Introduction
Glioblastoma is the most common and lethal
intracranial malignant tumor with a yearly incidence of 3–4 per
100,000 (1). Despite the use of
multimodal treatments, the prognosis of glioblastoma has been
little improved, with a typical median survival time of only 12–15
months (2). Due to the diffuse
infiltration of glioblastoma cells into the brain tissues,
resection of the bulk tumor is typically followed by tumor
reinitiation at the resection site or at another location in the
brain. The highly aggressive and invasive properties of
glioblastoma cells contribute significantly to the poor prognosis
of cases involving this type of tumor (3). Therefore, it has been proposed that
anti-invasion agents may play a crucial role in the treatment of
glioblastoma (4).
The invasion of malignant cancer cells is favored by
degradation of the extracellular matrix (ECM), upregulation of
proteolytic enzyme activity and an increase in tumor cell motility
and invasive ability (5,6). Accumulating evidence has suggested that
matrix metalloproteinases (MMPs) are capable of promoting tumor
invasion and metastasis via degradation of the ECM (7), among which two gelatinases, MMP-2 and
MMP-9, have attracted the most attention (8). Rho GTPases are a family of small
GTP-binding proteins that serve as molecular switches in a wide
variety of cellular signaling pathways, ultimately inducing changes
in the organization of the actin cytoskeleton and regulating cell
motility (9). It has been reported
that RhoA activity is significantly reduced in astrocytic tumors
(10–12), and increased RhoA activity in
glioblastoma cells has been associated with impaired cell migration
and invasion through the rearrangement of actin into stress fibers
and the induction of focal adhesions (13).
Resveratrol is a natural polyphenol that exists in
grapes, berries and peanuts (14).
Previous studies reported that resveratrol was able to induce
apoptosis and suppress proliferation in various cancer cell types
(15,16). In addition, resveratrol has been
demonstrated to inhibit cell proliferation and induce apoptosis in
glioblastoma cells (17,18). However, the effects of resveratrol on
the motility and invasiveness of glioblastoma cells is not well
documented. Therefore, the current study aimed to examine the
effects of resveratrol on cell migration and invasion in human
glioblastoma cells, and to explore the underlying molecular
mechanisms.
Materials and methods
Cell culture and chemicals
Human glioblastoma U87MG, T98G and U251 cells were
obtained from the cell bank of the Fourth Military Medical
University (Xi'an, China) and cultured in Dulbecco's modified
Eagle's medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 10% fetal bovine serum (FBS; Shanghai BioSun
Sci&Tech Co., Ltd., Shanghai, China) at 37°C in a humidified
atmosphere of 5% CO2 and 95% air. Resveratrol (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was dissolved in
dimethyl sulfoxide (DMSO; Thermo Fisher Scientific, Grand Island,
NY, USA) to produce a 100 mM stock solution, which was stored at
−20°C. Prior to the experiments, fresh DMEM was used to dissolve
resveratrol stock solution to working concentrations. C3
transferase (Cytoskeleton Inc., Denver, CO, USA) and Y-27362
(#sc-216067A; Santa Cruz Biotechnology, Inc.) were added directly
to the media at concentrations of 0.25 µg/ml and 5 µg/ml,
respectively, and cells were harvested or analyzed following
treatment for 48 h. For each experiment, control groups were
treated with fresh DMEM containing 0.1% DMSO.
The Upstate® Rho Activation Assay Kit (#17–294) was
purchased from EMD Millipore (Lake Placid, NY, USA). Tris-HCl,
bromophenol blue and Triton X-100 were purchased from
Sigma-Aldrich. CaCl2, ZnCl2 and methanol were
purchased from Tianjin Tianli Chemical Co., Ltd. (Tianjin,
China).
Methyl thiazolyl tetrazolium (MTT)
assay
The cytotoxicity of resveratrol was measured using
an MTT assay (Sigma-Aldrich). U87MG, T98G and U251 cells were grown
in 96-well plates (Greiner Bio-One GmbH, Frickenhausen, Germany) at
a density of 5×104 cells/well and cultured for 24 h.
Cells were then treated with resveratrol at various concentrations
(20–100 µM). Following incubation for 48 h, 20 µl MTT (5 mg/ml) was
added to each well and incubated at 37°C for 4 h. Finally, 150 µl
DMSO was added to solubilize the formazan crystals, and the amount
of formazan salt was determined by measuring the optical density
(OD) at 490 nm using a Bio-Rad 680 microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Cell viability was
expressed as the percent OD value of each group relative to that of
control cells.
Wound-healing assay
Cells were seeded in 6-well culture plates
(5×105 cells/well) and grown to 80–90% confluence.
Monolayer cells were wounded by scratching the surface with a
sterile 200-µl pipette tip to create a gap of constant width, and
the cells were then incubated with serum-free DMEM. Following
incubation for 12 h, the medium was aspirated and the cells were
exposed to resveratrol treatment. The speed of wound healing was
considered to represent the motile capacity of the cells. Wound
closure was monitored and photographed at 0, 24 and 48 h by
phase-contrast microscopy (TS100 microscope; Nikon Corporation,
Tokyo, Japan). The gap widths were counted in five random
fields.
Matrigel transwell assay
Cell invasion was determined with Matrigel-coated
transwell cell culture chambers (8 µm pore size; EMD Millipore,
Billerica, MA, USA). Cells were incubated for 12 h in serum-free
medium, then trypsinized and resuspended in serum-free DMEM and
placed in the upper chamber of the transwell insert
(4×104 cells/well). Medium containing 10% FBS was
applied to the lower chamber as a chemoattractant. After incubating
the cells for 6 h, medium in the upper chamber was aspirated and
replaced by resveratrol solution. The chamber was then incubated
for 48 h at 37°C. At the end of the incubation, the invaded cells
were fixed with methanol and stained with hematoxylin and eosin
solution. The number of cells in five random fields were counted
under a Nikon TS100 light microscope.
Gelatin zymography
Gelatin (#G9391; Sigma-Aldrich) was dissolved in
distilled water at a concentration of 1%, autoclaved, cooled and
stored at 4°C. Cells were incubated in serum-free DMEM for 48 h.
The conditioned medium was collected and measured using a
bicinchoninic acid assay (#P0012; Beyotime Institute of
Biotechnology, Shanghai, China) to detect the protein
concentration. Samples (21 µl) with equal protein concentrations
were mixed with 7 µl sample buffer containing 0.32 M Tris base, 4%
sodium dodecyl sulfate (SDS), 16% glycerin, and 0.1% bromphenol
blue (pH 7.6), and loaded onto 8% SDS-polyacrylamide gels that had
been copolymerized with 0.1% gelatin (SDS-PAGE gel preparation kit;
#P0012A; Beyotime Institute of Biotechnology). Electrophoresis was
performed at 4°C. The gels were then eluted in wash buffer (50 mM
Tris-HCl, pH 7.6; 5 mM CaCl2; 2.5% Triton X-100)to
remove SDS, rinsed in rinsing buffer (50 mM Tris-HCl, pH 7.6; 5 mM
CaCl2) and then incubated in reaction buffer [50 mM
Tris-HCl, pH 7.6; 5 mM CaCl2; 1 µM ZnCl2;
0.02% Brij-35 (Santa Cruz Biotechnology, Inc.)] at 37°C. The gel
was stained with 0.05% Coomassie Blue (Beyotime Institute of
Biotechnology) in 30% methanol/10% acetic acid for 3 h and
de-stained in 30% methanol/10% acetic acid. The activity of
gelatinases was observed as opaque unstained bands.
Western blot analysis
Western blot analysis was performed as previously
described (18). In brief, equivalent
amounts (25 µg) of protein lysates were loaded into each well,
separated by 8% SDS-PAGE and transferred to nitrocellulose blotting
membranes (0.22 µm; EMD Millipore). The membranes were probed with
rabbit polyclonal antibodies against human MMP-2 (#BS1236; Bioworld
Technology, Inc., St. Louis Park, MN, USA; dilution, 1:1,000),
phosphorylated (p) myosin phosphatase target subunit 1 (MYPT1;
#BS4114; Bioworld Technology, Inc.; dilution, 1:1,000) and β-actin
(#sc-130656; Santa Cruz Biotechnology, Inc.; dilution, 1:2,000),
followed by incubation for 1 h at room temperature with horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(#sc-2004; dilution, 1:5,000; Santa Cruz Biotechnology, Inc.). The
membranes were then developed using an ECL system (Cell Signaling
Technology, Beverly, MA, USA). The grayscale values of each band on
the blots were measured using BandScan software version 4.3 (Glyko
Biomedical Ltd., Novato, CA, USA); the integrated density of each
band was normalized to the corresponding human β-actin band.
Representative results from at least three independent experiments
are shown.
Measurement of RhoA activity
RhoA activity was measured using a pull-down assay
(Upstate® Rho Activation Assay Kit) according to the manufacturer
instructions. Briefly, protein lysates and Rhotekin agarose were
incubated for 45 min at 4°C with gentle agitation. Following
thorough washes, the samples were boiled for 5 min in Laemmli
buffer [60 mM Tris-HCl, pH 6.8; 2% SDS; 10% glycerin (Tianjin
Yongda Chemical Reagent Co., Ltd., Tianjin City, China); 5%
β-mercaptoethanol (Sigma–Aldrich); 0.1% bromphenol blue] to detach
active GTP-bound Rho and loaded on SDS-PAGE gels and immunoblotted
using the anti-RhoA antibody included with the assay kit. The
subsequent procedure was performed as for the western blot
analysis.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments, and were evaluated by one-way
analysis of variance using SPSS software version 13.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate
statistically significant differences.
Results
Resveratrol suppresses human
glioblastoma cell migration and invasion in vitro
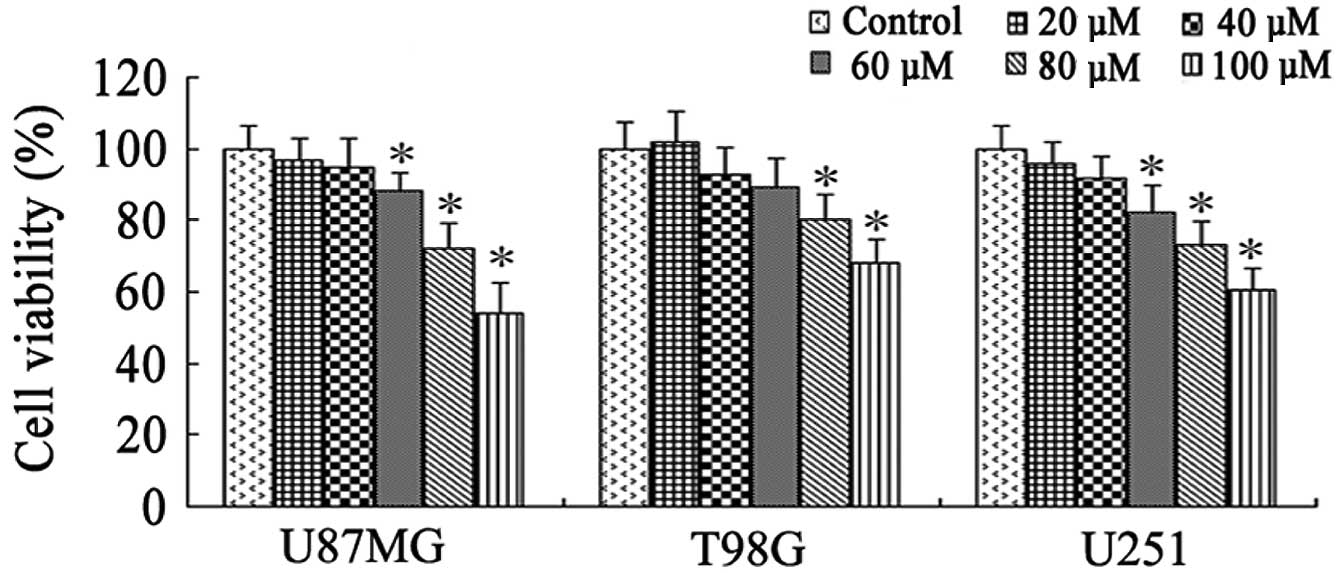
To rule out a cytotoxic effect on cell number
following resveratrol treatment, the viability of U87MG, T98G and
U251 cells treated with increasing concentrations of resveratrol
(20–100 µM) for 48 h was assessed by MTT assay. Resveratrol
inhibited the cell viability of glioblastoma cells in a
concentration-dependent manner; however, it had only marginal
effects on cell viability up to a concentration of 40 µM in all
three cell lines (P>0.05; Fig. 1).
However, treatment with 60 µM resveratrol demonstrated significant
cytotoxic effects on the cell viability of U87MG and U251 cells
(P<0.05; Fig. 1). Thus, the
non-cytotoxic concentration of 40 µM resveratrol was used in the
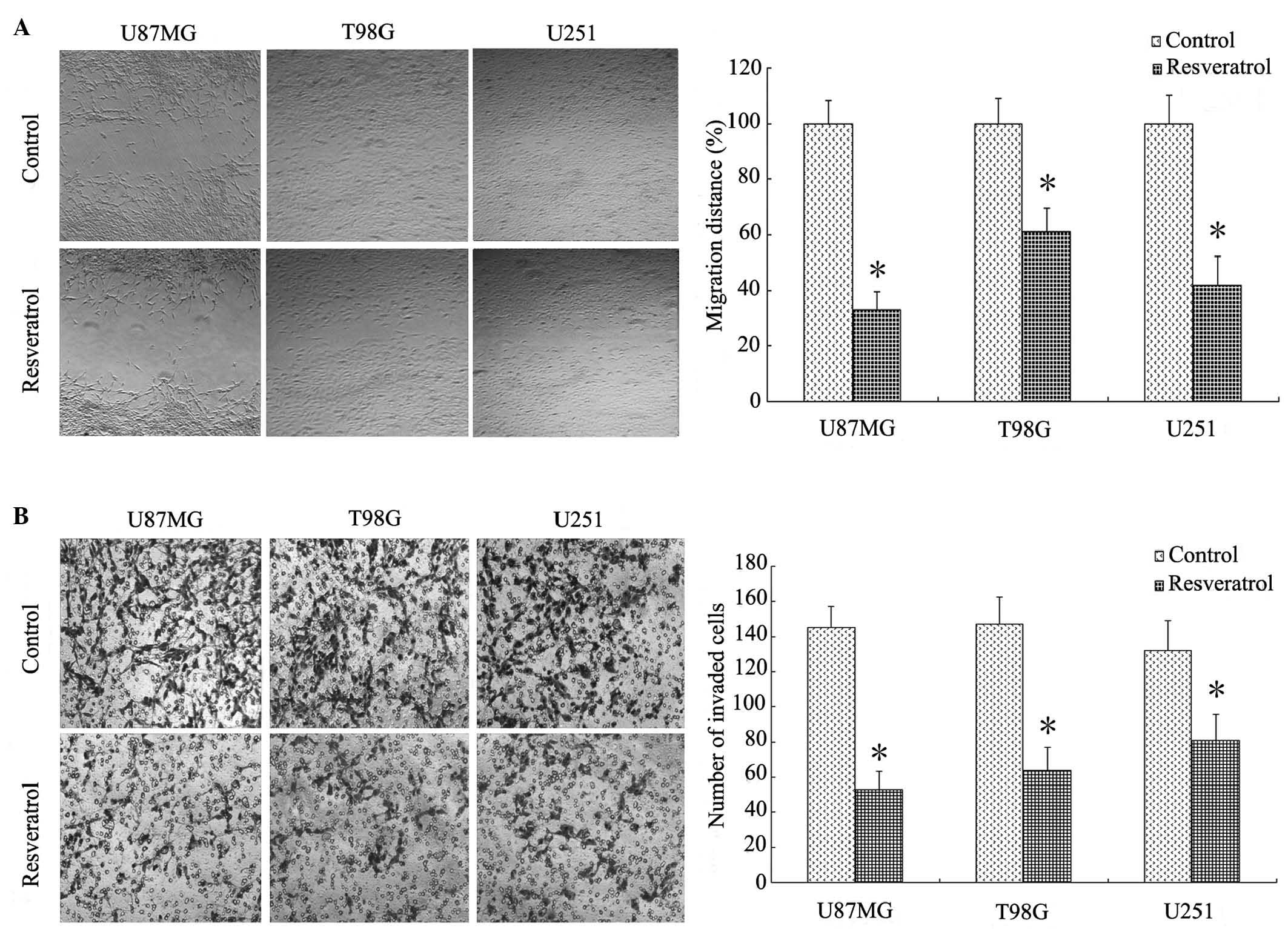
subsequent experiments. To evaluate the effects of resveratrol on
cell migration and invasion, wound-healing and Matrigel transwell
assays were performed, revealing that resveratrol significantly
inhibited human glioblastoma cell migration and invasion in
vitro. Specifically, resveratrol reduced the migration distance
of U87MG cells to 33.5±6.5% relative to the control group (Fig. 2A; P<0.001), and decreased the
number of U87MG cells invading through the matrigel by ~64%
(Fig. 2B; P<0.001) following
incubation for 48 h. Similar results were observed in T98G and U251
cells to a lesser degree (Fig.
2).
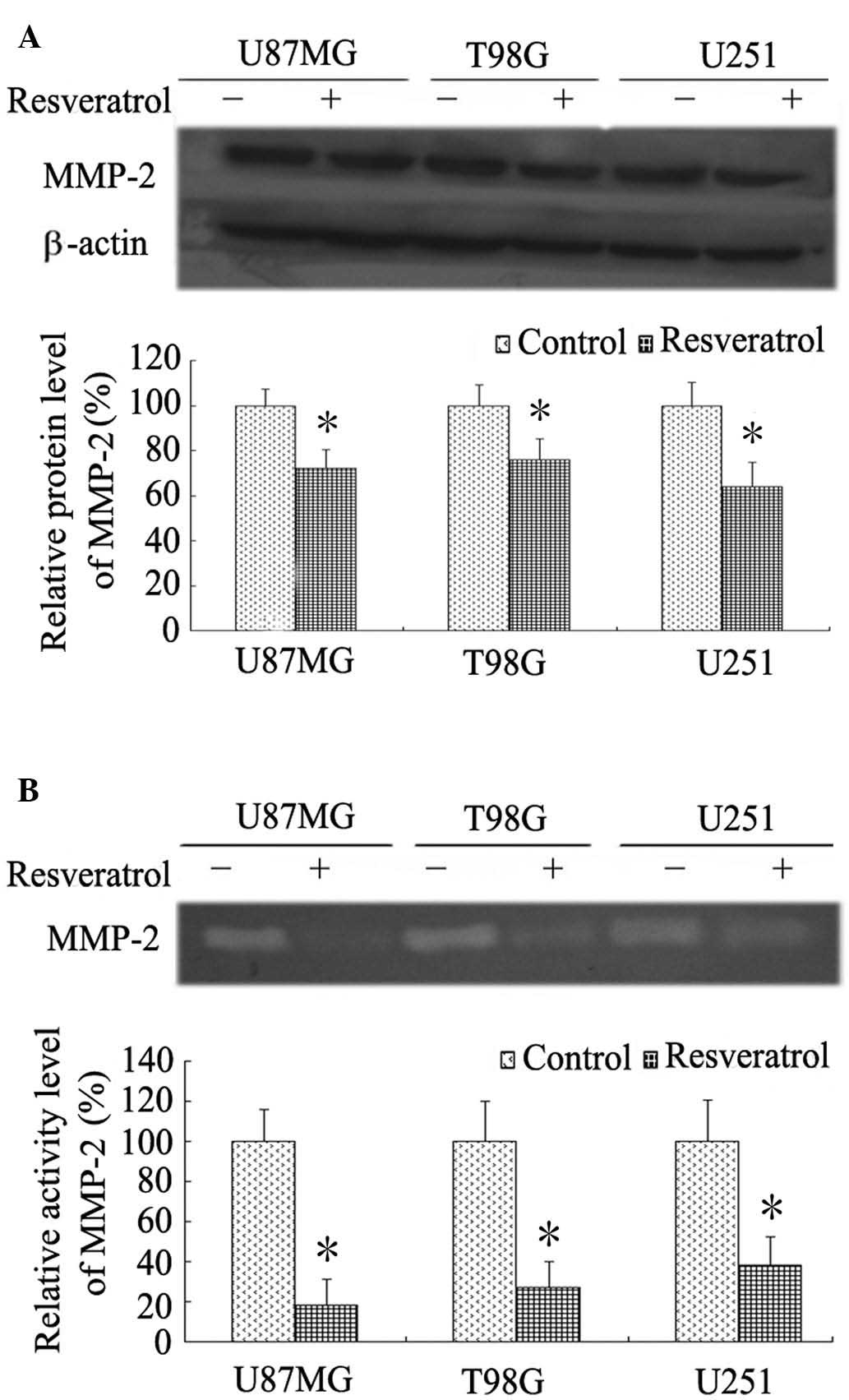
Resveratrol inhibits activity and
expression of MMP-2 in glioblastoma cells
MMPs, particularly MMP-2, are reported to be crucial
for cell migration and invasion in numerous types of tumor,
including glioblastoma (19). Using
western blot analysis, resveratrol was observed to induce a marked
reduction in MMP-2 protein expression in all three glioblastoma
cell lines (P<0.001; Fig. 3A).
Furthermore, gelatin zymographic analyses were performed to
determine the MMP-2 gelatinolytic activity. As indicated by
Fig. 3B, the suppression of MMP-2
activity due to resveratrol was confirmed in the three cell lines,
with a reduction to 20–40% relative to each control group
(P<0.001).
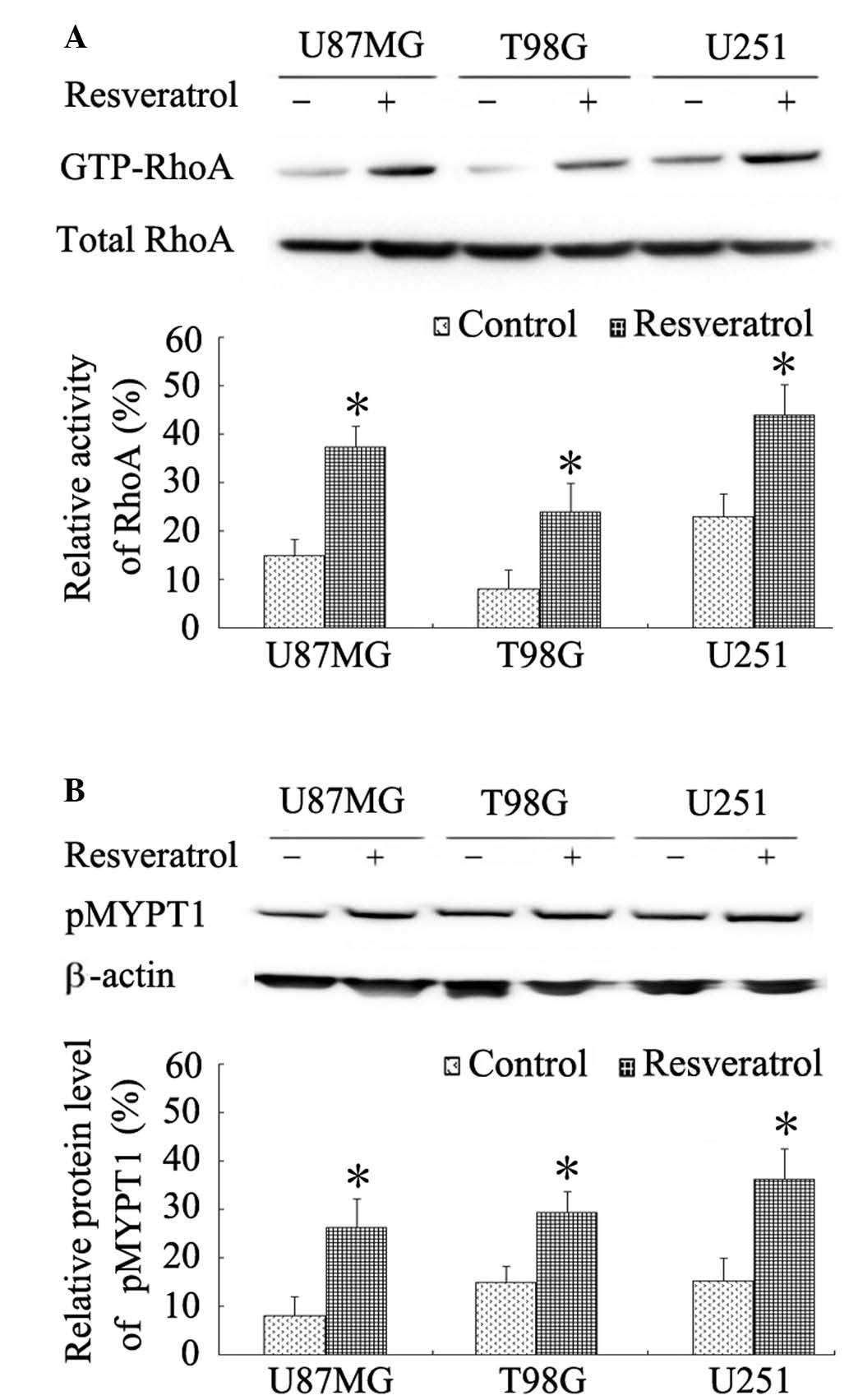
Resveratrol increases activation of
RhoA in glioblastoma cells
The Rho family GTPase RhoA has been implicated in
the regulation of cell contraction and retraction forces that are
required for cell migration and invasion (20). In the current study, the activity of
RhoA in glioblastoma cells in response to resveratrol was examined
using a pull-down assay. The results revealed that resveratrol
significantly increased activity of RhoA in glioblastoma cells
(P<0.001; Fig. 4A). Despite the
variations of basal RhoA activity among the different glioblastoma
cell lines, the increases in RhoA activity induced by resveratrol
were 2- to 3-fold higher than the baselines. Furthermore, to
confirm the activation of RhoA/Rho-associated kinase (ROCK)
signaling by resveratrol, the phosphorylation of MYPT1, a
downstream target of ROCK, was examined. The results revealed that
resveratrol significantly induced MYPT1 phosphorylation in
glioblastoma cells (P<0.001; Fig.
4B).
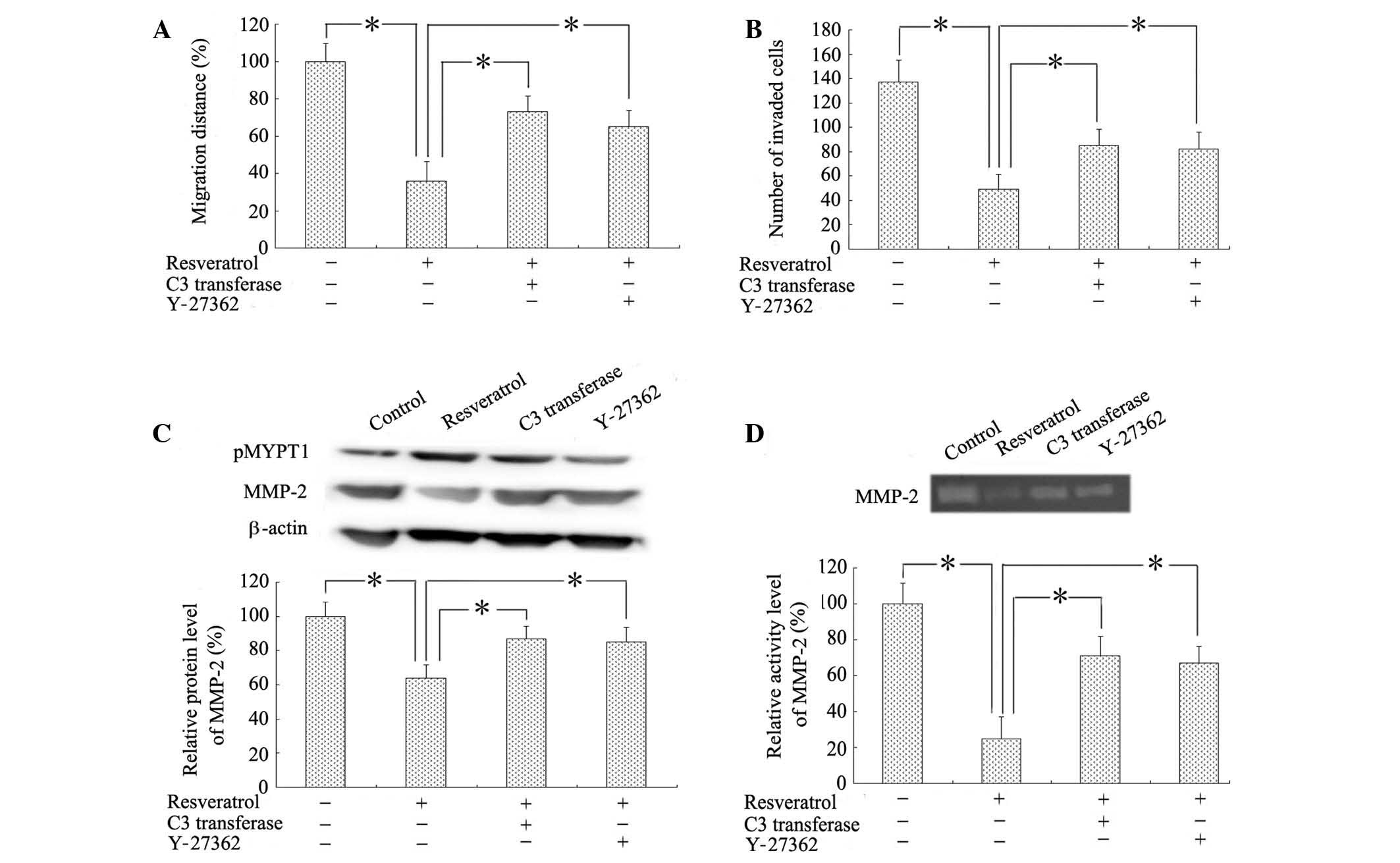
Blockade of the RhoA/ROCK pathway
reverses the inhibition of migration and invasion in glioblastoma
cells induced by resveratrol
As U87MG cells manifested more sensitive responses
to resveratrol treatment, they were selected for use in the
subsequent experiments. Wound-healing and Matrigel transwell assays
were performed using U87MG cells in the presence of resveratrol
alone or in combination with a specific cell-permeable RhoA
inhibitor (C3 transferase; 0.25 µg/ml), or ROCK inhibitor (Y-27362;
5 µg/ml). Consistently with our previous data, resveratrol led to
significant reductions in the rates of cell migration and invasion
relative to that of untreated controls; cell migration was reduced
to ~30% (P<0.001), whilst the number of matrigel-invaded cells
was decreased to ~40% (P<0.001). By contrast, inhibition of
either RhoA or ROCK attenuated the resveratrol-induced reductions
in cell migration and invasion, partially restoring the rates of
migration and invasion to that of untreated cells (Fig. 5A and B). Next, the ability of
inhibition of RhoA/ROCK signaling to interfere with MMP-2
expression and activity was investigated. As expected, western blot
and zymographic analyses revealed that blocking RhoA/ROCK signaling
partially rescued the decreased expression and activity of MMP-2
induced by resveratrol (P<0.001; Fig.
5C and D).
Discussion
The highly diffuse infiltration of tumor cells
typically prevents a successful local treatment for glioblastoma
(21). The present study demonstrated
the ability of resveratrol to reduce cell migration and invasion in
human glioblastoma cells in vitro. Furthermore, the results
indicated that activation of RhoA and inhibition of MMP-2 activity
may be responsible for the inhibitory effects of resveratrol on
cell migration and invasion in glioblastoma cell lines. Thus, this
natural dietary compound may be a promising therapeutic agent for
the treatment of glioblastoma. Resveratrol is a natural polyphenol
that is synthesized by a wide range of plant species. Over the past
decades, numerous studies have reported that resveratrol inhibits
cell proliferation, induces apoptosis in various kinds of cancer
cells in vitro, and retards the growth of implanted tumors
in vivo (15,16,22–24). More
recent evidence has indicated that resveratrol is able to sensitize
cancers to chemotherapy with cisplatin and other conventional
anticancer therapeutics (25). Our
previous studies had demonstrated that resveratrol was able to
inhibit cell proliferation in U87MG and T98G cells, and could also
reverse temozolomide resistance by downregulation of
O-6-methylguanine-DNA methyltransferase in T98G glioblastoma cells
(18,26,27). The
present study further indicated that resveratrol is able to inhibit
cell migration and invasion in U87MG, U251 and T98G glioblastoma
cells via activation of the RhoA/ROCK pathway.
Tumor cells achieve their invasive ability through
the secretion and activation of proteolytic enzymes, including
serine, metallo- and cysteine proteases, which can degrade ECM
components and break down other natural barriers to tumor invasion
(5,6).
A number of reports have revealed that elevated expression of MMPs
is closely associated with the invasion and aggressiveness of tumor
cells, including glioblastoma (8,28,29). Certain MMPs have become promising
therapeutic targets for the development of anticancer drugs
(30,31). The current results indicated that
resveratrol repressed the expression and secretion of MMP-2 in
glioblastoma cells, and that cell invasive ability was markedly
impaired following MMP-2 gene silencing (data not shown). In
accordance with these results, Gagliano et al (32) demonstrated that resveratrol is able to
decrease the expression of MMP-2 in primary cultured glioblastoma
cells. Notably, MMP-2 is not the only tumor invasion mediator
targeted by resveratrol. In our previous study, resveratrol
repressed YKL-40 expression and inhibited U87MG cell invasion
(26). Another study also
demonstrated that resveratrol reduced U373MG human glioma cell
invasion through decreasing plasminogen activator and its specific
receptor expression (33); urokinase
plasminogen activator (uPA) and the uPA receptor are important
mediators of cell migration and invasion in various cell types
(34).
Rho GTPases, which serve as binary switches that
cycle between an active GTP-bound form and an inactive GDP-bound
form, are a class of key regulators of the actin cytoskeleton
(9). Rho GTPases also coordinate the
regulation of other cellular activities, including gene
transcription, cell morphological changes and migration (35). RhoA, a well-known member of the Rho
protein family, has been reported to be significantly
underexpressed in astrocytoma and inversely correlated with tumor
malignancy (10). The increase of
RhoA activity in glioblastoma cells is reportedly linked with
impaired cell migration and invasion through the rearrangement of
actin into stress fibers and the induction of focal adhesions
(12,36). The role of RhoA is mediated through a
major effector, ROCK, activation of which has been shown to inhibit
migration and invasion of astrocytoma cells (37). In the current study, based on the
observation that resveratrol activated RhoA in glioblastoma cells,
and that blockade of the RhoA/ROCK pathway attenuated the
resveratrol-induced inhibition of cell migration and invasion, we
hypothesize that the inhibitory effect of resveratrol on migration
and invasion in glioblastoma cells may be mediated through the
RhoA/ROCK pathway. A similar finding was reported in human
umbilical vein endothelial cells, in which resveratrol inhibited
cell migration through a RhoA/ROCK-dependent mechanism (38). With regard to the effect of the
RhoA/ROCK pathway on MMP-2, however, there have been controversial
reports: Inhibition of RhoA/ROCK was observed to increase MMP-2
expression and activity in microvascular endothelial cells, whereas
MMP-2 activity was decreased in osteosarcoma cells (39,40). The
mechanisms underlying the regulation of MMPs by RhoA and the effect
on cell migration and invasion require further investigation.
Notably, in the present study, the inhibition of cell migration and
invasion by resveratrol was only partially restored by blocking the
RhoA/ROCK pathway, suggesting the existence of other signaling
pathways associated with resveratrol in glioblastoma cells.
In summary, the present findings indicate that
resveratrol may inhibit glioblastoma cell motility and invasiveness
via downregulation of MMP-2 and activation of the RhoA/ROCK
signaling pathway. These findings highlight resveratrol as a
promising therapeutic agent for glioblastoma patients, and form a
basis for further investigation of this natural dietary
compound.
Acknowledgements
The authors would like to thank Miss Juan Li (Xijing
Institute of Clinical Neuroscience, Xi'an, China) for her technical
assistance. This study was supported by the Chinese National
Natural Science Foundation, (grant no. 81471266).
Glossary
Abbreviations
Abbreviations:
|
ECM
|
extracellular matrix
|
|
MTT
|
methyl thiazolyl tetrazolium
|
|
MMP
|
matrix metalloproteinase
|
References
|
1
|
Taylor JW, Chi AS and Cahill DP: Tailored
therapy in diffuse gliomas: Using molecular classifiers to optimize
clinical management. Oncology (Williston Park). 27:504–514.
2013.PubMed/NCBI
|
|
2
|
Van Meir EG, Hadjipanayis CG, Norden AD,
et al: Exciting new advances in neuro-oncology: The avenue to a
cure for malignant glioma. CA Cancer J Clin. 60:166–193. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alves TR, Lima FR, Kahn SA, et al:
Glioblastoma cells: A heterogeneous and fatal tumor interacting
with the parenchyma. Life Sci. 89:532–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Munson J, Bonner M, Fried L, et al:
Identifying new small molecule anti-invasive compounds for glioma
treatment. Cell Cycle. 12:2200–2209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Westermarck J and Kähäri VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.PubMed/NCBI
|
|
6
|
Kleiner DE and Stetler-Stevenson WG:
Matrix metalloproteinases and metastasis. Cancer Chemother
Pharmacol. 43(Suppl): S42–S51. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forsyth PA, Wong H, Lainq TD, et al:
Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix
metalloproteinase-1 (MT1-MMP) are involved in different aspects of
the pathophysiology of malignant gliomas. Br J Cancer.
79:1828–1835. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hall A: Rho GTPases and the actin
cytoskeleton. Science. 279:509–514. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nutt CL, Mani DR, Betensky RA, et al: Gene
expression-based classification of malignant gliomas correlates
better with survival than histological classification. Cancer Res.
63:1602–1607. 2003.PubMed/NCBI
|
|
11
|
Forget MA, Desrosiers RR, Del M, Moumdjian
R, Shedid D, Berthelet F and Béliveau R: The expression of rho
proteins decreases with human brain tumor progression: Potential
tumor markers. Clin Exp Metastasis. 19:9–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malchinkhuu E, Sato K, Maehama T, Mogi C,
Tomura H, Ishiuchi S, Yoshimoto Y, Kurose H and Okajima F: S1P (2)
receptors mediate inhibition of glioma cell migration through Rho
signaling pathways independent of PTEN. Biochem Biophys Res Commun.
366:963–968. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldberg L and Kloog Y: A Ras inhibitor
tilts the balance between Rac and Rho and blocks
phosphatidylinositol 3-kinasedependent glioblastoma cell migration.
Cancer Res. 66:11709–11717. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brakenhielm E, Cao R and Cao Y:
Suppression of angiogenesis, tumour growth and wound healing by
resveratrol, a natural compound in red wine and grapes. FASEB J.
15:1798–1800. 2001.PubMed/NCBI
|
|
17
|
Zhang W, Fei Z, Zhen H, Zhang JN and Zhang
X: Resveratrol inhibits cell growth and induces apoptosis of rat C6
glioma cells. J Neurooncol. 81:231–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin H, Xiong W, Zhang X, Liu B, Zhang W,
Zhang Y, Cheng J and Huang H: Notch-1 activation-dependent p53
restoration contributes to resveratrol-induced apoptosis in
glioblastoma cells. Oncol Rep. 26:925–930. 2011.PubMed/NCBI
|
|
19
|
Philip S, Bulbule A and Kundu GC: Matrix
metalloproteinase-2: Mechanism and regulation of NF-kappaB-mediated
activation and its role in cell motility and ECM-invasion.
Glycoconj J. 21:429–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuoka T, Yashiro M, Kato Y, Shinto O,
Kashiwagi S and Hirakawa K: RhoA/ROCK signaling mediates plasticity
of scirrhous gastric carcinoma motility. Clin Exp Metastasis.
28:627–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giese A, Bjerkvig R, Berens ME and
Westphal M: Cost of migration: invasion of malignant gliomas and
implications for treatment. J Clin Oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu H, Liang X, Fang Y, Qin X, Zhang Y and
Liu J: Resveratrol inhibits hypoxia-induced metastasis potential
enhancement by restricting hypoxia-induced factor-1 alpha
expression in colon carcinoma cells. Biomed Pharmacother.
62:613–621. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kundu JK and Surh YJ: Cancer
chemopreventive and therapeutic potential of resveratrol:
Mechanistic perspectives. Cancer Lett. 269:243–261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodrigue CM, Porteu F, Navarro N, Bruyneel
E, Bracke M, Romeo PH, Gespach C and Garel MC: The cancer
chemopreventive agent resveratrol induces tensin, a cell-matrix
adhesion protein with signaling and antitumor activities. Oncogene.
24:3274–3284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
EI-Mowafy AM, EI-Mesery ME, Salem HA,
Al-Gayyar MM and Darweish MM: Prominent chemopreventive and
chemoenhancing effects for resveratrol: Unraveling molecular
targets and the role of C-reactive protein. Chemotherapy. 56:60–65.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang W, Murao K, Zhang X, Matsumoto K,
Diah S, Okada M, Miyake K, Kawai N, Fei Z and Tamiya T: Resveratrol
represses YKL-40 expression in human glioma U87 cells. BMC Cancer.
10:5932010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang H, Lin H, Zhang X and Li J:
Resveratrol reverses temozolomide resistance by downregulation of
MGMT in T98G glioblastoma cells by the NF-κB-dependent pathway.
Onclo Rep. 27:2050–2056. 2012.
|
|
28
|
Libra M, Scalisi A, Vella N, Clementi S,
Sorio R, Stivala F, Spandidos DA and Mazzarino C: Uterine cervical
carcinoma: Role of matrix metalloproteinases (review). Int J Oncol.
34:897–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rao JS, Steck PA, Mohanam S,
Stetler-Stevenson WG, Liotta LA and Sawaya R: Elevated levels of
M(r) 92,000 type IV collagenase in human brain tumors. Cancer Res.
53(10 Suppl): 2208–2211. 1993.PubMed/NCBI
|
|
30
|
Gabelloni P, Da Pozzo E, Bendinelli S,
Costa B, Nuti E, Casalini F, Orlandini E, Da Settimo F, Rossello A
and Martini C: Inhibition of metalloproteinases derived from
tumours: New insights in the treatment of human glioblastoma.
Neurosci. 168:514–522. 2010. View Article : Google Scholar
|
|
31
|
Kim SY, Lee EJ, Woo MS, Jung JS, Hyun JW,
Min SW, Kim DH and Kim HS: Inhibition of matrix metalloproteinase-9
gene expression by an isoflavone metabolite, irisolidone in U87MG
human astroglioma cells. Biochem Biophys Res Commun. 366:493–499.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gagliano N, Moscheni C, Torri C, Magnani
I, Bertelli AA and Gioia M: Effect of resveratrol on matrix
metalloproteinase-2 (MMP-2) and secreted protein acidic and rich in
cysteine (SPARC) on human cultured glioblastoma cells. Biomed
Pharmacother. 59:359–364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ryu J, Ku BM, Lee YK, Jeong JY, Kang S,
Choi J, Yang Y, Lee DH, Roh GS, Kim HJ, et al: Resveratrol reduces
TNF-α-induced U373MG human glioma cell invasion through regulating
NF-κB activation and uPA/uPAR expression. Anticaner Res.
31:4223–4230. 2011.
|
|
34
|
Guo J, Fan KX, Xie L, Xiao JJ, Chen K, Hui
LN and Xu ZH: Effect and prognostic significance of the KAI1 gene
in human gastric carcinoma. Oncol Lett. 10:2035–2042.
2015.PubMed/NCBI
|
|
35
|
Nobes CD and Hall A: Rho, rac and cdc42
GTPases regulate the assembly of multimolecular focal complexes
associated with actin stress fibers, lamellipodia and filopodia.
Cell. 81:53–62. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khalil BD, Hanna S, Saykali BA, El-Sitt S,
Nasrallah A, Marston D, El-Sabban M, Hahn KM, Symons M and El-Sibai
M: The regulation of RhoA at focal adhesions by StarD13 is
important for astrocytoma cell motility. Exp Cell Res. 321:109–122.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Salhia B, Rutten F, Nakada M, Beaudry C,
Berens M, Kwan A and Rutka JT: Inhibition of Rho-kinase affects
astrocytoma morphology, motility and invasion through activation of
Rac1. Cancer Res. 65:8792–8800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cicha I, Regler M, Urschel K,
Goppelt-Struebe M, Daniel WG and Garlichs CD: Resveratrol inhibits
monocytic cell chemotaxis to MCP-1 and prevents spontaneous
endothelial cell migration through Rho kinase-dependent mechanism.
J Atheroscler Thromb. 18:1031–1042. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ispanovic E, Serio D and Haas TL: Cdc42
and RhoA have opposing roles in regulating membrane type 1-matrix
metalloproteinase localization and matrix metalloproteinase-2
activation. Am J Physiol Cell Physiol. 295:C600–C610. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fromigué O, Hamidouche Z and Marie PJ:
Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces
osteosarcoma cell invasion. J Biol Chem. 283:30549–30556. 2008.
View Article : Google Scholar : PubMed/NCBI
|