Introduction
Prostate cancer (PCa) is one of the leading causes
of male urological cancer-associated mortality worldwide. Early
stage PCa is androgen-dependent, and may be treated effectively
using androgen ablation therapy, radiation and/or surgery. Although
these treatments have largely been observed to be successful, there
are associated disadvantages, including surgical injuries, drug
resistance and tumor recurrence (1–3). In
addition, all patients with PCa eventually progress to an
androgen-independent state, known as castration-resistant prostate
cancer (CRPC), which may lead to tumor metastasis or patient
mortality (4). At present, there are
no effective treatment options available for CRPC (5). Therefore, the development of novel
therapeutic strategies is critical (6).
Gene therapy may be utilized to transfer normal
target genes into abnormal cells, in order to achieve therapeutic
benefits (7). At present, gene
therapy is considered to be a novel method for the treatment of a
number of diseases, and may represent a promising strategy for the
treatment of cancer. In order to develop effective methods of gene
transfer, numerous experimental and clinical studies have been
undertaken (8). Tumor protein P53
(P53) has a critical role in a number of significant biological
processes, including DNA repair, apoptosis, cell cycle, autophagy,
senescence and angiogenesis (9).
There are two types of P53: Wild-type (wt-P53), which promotes cell
apoptosis, and mutant P53, which may enhance the growth of
cancerous cells (10). Inactivation
of the P53 system is associated with the onset and progression of
cancer, and is a typical alteration characteristic of cancer cells:
When this occurs, a P53-activating agent does not appear to work
(11,12). A number of studies have also
demonstrated that P53 is capable of stimulating autophagy (13–15).
ULK1 is part of a ULK1-complex, which contributes to
various physiological and pathological processes in mammals and is
an important protein in the early stages of autophagy (16). The upregulation of ULK1 allows for
sustained autophagy and leads to an increase in non-apoptotic cell
death. Notably, previous studies have indicated that ULK1 is a
transcriptional target of p53 following DNA damage (17).
Previous studies have revealed that ultrasound,
electric, laser and microinjection techniques may assist in the
delivery of various therapeutic molecules, including genes and
drugs (18–20). However, ultrasound is considered to be
a ‘gentle’ technique. A number of studies have been undertaken to
investigate how ultrasound may be capable of enhancing permeability
of cells. The main mechanism is considered to be sonoporation.
Sonoporation is similar to electroporation and refers to the
generation of small pores in the cell membrane following
irradiation by ultrasound and this has been revealed by high speed
camera images (21); sonoporation is
a non-invasive method that allows drugs or genes to enter cells
(22,23). Sonoporation may result from
oscillations of the microbubbles, which cause cavitation and
subsequent disruption of membrane that allows to enhance
permeability (24). These
microbubbles, oscillating in the presence of US, create localized
shear stress or ‘microstreaming’ or they may expand and collapse
(‘transient cavitation’) to create intense local heating and
pressure (25). This type of
transient cavitation effect is considered to occur more at low
frequencies (26). Previous research
has suggested that the use of ultrasound combined with microbubbles
may enhance the acoustic cavitation effect (27). However, further study is required in
order to enhance transfection efficiency whilst simultaneously
reducing harm to the body.
Materials and methods
Cell lines
The PC3 human prostate cancer cell line (The Cell
Bank of the Chinese Academy of Sciences, Shanghai, China) was used
in all experiments. Cells were incubated at 37°C in an atmosphere
of 5% CO2. Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; Gibco, Life
Technologies, Carlsbad, CA, USA) was used as culture medium and
replaced every second day.
Ultrasound apparatus and
microbubbles
The present experiment was performed using an FS-450
ultrasonic processor, supplied by the Shanghai Institute of
Ultrasound in Medicine (Shanghai, China). In all experiments, the
probe frequency was fixed at 21 kHz, the intensity was 4.6
mW/cm2 and the duty cycle was 20% (2 sec ‘on’ time and 8
sec ‘off’ time) with a total exposure time of 5 min. The SonoVue™
microbubble echo-contrast agent (Bracco S.p.A, Milan, Italy) was
reconstituted in 5 ml phosphate-buffered saline, 200 µl of which
was used in each experimental group.
In the present study, cells were divided into three
groups: A control group without treatment (Group A), a group
treated with Lipofectamine® (Invitrogen Life Technologies,
Carlsbad, CA, USA) and wt-P53/enhanced green fluorescent protein
(EGFP) plasmid (Group B), and a group treated with Lipofectamine,
wt-P53/EGFP plasmid and ultrasound combined with microbubbles
(Group C). Experiments were performed in triplicate.
Preparation of plasmid DNA
The pEGFP plasmid (donated by the Center Laboratory
of Shanghai Jiao Tong University Affiliated Sixth People's
Hospital, Shanghai, China) DNA was used as a marker to indicate
transfection efficiency and was prepared with the E.ZN.A Plasmid
Miniprep kit II (Omega Bio-Tek, Inc., Norcross, GA, USA) in an
identical manner to the wt-P53 plasmid DNA. The lysing solution
from the Plasmid Miniprep kit was able to generate and lyse DH5α
Escherichia coli transformants, which were of a density
capable of expressing the target plasmid (28). The DNA-specific resin in a column was
subsequently used to isolate plasmid DNA from genomic DNA, and the
plasmid DNA was collected. The purity of the extracted pEGFP
plasmid DNA was measured using an ultraviolet spectrophotometer
(DU800; Beckman Coulter, Inc., Brea, CA, USA) whose optical density
at 260/280 nm was 1.8. A digestive enzyme (SalI or
XhoI) and subsequent electrophoresis was used to identify
pEGFP plasmid DNA (BD Biosciences, ClonTech, Palo Alto CA). Two
restriction sites (SalI and XhoI) were included to
verify that the obtained plasmid was pEGFP when the genetic map was
analyzed.
Gene transfer
Transfection experiments were performed according to
the manufacturers's instructions. Transfection reagents were
prepared according to the protocol of the Lipofectamine® 2000 kit
(Invitrogen Life Technologies); the ratio of plasmid DNA (µg) to
liposome (µl) was 1:2. Group A were the control cells, group B were
the cells transfected with pEGFP/wt-P53 plasmid, and group C were
the cells transfected with pEGFP/wt-P53 plasmid and treated with
ultrsound combined with microbubble. Prior to ultrasound
irradiation, the reagent was added to the suspension of PC3 cells
in Group C. The density of the PC3 cells in polystyrene sample test
tubes (Beijing Donglinchangsheng Biotechnology Co., Ltd., Beijing,
China) was 1×105 cells/ml. Subsequently, the cell
suspension was gently mixed with diluted plasmid DNA in 100 µl DMEM
without FBS. Prior to use, Lipofectamine 2000 for Groups B and C
was mixed gently and the appropriate amount was diluted in 100 µl
DMEM without FBS. Following incubation for 5 min at room
temperature, the diluted plasmid DNA (1 µg/100 µl) was combined
with diluted Lipofectamine 2000 (2.5 µg/100 µl), mixed gently and
incubated for 20 min at room temperature. Following 5 min of
exposure to ultrasound with microbubbles, the cell suspensions were
seeded into 6-well plates and 4 h later the serum-free DMEM was
replaced by medium containing 10% FBS.
Detection of gene transfection
efficiency
Following 24 h of incubation, pEGFP transfection
efficiency was detected in the three groups using fluorescence
microscopy and flow cytometry using a flow cytometer (Beckman
Coulter, Fullerton, CA, USA). Images were captured using an A1RMP
fluorescence microscope (Nikon Corporation, Tokyo, Japan). Cells
were observed to express green fluorescence when successfully
transfected with pEGFP DNA.
Measurement of cell proliferation by
MTT assay
Following treatment, each group of cells was grown
in 96-well plates for 24 h, and subsequently viability was assessed
by MTT assay (Wellscan MK3; Ani Labsystems, Ltd., Oy, Vantaa,
Finland). The assay was conducted as follows: 50 µl MTT reagent was
incubated with cells for 4 h at 37°C and subsequently removed.
Following this, 150 µl dimethyl sulfoxide was added to each well
and agitated for 15 min. The optical density was measured at a
wavelength of 492 nm using a BIO-TEK Synergy HT microculture plate
reader (Bio-Tek Instruments, Inc., Winooski, VT, USA). The result
was calculated as follows: Viability (%)=absorbance of experimental
group/absorbance of control group × 100. Viability was calculated
based on the mean percentage of triplicate experiments.
Transmission electron microscopy
PC3 cells from the three groups were sliced into
ultra-thin specimens according to the conventional method (29). Specimens were then observed and images
were captured using transmission electron microscopy (Hitachi
S-4800; Hitachi, Ltd., Tokyo, Japan) at magnification, ×24,500.
Autophagosomes were identified by the characteristic structure of a
double- or multi-lamellar smooth membrane completely surrounding
compressed mitochondria, or membrane-bound electron-dense material.
The investigator was blinded, and the numbers of autophagosomes in
cells of each of the three groups were counted in 10 fields of view
from various microscopic fields.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Once grown to ~85% confluence, 5×106
cells were harvested and the RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer's
instructions. The first strand of cDNA was generated from 2 mg
total RNA using the SuperScript III Reverse Transcriptase kit
(Invitrogen Life Technologies) according to the manufacturer's
instructions. Following purification, RNA was subjected to PCR
analysis on a Bio-Rad iQ5 multicolor detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), using the following
primers: ULK1, F 5′-TCGAGTTCTCCCGCAAGG-3′ and CGTCTGAGACTTGGCGAGGT.
The probe for ULK1 was CACCGCGAGAAGCACGATTTGGA. PCR was performed
under the following conditions: 95°C for 3 min followed by 40
cycles of 95°C for 10 sec and 60°C for 30 sec. GAPDH expression
levels served as a control. The relative expression levels were
calculated using the relative quantitative 2−ΔΔCt method
(30). The primer sequences used are
indicated in Table I.
 | Table I.Oligonucleotide primer sequences for
reverse transcription-polymerase chain reaction. |
Table I.
Oligonucleotide primer sequences for
reverse transcription-polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| ULK1 |
|
|
Forward |
AAGTTCGAGTTCTCTCGCAAG |
|
Reverse |
ACCTCCAGGTCGTGCTTCT |
| GAPDH |
|
|
Forward |
AATGGATTTGGACGCATTGGT |
|
Reverse |
TTTGCACTGGTACGTGTTGAT |
Western blot analysis
Cells were harvested using lysis buffer
(radioimmunoprecipitation assay buffer), containing 20 mM Tris (pH
7.5), 150 mM sodium chloride, 1 mM EDTA, 1 mM ethylene glycol
tetraacetic acid, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1
mM β-glycerophosphate, 1 mM sodium orthovanadate and protease
inhibitors (10 µg/ml aprotinin, 1 µg/ml leupeptin and 0.1 mM
phenylmethylsulfonyl fluoride) (Beyotime Institute of
Biotechnology). Harvesting was performed when cells had grown to
80% confluence. Protein samples were collected, quantified by
SDS-PAGE (10–15%) and transferred onto a nitrocellulose membrane.
The membranes were blocked using Tris-buffered saline containing
0.1% Tween-20 and 5% non-fat milk for 1 h. Protein samples were
probed with rabbit polyclonal antibody specific for ULK1 (Santa
Cruz Biotechnology, Santa Cruz, CA; 1:1,000) overnight at 4°C and
subsequently incubated with HRP-conjugated anti-rabbit IgG (Cell
Signalling Technology, Boston, MA; 1:1,000) for 1 h at 25°C.
β-actin served as a control. Protein levels were visualized using
an enhanced chemiluminescence system and band intensity was
quantified using the SuperSignal chemiluminescent substrate
(PerkinElmer, Inc., Waltham, MA, USA).
Statistical analysis
One-way analysis of variance was performed using
SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant difference.
All experiments were performed at least in triplicate. Data are
expressed as the mean ± standard deviation.
Results
Ultrasound treatment provides the most
efficient transfection of P53
Gene transfection efficiency was detected using flow
cytometry (Fig. 1A) and fluorescence
microscopy (Fig. 1B). The results
revealed that the wt-P53-EGFP-positive rate was highest in Group C
(P<0.05). This suggested that the transfection efficiency of
Group C was increased compared with that of Groups A and B. A
significant difference in transfection efficiency was also observed
between Groups A and B (P<0.05).
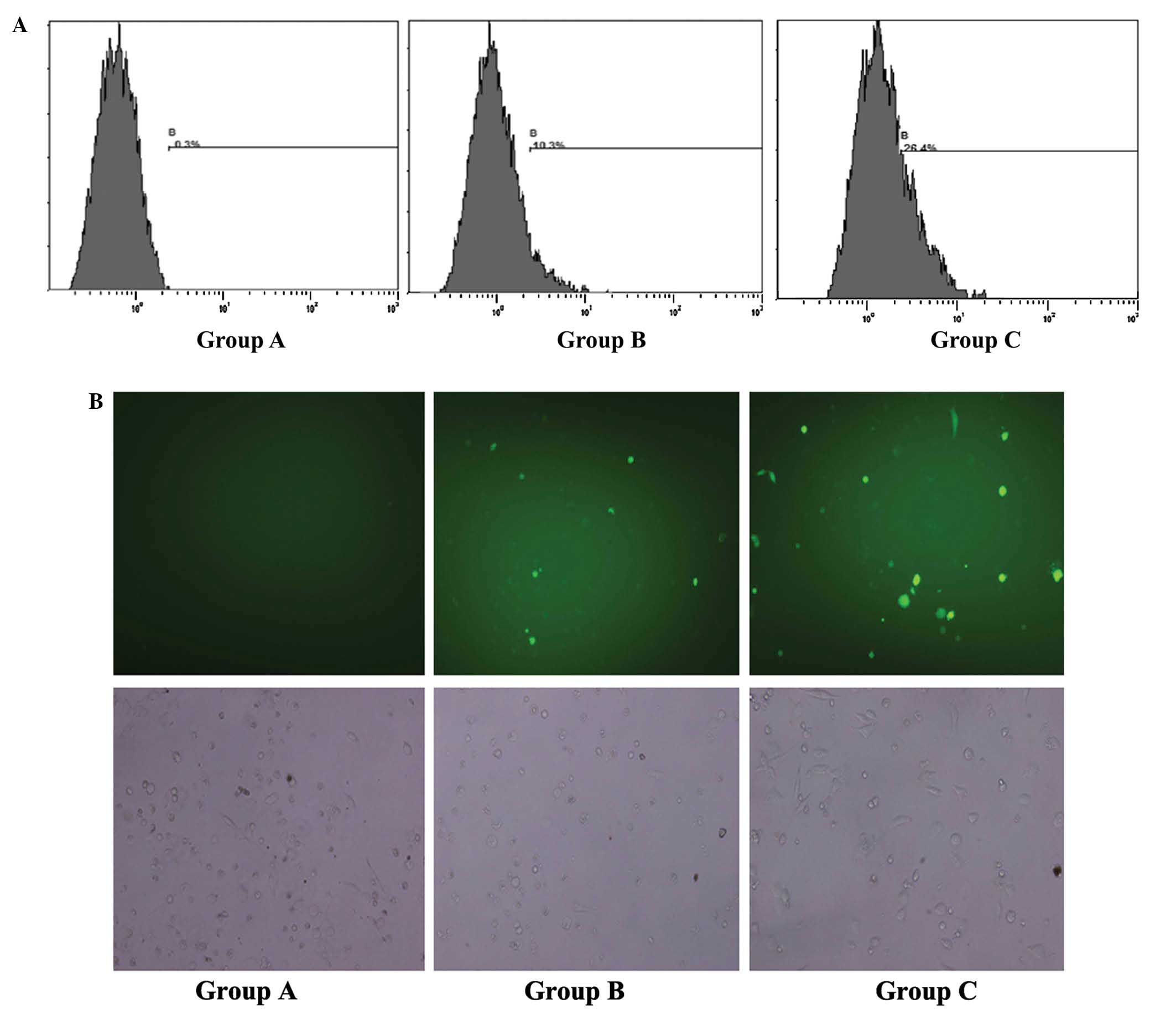 | Figure 1.Gene transfection efficiency detected
by flow cytometry and fluorescence microscopy. EGFP-positive rate
of PC3 cells detected by (A) flow cytometry and (B) fluorescence
microscopy (magnification, ×200) 24 h following treatment in: Group
A, no treatment; Group B, cells treated with Lipofectamine® and
wt-P53/EGFP plasmid; and Group C, cells treated with Lipofectamine,
wt-P53/EGFP plasmid and ultrasound combined with microbubbles. Top
panel, green stain indicates successful transfection; bottom panel,
brightfield view. EGFP, enhanced green fluorescent protein; P53,
tumor protein P53; wt-P53, wild-type P53. |
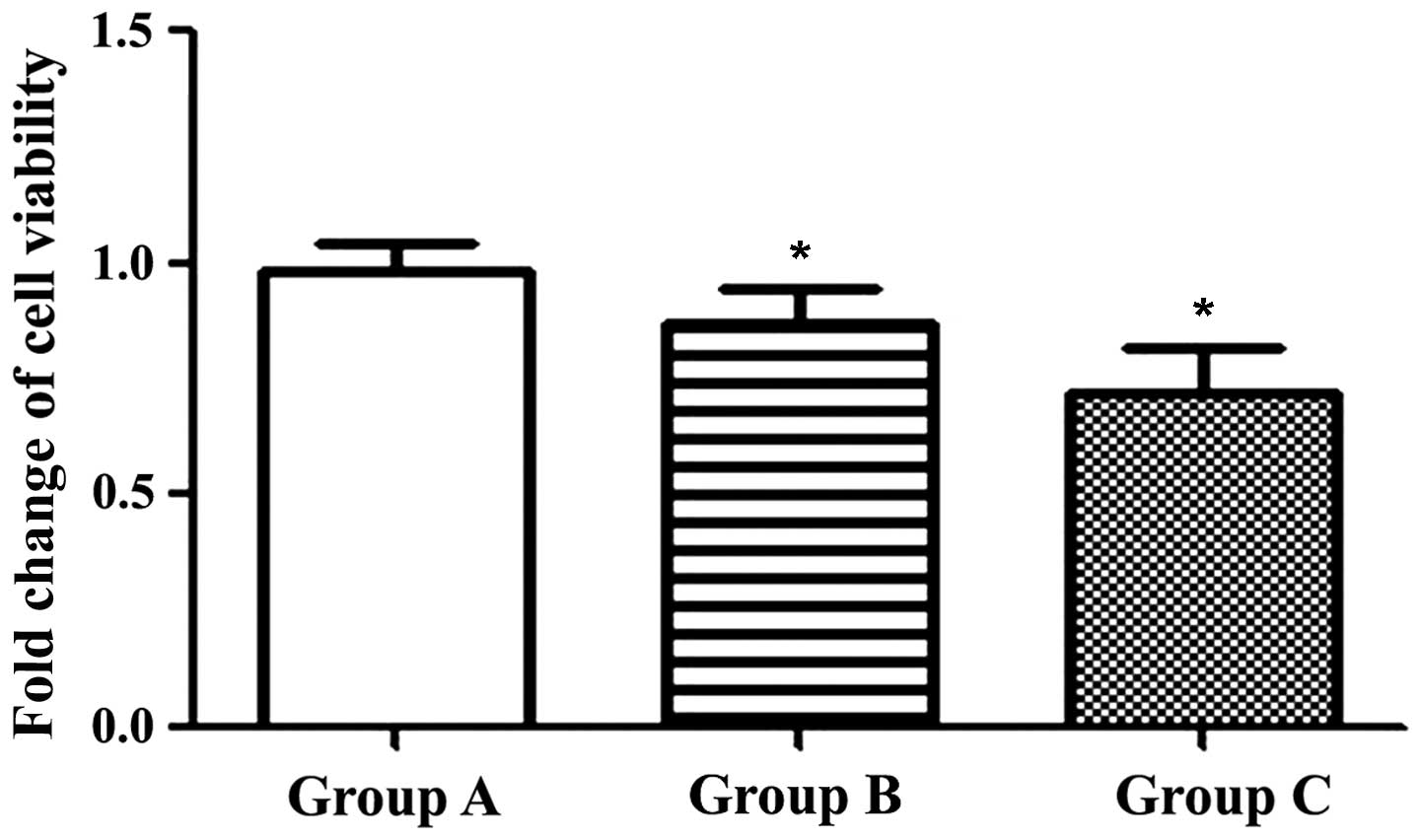
Transfection of P53 reduces cell
viability
Following transfection, an MTT assay was used to
evaluate cell viability. The results revealed that cell viability
was 99.7±5.7% in Group A, 87.0±8.0% in Group B and 72.3±9.6% in
Group C (Fig. 2). This demonstrated
that wt-P53 was capable of inhibiting the growth of PC3 cells, and
that ultrasound combined with microbubbles may enhance this
effect.
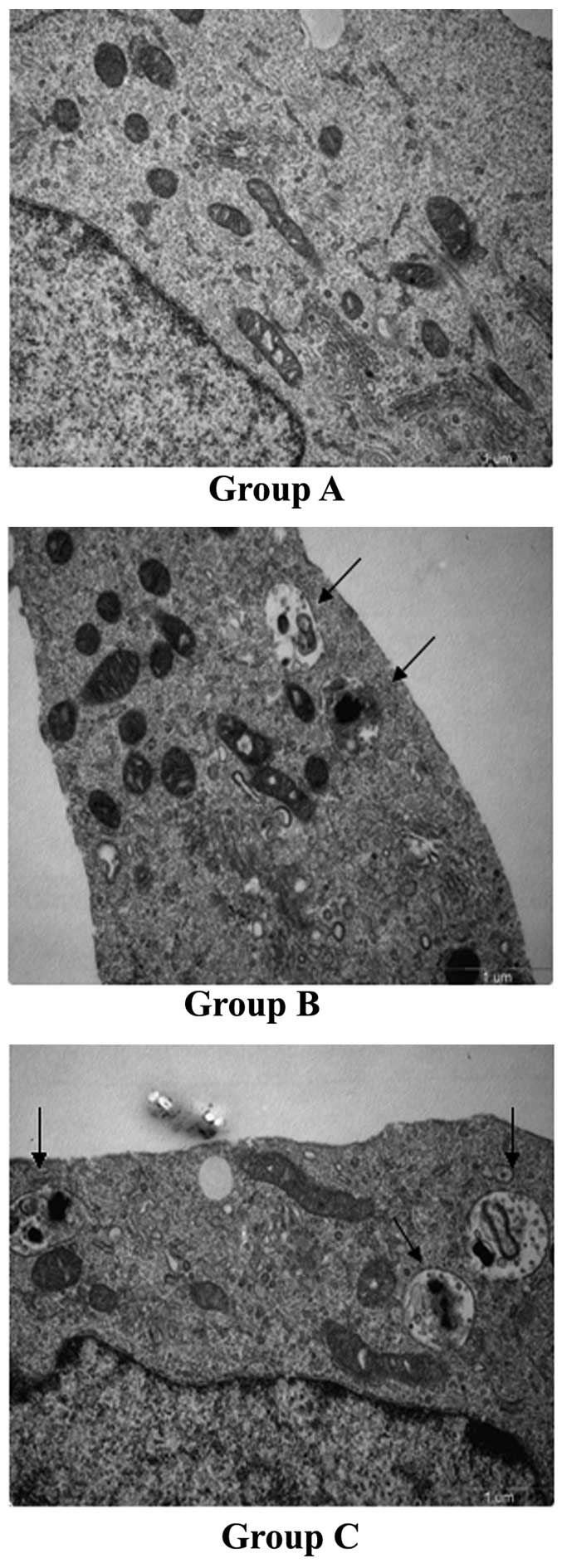
Greater numbers of autophagosomes are
present in Groups B and C
Autophagosomes have a characteristic structure of a
double- or multi-lamellar membrane, which contains organelles or
other decomposed residues (31). The
numbers of autophagosomes in cells of each of the three groups were
counted in 10 visions from various microscopic fields at a
magnification of ×24,500. Compared with Group A, the numbers of
autophagosomes in Groups B and C were significantly increased
(0.2±0.42 vs. 1.80 ±1.62 and 2.6±2.12; P<0.05; Fig. 3).
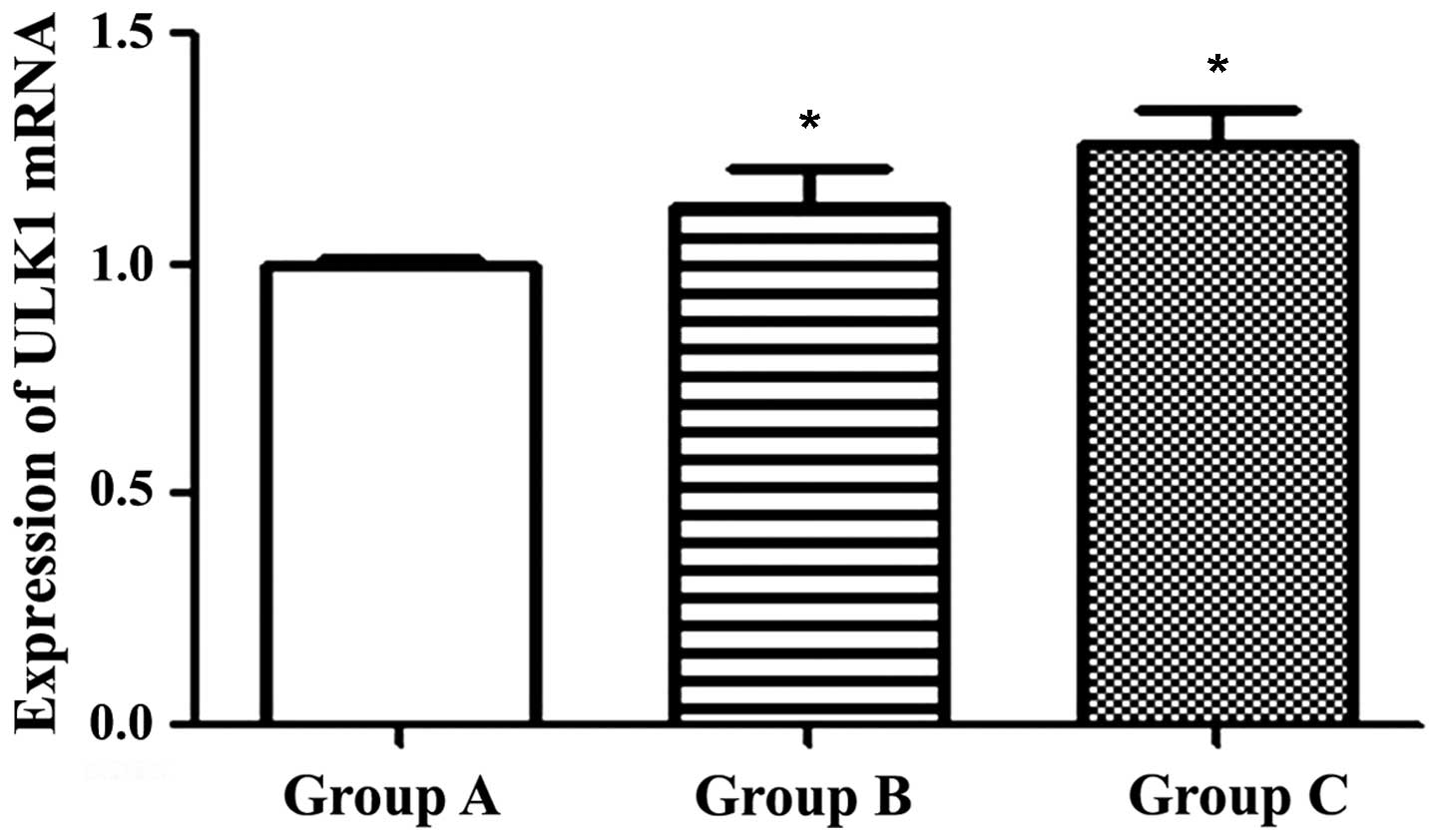
Serine/threonine-protein kinase ULK1
induces autophagy in PC3 cells
Levels of autophagy-associated gene ULK1 messenger
RNA (mRNA) were significantly increased in Groups B and C, compared
with Group A (Fig. 4). Levels of ULK1
mRNA were 1.00±0.02 in Group A, 1.13±0.08 in Group B and 1.26±0.08
in Group C (P<0.05).
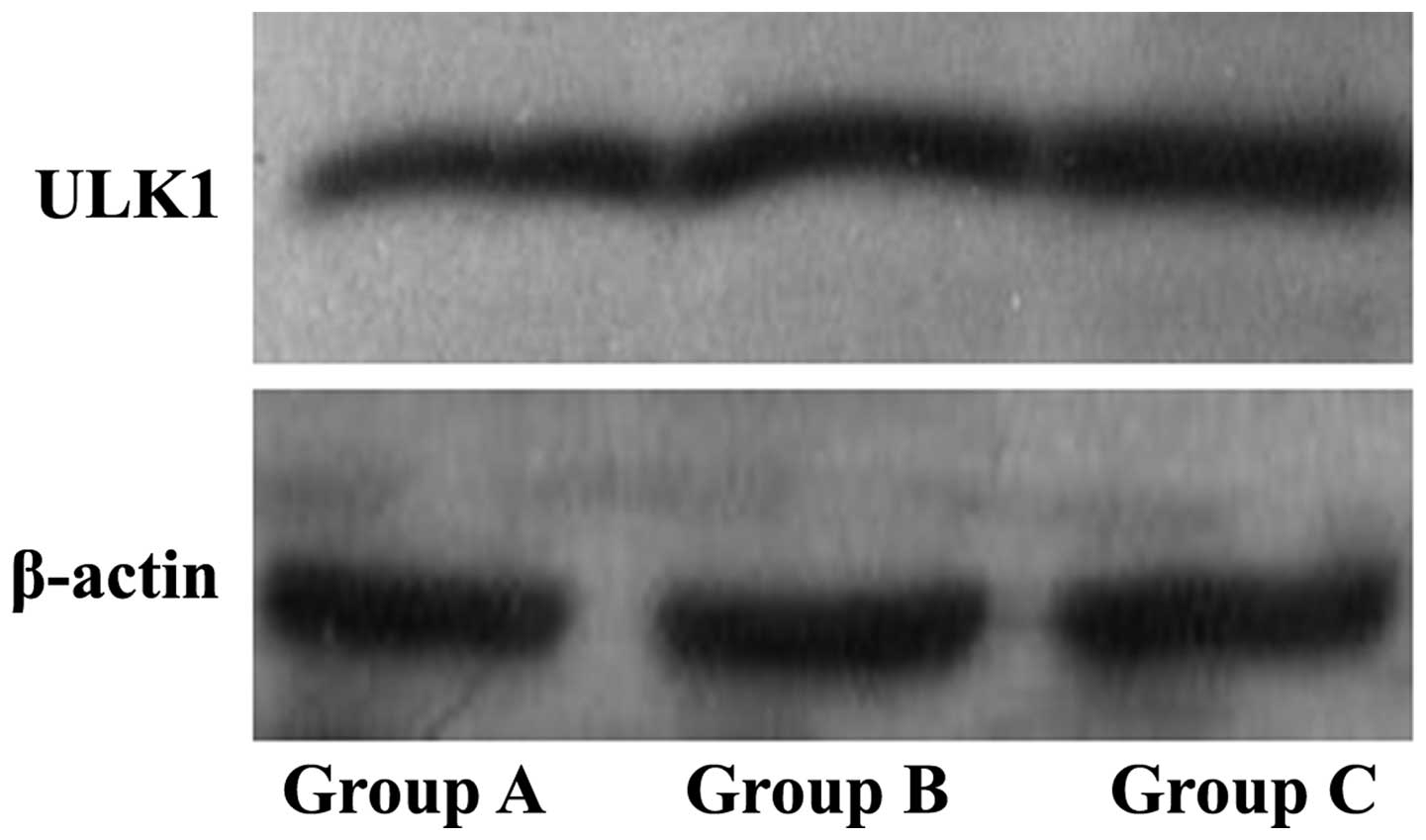
Autophagy-related protein expression
is enhanced in Groups B and C
Western blotting was used to assess
autophagy-associated protein expression. ULK1 protein expression
levels of the Groups B and C were observed to be increased compared
with those of the control Group A (Fig.
5; P<0.05). Therefore, ultrasound combined with microbubble
increased the expression levels of ULK1 protein.
Discussion
At present, ultrasound is not only widely used for
the purpose of examination, but is also used as a treatment
strategy, as it is non-invasive, safe and low cost (32). Microbubbles may be capable of
enhancing the biological effects of ultrasound, by processes
including increasing cell permeability, inhibiting tumor cell
proliferation and inducing cell apoptosis (33). Cavitation is a significant effect of
ultrasound. When microbubbles are irradiated by ultrasound,
oscillation expansion, contraction and a series of other dynamic
processes occur, leading to the ‘cavitation effect’. Large shear
forces and shock waves created by the cavitation effect generate
holes in the membrane (34). This
phenomenon is known as the ‘sonoporation effect’, and allows
molecules outside the membrane to penetrate into the cells, and may
subsequently improve the efficiency of delivery of therapeutic
molecules into cells (35,36). Marmottant and Hilgenfeldt (37) suggest that low intensity ultrasonic
irradiation, which induces vacuole vibration and microsound flow,
may have the potential to alter cell membrane permeability.
Prentice et al (38)
hypothesized that irreversible sound perforation effects may be
used in the treatment of cancer.
Three prostate cancer cell lines are known to
possess varying P53 statuses: LNCaP is wild-type for P53, PC3 is
null for P53 and DU145 is mutant-type for P53. PC3 cells were
selected for use in the present study due to this absence of P53.
Transfection assays of wt-P53-GFP plasmid were designed in order to
detect whether ultrasound combined with microbubbles was able to
enhance transfection. The flow cytometry and fluorescence
microscopy results of the present study indicated that ultrasound
combined with microbubbles was able to enhance transfection
efficiency. An MTT assay was performed to detect whether this
transfection induced cytotoxic effects and reduced the
proliferation of tumor cells. Twenty-four hours following
transfection, the cytotoxic effect of wt-P53 was found to be
enhanced by ultrasound irradiation combined with microbubbles, due
to enhanced rates of transfection and increased levels of wt-P53 in
PC3 cells.
As a well-known tumor suppressor gene, wt-P53 may
repair damaged genes in tumor cells and has been revealed to have a
significant role in the prevention of cancer onset and progression
(39). In addition, wt-P53 has a key
role in the regulation of autophagosome formation (40). In the present study it was observed
that, following successful transfection, P53-induced autophagy
occurred. Results from transmission electron microscopy also
suggested that autophagosome numbers were increased in Groups B and
C, compared with those of Group A. Subsequently, western blot
analysis and RT-PCR were performed to investigate ULKl expression.
ULKl is a downstream target gene of wt-P53. When DNA is damaged,
wt-P53 is able to adjust ULKI expression levels. Raised levels of
the ULKl/Atg13 complex induced by wt-P53 are essential in order for
autophagy to take place (17). To a
certain extent, enhanced autophagy levels may promote cell
apoptosis. In mammalian cells, ULK1-induced autophagy may inhibit
certain types of cancer and increase the efficiency of toxic
chemotherapy drugs (17). In the
present study, it was observed that ULK1 levels were upregulated in
Groups B and C, and were highest in Group C. This confirmed that
ultrasound combined with microbubbles was able to enhance the
efficiency of the P53 gene, whose expression was not altered.
A number of studies have revealed that ultrasound
combined with microbubbles is able to increase the efficacy of
various types of therapeutic agents in vivo and that it is
safe for normal tissues to be exposed to therapeutic techniques
involving ultrasound. The present study represents an initial step
towards the development of combination therapy for PCa. Further
research may be required in order to gain an increased
understanding of the underlying mechanisms of this technique and
further development is required for these therapies to be
translated into a clinical setting.
Acknowledgments
The present study was supported by the major
infrastructure projects of Shanghai Science and Technology (grant
no. 10JC1412600) and by the National Natural Science Foundation of
China (grant nos. 81271597 and 81401421).
References
|
1
|
Tanaka G, Hirata Y, Goldenberg SL,
Bruchovsky N and Aihara K: Mathematical modelling of prostate
cancer growth and its application to hormone therapy. Philos
Transact A Math Phys Eng Sci. 368:5029–5044. 2010. View Article : Google Scholar
|
|
2
|
Lecornet E, Ahmed HU, Moore C and Emberton
M: Focal therapy for prostate cancer: A potential strategy to
address the problem of overtreatment. Arch Esp Urol. 63:845–852.
2010.PubMed/NCBI
|
|
3
|
Baumert H: Salvage treatments for
prostatic radiation failure. Cancer Radiother. 14:442–445. 2010.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chaturvedi S and Garcia JA: Novel agents
in the management of castration resistant prostate cancer. J
Carcinog. 13:52014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shin SW, Kim SY and Park JW: Autophagy
inhibition enhances ursolic acid-induced apoptosis in PC3 cells.
Biochim Biophys Acta. 1823:451–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jácome-Pita F, Sánchez-Salas R, Barret E,
Amaruch N, Gonzalez-Enguita C and Cathelineau X: Focal therapy in
prostate cancer: The current situation. Ecancermedicalscience.
8:4352014.PubMed/NCBI
|
|
7
|
Wang W, Li W, Ma N and Steinhoff G:
Non-viral gene delivery methods. Curr Pharm Biotechnol. 14:46–60.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Podolska K, Stachurska A, Hajdukiewicz K
and Małecki M: Gene therapy prospects - intranasal delivery of
therapeutic genes. Adv Clin Exp Med. 1:525–534. 2012.
|
|
9
|
Tasdemir E, Maiuri MC, Orhon I, Kepp O,
Morselli E, Criollo A and Kroemer G: p53 represses autophagy in a
cell cycle-dependent fashion. Cell Cycle. 7:3006–3011. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rozan LM and El-Deiry WS: p53 downstream
target genes and tumor suppression: A classical view in evolution.
Cell Death Differ. 14:3–9. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vousden KH and Lane DP: p53 in health and
disease. Nat Rev Mol Cell Biol. 8:275–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amaravadi RK, Yu D, Lum JJ, Bui T,
Christophorou MA, Evan GI, Thomas-Tikhonenko A and Thompson CB:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crighton D, Wilkinson S, O'Prey J, Syed N,
Smith P, Harrison PR, Gasco M, Garrone O, Crook T and Ryan KM:
DRAM, a p53-induced modulator of autophagy, is critical for
apoptosis. Cell. 126:121–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng Z, Hu W, de Stanchina E, Teresky AK,
Jin S, Lowe S and Levine AJ: The regulation of AMPK beta1, TSC2,
and PTEN expression by p53: Stress, cell and tissue specificity,
and the role of these gene products in modulating the
IGF-1-AKT-mTOR pathways. Cancer Res. 67:3043–3053. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizushima N: The role of the Atg1/ULK1
complex in autophagy regulation. Curr Opin Cell Biol. 22:132–139.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao W, Shen Z, Shang L and Wang X:
Upregulation of human autophagy-initiation kinase ULK1 by tumor
suppressor p53 contributes to DNA-damage-induced cell death. Cell
Death Differ. 18:1598–1607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng CX, Sieling F, Pan H and Cui J:
Ultrasound-induced cell membrane porosity. Ultrasound Med Biol.
30:519–522. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lawrie A, Brisken AF, Francis SE,
Cumberland DC, Crossman DC and Newman CM: Microbubble-enhanced
ultrasound for vascular gene delivery. Gene Ther. 7:2023–2024.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HJ, Greenleaf JF, Kinnick RR, Bronk JT
and Bolander ME: Ultrasound-mediated transfection of mammalian
cells. Hum Gene Ther. 7:1339–1346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Z, Raghavan M, Hall TL, Chang CW, Mycek
MA, Fowlkes JB and Cain CA: High speed imaging of bubble clouds
generated in pulsed ultrasound cavitational therapy - histotripsy.
IEEE Trans Ultrason Ferroelectr Freq Control. 54:2091–2101. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tachibana K and Tachibana S: Transdermal
delivery of insulin by ultrasonic vibration. J Pharm Pharmacol.
43:270–271. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang W, Li W, Ma N and Steinhoff G:
Non-viral gene delivery methods. Curr Pharm Biotechnol. 14:46–60.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Husseini GA and Pitt WG: The use of
ultrasound and micelles in cancer treatment. J Nanosci Nanotechnol.
8:2205–2215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Juffermans LJ, van Dijk A, Jongenelen CA,
Drukarch B, Reijerkerk A, de Vries HE, Kamp O and Musters RJ:
Ultrasound and microbubble-induced intra- and intercellular
bioeffects in primary endothelial cells. Ultrasound Med Biol.
35:1917–1927. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoskins P, Thrush A, Martin K and
Whittingam T: Diagnostic Ultrasound: Physics and Equipment (2nd).
New York, NY: Cambridge University Press. 2010. View Article : Google Scholar
|
|
27
|
Nyborg WL: Acoustic streaming. Nonlinear
Acoustics. Hamilton NF and Blackstock DT: (San Diego). Blackstock
Academic Press. 207–232. 1998.
|
|
28
|
Wooley REI, Gibbs PS, Dickerson HW, Brown
J and Nolan LK: Analysis of plasmids cloned from a virulent avian
Escherichia coli and transformed into Escherichia coli DH5 alpha.
Avian Dis. 40:533–539. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hinescu ME, Gherghiceanu M, Suciu L and
Popescu LM: Telocytes in pleura: Two- and three-dimensional imaging
by transmission electron microscopy. Cell Tissue Res. 343:389–397.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu YI, Chen X and Wu Y, Wang Y, He Y and
Wu Y: Directly transforming PCR-amplified DNA fragments into plant
cells is a versatile system that facilitates the transient
expression assay. PLoS One. 8:e571712013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eng KE, Panas MD, Murphy D, Karlsson
Hedestam GB and McInerney GM: Accumulation of autophagosomes in
Semliki Forest virus-infected cells is dependent on expression of
the viral glycoproteins. J Virol. 86:5674–5685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng X, Ji P and Hu J: Sonoporation using
microbubbles promotes lipofectamine-mediated siRNA transduction to
rat retina. Bosn J Basic Med Sci. 11:147–152. 2011.PubMed/NCBI
|
|
33
|
Tsunoda S, Mazda O, Oda Y, Iida Y, Akabame
S, Kishida T, Shin-Ya M, Asada H, Gojo S, Imanishi J, et al:
Sonoporation using microbubble BR14 promotes pDNA/siRNA
transduction to murine heart. Biochem Biophys Res Commun.
336:118–127. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Geers B, Lentacker I, Alonso A, Sanders
NN, Demeester J, Meairs S and De Smedt SC: Elucidating the
mechanisms behind sonoporation with adeno-associated virus-loaded
microbubbles. Mol Pharm. 8:2244–2251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nomikou N and McHale AP: Exploiting
ultrasound-mediated effects in delivering targeted, site-specific
cancer therapy. Cancer Lett. 296:133–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Richardson ES, Pitt WG and Woodbury DJ:
The role of cavitation in liposome formation. Biophys J.
93:4100–4107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marmottant P and Hilgenfeldt S: Controlled
vesicle deformation and lysis by single oscillating bubbles.
Nature. 423:153–156. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Prentice PA, McLean D, Cuschieri A,
Dholakia K and Campbell PA: Spatially controlled sonoporation of
prostate cancer cells via ultrasound activated microbubble
cavitation. 3rd IEEE/EMBS Special Topic Conference on
Microtechnology in Medicine and Biology. IEEE. (New York). 158–159.
2005. View Article : Google Scholar
|
|
39
|
Xu J, Singh A and Amiji MM:
Redox-responsive targeted gelatin nanoparticles for delivery of
combination wt-p53 expressing plasmid DNA and gemcitabine in the
treatment of pancreatic cancer. BMC Cancer. 14:752014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Green DR and Kroemer G: Cytoplasmic
functions of the tumor suppressor p53. Nature. 458:1127–1130. 2009.
View Article : Google Scholar : PubMed/NCBI
|