubMed/NCBI
|