Introduction
A solitary fibrous tumor (SFT) is a rare form of
tumor that typically develops in the pleura. SFTs that establish in
the salivary gland are particularly rare, with imaging studies
describing only a few known cases (1,2). Due to
the rarity of the tumor, the incidence of such in the salivary
gland remains unclear. The association between pathological
morphology and clinical symptoms is non-specific. SFTs are
unpredictable and the diagnosis remains challenging. Additionally,
10–15% of tumors advance to become malignant or metastasize, and
can recur (3). SFTs in the head and
neck region are also uncommon, with the characteristics of tumors
in this location not clearly understood (4). Magnetic resonance imaging (MRI) features
are also relatively non-specific. Wignall et al (5) described that 89% of tumors presented
with high signal intensities on T2-weighted images and intermediate
signal intensities on T1-weighted images in a review of previous
studies. Areas of low signal intensity were observed in larger
tumors, resulting from flow voids in prominent perilesional
vessels. Collagen fibroblasts and tumor degeneration have been
suggested to be accountable for such variable signal intensities
(6). Previous studies have reported
linear or curvilinear hypointense areas within SFTs on T2-weighted
images, and such lesion morphology has been demonstrated to
correlate with hypocellular densities near the collagenous
sclerotic area (6,7).
Although further investigation is required on the
incidence of SFTs in this location, the present study describes a
lesion in the right salivary gland and the subsequent MRI
findings.
Case report
In October 2013, a 48-year-old woman was referred to
the Subei People's Hospital (Yangzhou, China), exhibiting a large,
soft salivary mass that had been identified 20 days earlier. The
physical examination was unremarkable, with the exception of the
palpable mass in the right jaw. The tumor extended from under the
jaw to the right side of the bottom of the mouth, and its quality
reflected a well-defined, poorly-mobilized lesion with no facial
nerve symptoms. The patient maintained a bilateral symmetrical face
with normal mouth opening and occlusion.
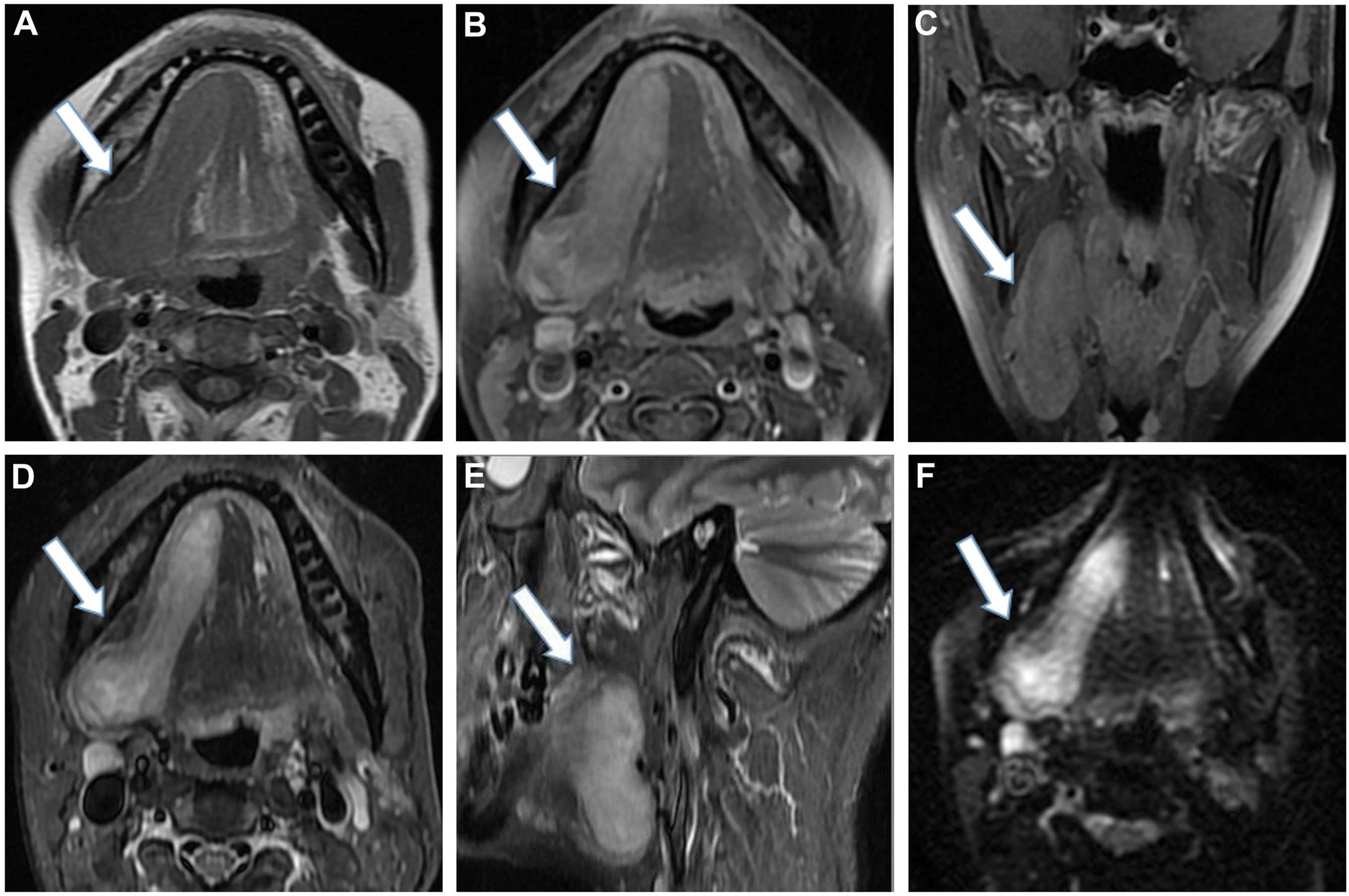
A plain MRI scan confirmed the presence of a
well-circumscribed, irregular, soft-tissue mass measuring 71×29 mm,
located in the right salivary area, associated with the right
salivary gland (Fig. 1). The tumor
exhibited marked and homogeneous enhancement following intravenous
injection of contrast material. The adjacent bone was not involved
and the lymph nodes in the carotid sheath area were observed. A
benign tumor was suspected and subsequently removed by local
excision. The surgical specimen was examined and an
irregular-shaped mass with a complete capsule was detected.
Microscopic examination demonstrated spindle cells and a collagen
component in the mesenchyme, with reduced cytoplasm (Fig. 2A and B). Immunohistochemical analysis
indicated neoplastic cells that were strongly positive for cluster
of differentiation (CD)34 monoclonal antibody (Fig. 2C and D). The cytoplasm (brown) was
identified as strongly positive for vimentin monoclonal antibody.
Thus, the case was diagnosed as an SFT. Written informed consent
was obtained from the patient for publication of this study.
Discussion
SFTs primarily occur in adults ranging from 30 to 64
years old (6). SFTs are rare lesions
that usually occur in the pleura and were first described by
Klemperer and Rabin in 1931 (8).
Previous studies have also reported such lesions in the head and
neck regions, and ~80 cases have been described regarding SFTs
located in the oral cavity (9). The
risks of local recurrence and metastasis are associated with tumor
size and histological grade of surgical resection margins (3,5,10).
In general, 79–100% positivity for CD34 on
immunohistochemistry is considered a highly specific marker of SFTs
(11). In addition, malignant SFTs
tend to exhibit decreased CD34 immunoreactivity and overexpress
p53, S-100, keratin and vimentin (12–14).
However, separating SFTs arising in the head and neck region from
those arising at other sites by histopathological or
immunophenotypical features is not straightforward (15).
In the present case, the salivary gland SFT
presented as a well-circumscribed, submucosal mass that was
asymptomatic and slow-growing, with normal staining. SFTs can often
be confused with other salivary gland conditions, including
submandibular gland inflammation and sublingual gland cysts
(1).
In the head and neck regions, the radiological
findings of SFTs are not specific. However, a number of SFTs are
detected incidentally through radiological examinations, or when
the symptoms appear associated with a mass in the surrounding
tissues (6). The components of the
tumor itself may cause the variable intensity observed on MRI
images (6). However, radiological
differentiation of SFTs from other salivary gland tumors, including
pleomorphic adenoma, is difficult. Therefore, in clinical practice,
imaging techniques combined with immunohistochemical and
pathological results may be advantageous for diagnosis.
SFTs are often observed as a soft-tissue attenuation
on imaging, comprised of a well-marginated, lobulated mass when the
tumor has prominent collateral feeding vessels or a visible fatty
component. Such features alert the radiologist to a possible SFT
diagnosis. When the tumor is ≥10 cm in size with central necrosis
and effusion, it is likely to be a malignant tumor (5,16,17).
The most important prognostic factor in treating
SFTs is treatment with a complete resection (18,19). Due
to the high probability of late recurrence, long-term follow-ups
are required (18).
In conclusion, the present study findings suggest
that an SFT should be considered in the differential diagnosis of a
well-marginated lesion with salivary gland association, a
soft-tissue component, isointensity to hyperintensity on
T2-weighted images and a high signal intensity on
diffusion-weighted images, including homogeneous
contrast-enhancement.
References
|
1
|
Ferreiro JA and Nascimento AG: Solitary
fibrous tumor of the major salivary glands. Histopathology.
28:261–264. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cho KJ, Ro JY, Choi J, Choi SH, Nam SY and
Kim SY: Mesenchymal neoplasms of the major salivary glands:
Clinicopathologcal features of 18 cases. Eur Arch Otorhinolaryngol.
265(Suppl 1): S47–S56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garcia-Bennett J, Olivé CS, Rivas A,
Domínguez-Oronoz R and Huguet P: Soft tissue solitary fibrous
tumor. Imaging findings in a series of nine cases. Skeletal Radiol.
41:1427–1433. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamada H, Hamada Y, Fujihara H, Fukami K,
Mishima K, Nakaoka K, Kumagai K and Imamura E: Solitary fibrous
tumor of the buccal space resected in combination with
coronoidectomy. Oral Surg Oral Med Oral Pathol Oral Radiol.
114:e9–e14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wignall OJ, Moskovic EC, Thway K and
Thomas JM: Solitary fibrous tumors of the soft tissues: Review of
the imaging and clinical features with histopathologic correlation.
AJR Am J Roentgenol. 195:W55–W62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim HJ, Lee HK, Seo JJ, Kim HJ, Shin JH,
Jeong AK, Lee JH and Cho KJ: MR imaging of solitary fibrous tumors
in the head and neck. Korean J Radiol. 6:136–142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeong AK, Lee HK, Kim SY and Cho KJ:
Solitary fibrous tumor of the parapharyngeal space: MR imaging
findings. AJNR Am J Neuroradiol. 23:473–475. 2002.PubMed/NCBI
|
|
8
|
Klemperer P and Rabin CB: Primary neoplasm
of the pleura: A report of 5 cases. Arch Pathol (Chic). 1:11–28.
1931.
|
|
9
|
Amico P, Colella G, Rossiello R, Maria
Vecchio G, Leocata P and Magro G: Solitary fibrous tumor of the
oral cavity with a predominant leiomyomatous-like pattern: A
potential diagnostic pitfall. Pathol Res Pract. 206:499–503. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Daigeler A, Lehnhardt M, Langer S,
Steinstraesser L, Steinau HU, Mentzel T and Kuhnen C:
Clinicopathological findings in a case series of extrathoracic
solitary fibrous tumors of soft tissues. BMC Surg. 6:102006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ali SZ, Hoon V, Hoda S, Heelan R and
Zakowski MF: Solitary fibrous tumor. A cytologic-histologic study
with clinical, radiologic, and immunohistochemical correlations.
Cancer. 81:116–121. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Civin CI, Strauss LC, Brovall C, Fackler
MJ, Schwartz JF and Shaper JH: Antigenic analysis of hematopoiesis.
III. A hematopoietic progenitor cell surface antigen defined by a
monoclonal antibody raised against KG-1a cells. J Immunol.
133:157–165. 1984.PubMed/NCBI
|
|
13
|
de Perrot M, Kurt AM, Robert JH, Borisch B
and Spiliopoulos A: Clinical behavior of solitary fibrous tumors of
the pleura. Ann Thorac Surg. 67:1456–1459. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yokoi T, Tsuzuki T, Yatabe Y, Suzuki M,
Kurumaya H, Koshikawa T, Kuhara H, Kuroda M, Nakamura N, Nakatani Y
and Kakudo K: Solitary fibrous tumour: Significance of p53 and CD34
immunoreactivity in its malignant transformation. Histopathology.
32:423–432. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Oliveira DH, Albuquerque AF, de Araújo
Barreto MD, Nonaka CF, da Silva JS, Germano AR and Queiroz LM:
Large solitary fibrous tumor of the oral cavity - report of a case.
Pathol Res Pract. 210:1064–1067. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferretti GR, Chiles C, Choplin RH and
Coulomb M: Localized benign fibrous tumors of the pleura. AJR Am J
Roentgenol. 169:683–686. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee SC, Tzao C, Ou SM, Hsu HH, Yu CP and
Cheng YL: Solitary fibrous tumors of the pleura: Clinical,
radiological, surgical and pathological evaluation. Eur J Surg
Oncol. 31:84–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magdeleinat P, Alifano M, Petino A, Le
Rochais JP, Dulmet E, Galateau F, Icard P and Regnard JF: Solitary
fibrous tumors of the pleura: Clinical characteristics, surgical
treatment and outcome. Eur J Cardiothorac Surg. 21:1087–1093. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Altinok T, Topçu S, Tastepe AI, Yazici U
and Cetin G: Localized fibrous tumors of the pleura: Clinical and
surgical evaluation. Ann Thorac Surg. 76:892–895. 2003. View Article : Google Scholar : PubMed/NCBI
|