Introduction
Malignant melanoma is a cancer that exhibits an
increasing incidence and mortality rate, in addition to possessing
a high risk of metastasis (1).
Malignant melanoma is not sensitive to radiotherapy or
chemotherapy; therefore, clinical treatment is an issue associated
with malignant melanoma (2). In
addition, the therapeutic effect of traditional chemotherapy is not
sufficient to treat this disease, and the side effects of the
chemotherapy drugs may cause marked damage to the patient (2). Therefore, effective and low-toxicity
compounds that treat malignant melanoma are required to be
identified or developed. Previous studies have found that several
tumor cells exhibit differentiation defects (3,4); however,
treating tumor cells with compounds may cause normal
differentiation and reduce tumor malignancy, for example, all-trans
retinoic acid (atRA) is used in the differentiation therapy of
acute promyelocytic leukemia (4).
Sansalvamide A, which is a cyclic depsipeptide derived from a
marine fungus of the Fusarium genus, exhibits significant
antiproliferative effects in the 60 cancer cell line panel of the
National Cancer Institute (5).
Synthesis of sansalvamide A derivatives has received increasing
attention and novel sansalvamide A derivatives may be valuable
therapeutic agents (6–8). The effect of the novel sansalvamide A
derivative and a cyclic pentapeptide H-15 on the growth and
differentiation of murine malignant melanoma B16 cells was
investigated in the present study. H-15 possesses a molecular
formula and molecular weight of
C29H44BrN5O6 and
637.2475, respectively (Fig. 1). In
the present study, the results may provide a basis for additional
studies of this novel compound.
Materials and methods
Materials
Gibco RPMI-1640 and
trypsin-ethylenediaminetetraacetic acid (EDTA) solution were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine
serum (FBS) was purchased from Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd. (Zhejiang, China). Dimethyl
sulfoxide (DMSO) was purchased from Tianjin Yongda Chemical
Reagents Development Center (Tianjin, China) H-15 was provided by
the Hebei Province Key Laboratory of Molecular Chemistry for Drug
(Shijiazhuang, Hebei, China). Sulforhodamine B (SRB) was purchased
from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) and
bicinchoninic acid (BCA) kit was acquired from Shanghai Generay
Biotechnology Co., Ltd. (Shanghai, China). Polyvinylidene fluoride
(PVDF) membranes were purchased from Shanghai Generay Biotechnology
Co., Ltd., and the polyclonal rabbit anti-mouse
glyceraldehyde-3-phosphate dehydrogenase (GAPDH; cat no. 2118s)
antibody was obtained from Hangzhou Goodhere Biotechnology Co.,
Ltd. (Hangzhou, Zhejiang, China). The monoclonal rabbit anti-mouse
tyrosinase (TYR; cat no. sc15341) antibody was purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA), and the secondary
polyclonal goat anti-rabbit fluorescence-conjugated antibody was
purchased from LI-COR Biosciences, Ltd. (Cambridge, UK; cat no.
926–32211). The B16 cell line was stored at the Research Center of
the Fourth Hospital of Hebei Medical University (Shijiazhuang,
Hebei, China).
Cell culture
The B16 cells were cultured in RPMI-1640 medium,
together with 10% heat-inactivated FBS and 100 U/ml penicillin and
100 µg/ml streptomycin. The B16 cell line was grown in 25
cm2 flasks in a humidified atmosphere of 5%
CO2 at 37°C, and the media were changed every 2–3 days.
The B16 cells were allowed to grow to 80–90% confluency, and the
cells were then digested using trypsin-EDTA. The cells were
subsequently plated in 25 cm2 flasks and in 24- or
96-well plates for generation of the cells and use in additional
experiments.
Concentration-dependent effect of H-15
on B16 cell growth inhibition
H-15 was dissolved in dimethyl sulfoxide (DMSO) and
diluted with a serum-free medium to prepare solution concentrations
of 1,000, 500, 100, 10 and 1 µM. Single-cell suspensions of B16
cells were prepared and adjusted based on the indicated
concentrations. The cells were then inoculated in 96-well plates,
with 90 µl of cell solution and ~2,000 cells/well. The cells were
allowed to adhere to the plates for 4 h, and 10 µl H-15 was added
to each well to produce the final concentrations of 100, 50, 10, 1
and 0.1 µM H-15. Each concentration was placed in three wells, and
a 1% DMSO group was simultaneously prepared as the control group.
The percentage growth of the B16 cells treated with various
concentrations of the H-15 for 48 h was calculated using the SRB
colorimetric method.
SRB colorimetric method
The cells were fixed with trichloroacetic acid (TCA)
following treatment with H-15 for 48 h, and the intracellular
protein was stained with SRB. A total of 100 µl of Trisbase was
then added to each well. The dissolved SRB was detected using a
microplate reader (Thermo Fisher Scientific, Inc.), wherein the
values indirectly indicated the numbers of living cells. The medium
in the 96-well plates was discarded, and 100 µl TCA was added at a
temperature of 4°C for 30 min. The TCA was then discarded, and the
cells were washed three times (30 sec washes) with distilled water
prior to drying at room temperature for 1 h. A total of 100 µl of
0.4% SRB was then added to the cells, and the cells were agitated
for 20 min. The dye solution was discarded, and the cells were
washed three times with 1% acetic acid and dried at room
temperature for >6 h. Finally, 100 µl Trisbase was added, and
the cells were again agitated for 5 min. The optical density (OD)
was obtained at a wavelength of 490 nm using a Multiskan Go
microplate reader (Thermo Fisher Scientific, Inc.).
Time-dependent effect of H-15 on B16
cell growth inhibition
The cells were harvested at 80–90% confluency using
trypsin, and a serum-free medium (RPMI-1640 medium without FBS)
were used to produce a single-cell suspension (10,000 cells/ml).
The cells were seeded in 24-well plates at a concentration of
20,000 cells/well. The medium and FBS in the wells was replaced
with fresh medium and FBS subsequent to 24 h. The wells were then
treated with 50 µM H-15, and the cell numbers were counted
following 24, 48, 72, 96, 120 and 144 h of treatment. A control
group (with equal volume of serum-free RPMI-1640 medium) was
simultaneously prepared, and a growth curve was generated.
Detection of melanin content of B16
cells
The cells were harvested at 80–90% confluency using
trypsin, and a serum-free medium was used to produce a single-cell
suspension. The cells were then seeded in 25 cm2 flasks
with ~50,000 cells/flask. The cells were allowed to adhere in the
flask for 4 h, and H-15 was added to the test flasks. The final
concentration in the flask was then adjusted to 50 µM. The cells
were treated for 48 h and harvested using trypsin. The cell pellets
were collected and washed twice with 0.9% NaCl. The pellets were
then dissolved in 200 µl of 1 M NaOH solution containing 10% DMSO,
and were then placed in a water bath at 80°C for 2 h. The pellets
were agitated for 30 sec to dissolve the melanin and centrifuged at
300 × g for 5 min, to remove the precipitates. The liquid was
transferred onto 96-well plates, and the optical density was
obtained at a wavelength of 490 nm using a Multiskan Go microplate
reader. Cell viability was calculated as follows: Viability
(%)=[experimental group (OD) - blank group (OD)]/[control group
(OD) - blank group (OD)] X 100.
Detection of TYR expression by western
blot analysis
Once the cells reached 80–90% confluency, they were
treated with 50 µM H-15 for 24 h, and a control group (without
H-15) was prepared. The cellular protein was extracted using a
radioimmunoprecipitation assay lysis buffer (BestBio company,
Shanghai, China), and the concentration of the extracted protein
was measured using a BCA kit. A total of 50 µg protein was
electrophoretically separated on a 10% polyacrylamide gel. The
proteins were transferred to a PVDF membrane with 90 V and 200 mA
for 60 min. The membranes were then incubated with rabbit
anti-mouse TYR and GAPDH antibodies (dilution, 1:500) overnight at
4°C. The blots were then incubated with the secondary
fluorescence-conjugated antibody (dilution, 1:5,000) for 2 h in the
dark, and the results were obtained using an Odyssey infrared
imager (LI-COR, Inc., Lincoln, NE, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). The data
were presented as the mean ± standard error of the mean and were
analyzed by paired t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
H-15 exerts a concentration-dependent
effect on B16 cell growth
No significant difference was observed between in
the proliferation rate of the 1% DMSO and the control groups
(P>0.05). The proliferation rate of the B16 cells gradually
decreased subsequent to the treatment of the cells with increasing
concentrations of H-15 (0.1, 1, 10, 50 and 100 µM) for 48 h
compared with the proliferation rate of the control group cells.
The proliferation rate of the B16 cells treated with 100 and 50 µM
H-15 was also significantly decreased (100 µM, P<0.01; 50 µM,
P<0.01; Fig. 2) compared with the
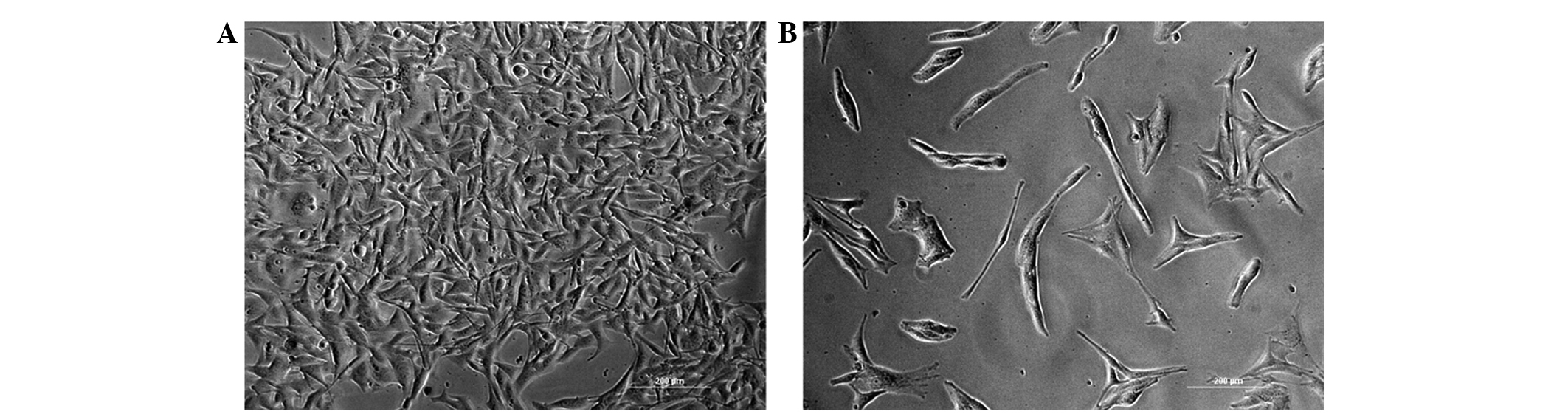
proliferation rate of the control group. Morphological changes in
the cell were observed under light microscopy (Fig. 3). The B16 cells treated with 50 µM
H-15 for 48 h exhibited marked morphological changes, including
decreased cell density, increased cell volume and more evident cell
processes.
H-15 exhibits a time-dependent effect
on the growth of B16 cells
The time-dependent effect of H-15 on cell
proliferation was measured in terms of the cell number. The number
of B16 cells was counted subsequent to treatment of the cells with
50 µM H-15 for 24, 48, 72, 96, 120 and 144 h. These cell numbers
were then compared with those of the control group. The results
indicated that H-15 inhibited the growth of the B16 cells in a
time-dependent manner (Fig. 4).
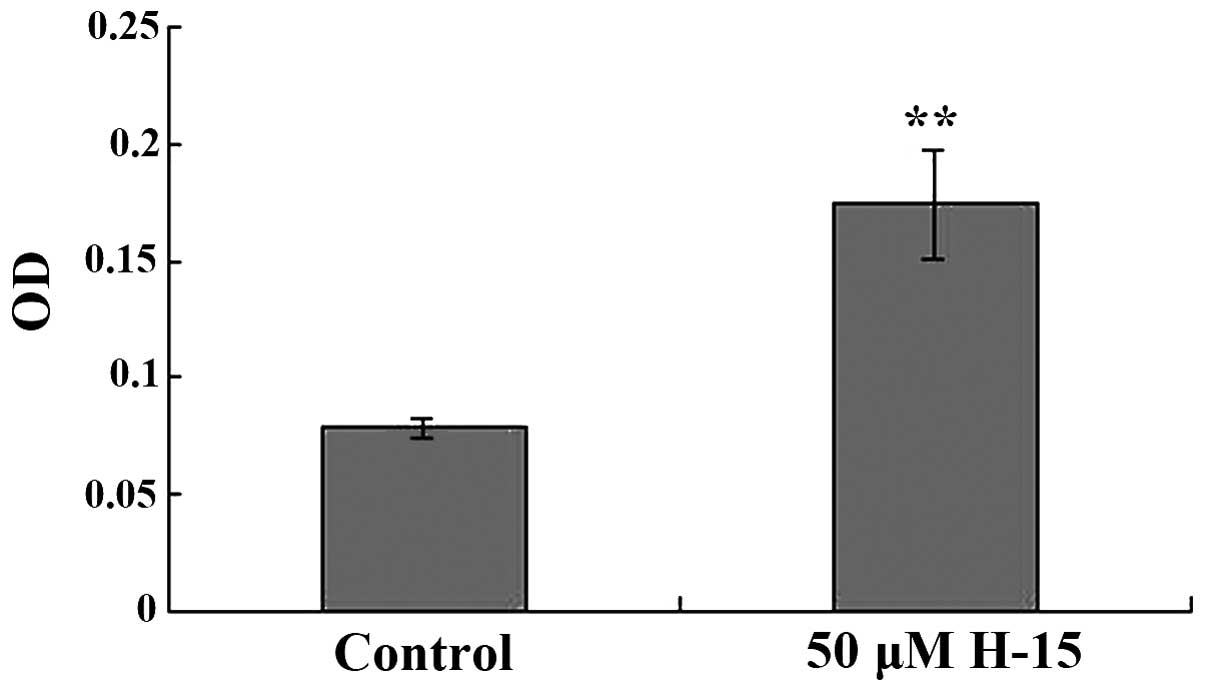
H-15 caused B16 cells to increase
production of melanin
The melanin content in the cells treated with 50 µM
H-15 for 48 h was increased compared with the melanin content of
the control group. The OD value of the B16 cells following
treatment with 50 µM H-15 for 48 h was 0.1743±0.0227, whereas the
OD of the control group was 0.0788±0.0039. The difference in the
results was statistically significant (P<0.05; Fig. 5).
H-15 may increase the expression of
TYR
TYR is an enzyme that plays an important role in
melanin production. The cells treated with 50 µM H-15 for 48 h
exhibited increased expression of TYR (Fig. 6). These results indicated that H-15
may induce the differentiation of B16 cells.
Discussion
Malignant melanoma is a cancer that exhibits an
increasing incidence and mortality rate, a high metastasis rate and
a strong resistance to chemotherapy and radiotherapy (1). Poor prognosis is evident in patients
with malignant melanoma. Currently, effective methods of treatment,
or drugs that treat malignant melanoma, are not available (1). Therefore, novel methods and drugs are
necessary for the treatment of this disease. Sansalvamide A, which
is a cyclic depsipeptide isolated from a marine fungus of the
Fusarium genus, has exhibited marked anti-tumor effects in
the 60 cancer cell line panel of the National Cancer Institute
(5). Various sansalvamide A
derivatives have been synthesized, and these sansalvamide A
derivatives have demonstrated strong antitumor ability and good
stability (9,10).
Malignant melanoma, similar to stem cells, shows an
extremely strong proliferation capability. At present, induced
differentiation therapy for cancer has received increasing
attention (11). Inducing tumor cells
to lose stem cell properties and enabling these cells to exhibit
the specific functions of differentiated cells is the theoretical
basis of induced differentiation therapy, such as inducing
melanocytes to produce melanin. Normal or malignant cells exhibit a
decreased proliferative ability following differentiation. The
present study showed that H-15 significantly inhibited the
proliferation rate of B16 cells, and the time-dependent analysis
confirmed that H-15 demonstrated a long-lasting suppression effect
on the growth of the B16 cell line.
At present, induction differentiation therapy for
the treatment of cancer is receiving increasing attention (3,4). Inducing
tumor cells to lose stem cell properties and enabling these cells
to exhibit the specific functions of differentiated cells is the
theoretical basis of induced differentiation therapy, and results
in the cells losing the ability for proliferation and invasion
(12). Melanin pigments are released
by melanocytes, and the color of skin and hair are largely
determined by melanin. Melanin is derived from the precursor
dopaquinone that is formed by TYR oxidation of L-tyrosine, and
therefore, TYR plays an important role in melanin synthesis
(13). The ability to produce melanin
and the upregulation of TYR were proposed to be responsible for the
differentiation of B16 cells. In the present study, the melanin
content of cells was evaluated and the results showed that the
melanin level was higher in cells subsequent to treatment with 50
µM H-15 for 48 h compared with the control cells. TYR is a
important component in the production of melanin, and following
treatment with 50 µM H-15 for 48 h, the results of the western blot
analysis revealed an ascendant trend in the expression of TYR.
These results indicated that H-15 may induce the differentiation of
B16 cells.
In conclusion, the results of the present study
indicated that H-15 may induce the differentiation of murine
melanoma B16 cells. In addition, this novel compound may improve
the therapeutic approach for the treatment of melanoma.
Acknowledgements
The present study was supported by the National
Basic Research Program of China (grant nos. 2011CB512007 and
2012CB723501) and National Natural Science Foundation of China
(grant no. 30873139).
References
|
1
|
Gray-Schopfer V, Wellbrock C and Marais R:
Melanoma biology and new targeted therapy. Nature. 445:851–857.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berk LB: Radiation therapy as primary and
adjuvant treatment for local and regional melanoma. Cancer Control.
15:233–238. 2008.PubMed/NCBI
|
|
3
|
MacQuarrie KL, Yao Z, Fong AP, Diede SJ,
Rudzinski ER, Hawkins DS and Tapscott SJ: Comparison of genome-wide
binding of MyoD in normal human myogenic cells and
rhabdomyosarcomas identifies regional and local suppression of
promyogenic transcription factors. Mol Cell Biol. 33:773–784. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manu D.T, Sarika M, Jadhav S and Jayesh
RB: All-trans retinoic acid loaded block copolymer nanoparticles
efficiently induce cellular differentiation in HL-60 cells. Eur J
Pharm Sci. 44:643–652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belofsky GN, Jensen PR and Fenical W:
Sansalvamide A new cytotoxic cyclic depsipeptide produced by a
marine fungus of the genus Fusarium. Tetrahedron Lett.
40:2913–2916. 1999. View Article : Google Scholar
|
|
6
|
Liu S, Gu W, Lo D, Ding XZ, Ujiki M,
Adrian TE, Soff GA and Silverman RB: N-methylSansalvamide A peptide
analogues. Histopathology. J Med Chem. 48:3630–3638. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heiferman MJ, Salabat MR, Ujiki MB,
Strouch MJ, Cheon EC, Silverman RB and Bentrem DJ: Sansalvamide
induces pancreatic cancer growth arrest through changes in the cell
cycle. Anticancer Res. 30:73–78. 2010.PubMed/NCBI
|
|
8
|
Styers TJ, Kekec A, Rodriguez R, Brown JD,
Cajica J, Pan PS, Parry E, Carroll CL, et al: Synthesis of
Sansalvamide A derivatives and their cytotoxicity in the MSS colon
cancer cell line HT-29. Bioorg Med Chem. 14:5625–5631. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amidon GL and Lee HJ: Absorption of
peptide and peptidomimetic drugs. Annu Rev Pharmacol Toxicol.
34:321–341. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang G, Liu S, Liu YJ, Wang F, Ren J, Gu
J, Zhou K and Shan B: A novel cyclic pentapeptide, H-10, inhibits
B16 cancer cell growth and induces cell apoptosis. Oncology Lett.
8:248–252. 2014.
|
|
11
|
Serra JM, Gutiérrez A, Alemany R, Navarro
M, Ros T, Saus C, Ginés J, Sampol A, Amat JC, Serra-Moisés L, et
al: Inhibition of c-Myc down-regulation by sustained extracellular
signal-regulated kinase activation prevents the antimetabolite
methotrexate- and gemcitabine-induced differentiation in
non-small-cell lung cancer cells. Mol Pharmacol. 73:1679–1687.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leszczyniecka M, Roberts T, Dent P, Grant
S and Fisher PB: Differentiation therapy of human cancer, Basic
science and clinical applications. Pharmacol Ther. 90:105–156.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Z, Xu J, Long M, Tu Z, Yang G and He
G: 2,3,5, 4′-tetrahydroxystilbene-2-O-beta-D-glucoside (THSG)
induces melanogenesis in B16 cells by MAP kinase activation and
tyrosinase upregulation. Life Sci. 85:345–350. 2009. View Article : Google Scholar : PubMed/NCBI
|