Introduction
Metformin is an oral biguanide that is used
worldwide for the treatment of type 2 diabetes (1). Previous studies have provided evidence
that long-term administration of metformin may reduce the
carcinogenic risk in various organs, and may have an inhibitory
effect on cell growth in breast and colon cancer, as well as glioma
(2,3).
The mechanism underlying the antitumor effect of metformin is
considered to involve the activation of adenosine
monophosphate-activated protein kinase (AMPK) and inhibition of
mammalian target of rapamycin (mTOR), which reduces cell growth
(4). Focusing on gynecological
cancer, as carcinogenesis in endometrial cancer appears to be
associated with obesity, type 2 diabetes and hyperestrogenic
conditions, metformin may be effective for prevention and
improvement of prognosis in endometrial cancer (5). Thus, the effect of metformin on
gynecological tumors, particularly endometrial cancer, is currently
under investigation.
The aim of drug repositioning is to identify novel
pharmacological effects for conventional drugs, in which human
safety and pharmacokinetics are already established, and to expand
the application of the drug for the treatment of additional
diseases (6). As the adverse
reactions of the repositioned drugs are known from previous
clinical trials, safety is guaranteed, and the time and cost of
drug discovery are considerably alleviated (6). Despite recent efforts, the efficacy of
the existing antitumor drugs requires improvement, since they
frequently cause adverse reactions, including nausea, vomiting,
hair loss, nephrotoxicity and myelosuppression, which may limit
their use. We hypothesize that by combining traditional antitumor
drugs with novel antitumor agents identified by drug repositioning,
improved therapeutic efficacy and reduced adverse reactions may be
achieved. In the present review, the clinical application of
metformin for the treatment of different types of gynecological
cancer is evaluated from the perspective of drug repositioning.
Metformin in the treatment of type 2
diabetes
Metformin is an oral biguanide that is safe and
cost-effective for the treatment of type 2 diabetes (1). Structurally, metformin contains two
conjugated guanidine groups and an additional amine (7) (Fig. 1).
Metformin is one of the first-line agents prescribed worldwide for
the treatment of type 2 diabetes (1,8), based on
its inhibition of insulin-dependent hepatic gluconeogenesis,
promotion of glucose uptake into surrounding cells by improvement
of insulin resistance and reduction of free fatty acids by
inhibition of lipolysis (9–11). Metformin additionally inhibits the
development of macroangiopathy to a greater extent than
sulfonylureas do, which may be utilized for the treatment of type 2
diabetes (12).
Metformin enhances glucose consumption in the
intestine and produces lactic acid, which is used in hepatic
gluconeogenesis (13). This causes
adverse reactions, including lactic acidosis, intestinal symptoms
such as diarrhea and abdominal pain, and vitamin B12 deficiency
(14). Lactic acidosis increases the
risk of impaired hemodynamics due to ischemia and shock,
nephropathy, hepatic dysfunction, alcoholism and heart failure
(15–18). Therefore, it is clinically important
to consider the balance between the therapeutic effects and the
risks of adverse reactions when using metformin (17). Nevertheless, the incidence of lactic
acidosis with metformin is 9/100,000 patients/year, whereas with
phenformin, an alternative drug used for the treatment of type 2
diabetes, 40–64/100,000 patients/year experience lactic acidosis
(19). Therefore, metformin is
generally considered to be safe, compared with alternative
antidiabetic drugs (19).
Effect of metformin on carcinogenic
risk
Type 2 diabetes and insulin resistance increase the
carcinogenic risk in the large intestine, lung, breast, prostate
gland and pancreas (20–25). A number of studies have evaluated the
effects of metformin on cancer prevention. In a population-based
study including 11,876 patients with type 2 diabetes, Evans et
al (26) reported a reduced
incidence of cancer in patients treated with metformin, compared
with patients not treated with metformin [odds ratio (OR), 0.79;
95% confidence interval (CI), 0.67–0.93]. Bowker et al
(27) compared patients with type 2
diabetes in the metformin (monotherapy or combined) group and
sulfonylurea monotherapy group, and reported that the cancer
mortality rate was significantly decreased in the metformin group
[hazard ratio (HR), 0.80; 95% CI, 0.65–0.98; P=0.03), compared with
the sulfonylurea group. In a study of 4,085 patients exhibiting
type 2 diabetes, Libby et al (28) identified that the incidence of cancer
in patients treated with metformin (7.3%) was significantly lower
than that observed in patients treated with alternative drugs
(11.6%). Following adjustment for confounding factors, the authors
observed that the use of metformin significantly reduced the risk
of cancer (HR, 0.63; 95% CI, 0.53–0.75). The results of the
aforementioned studies suggest that metformin is able to reduce the
carcinogenic risk in patients with type 2 diabetes.
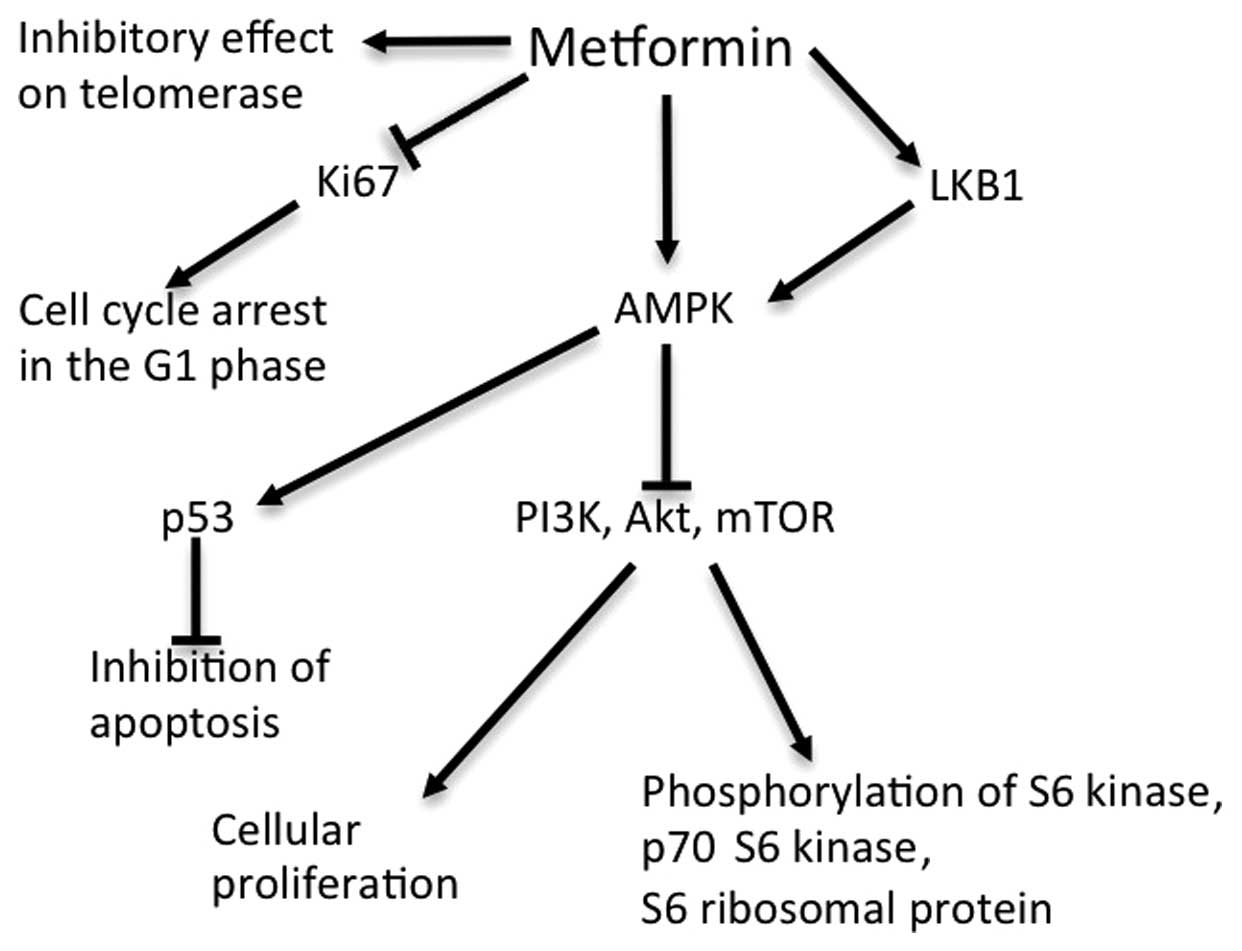
The antitumor effect of metformin
Multiple pathways are considered to be involved in
the antitumor activity of metformin (4,29–32) (Fig. 2).
The primary action of metformin occurs via activation of AMPK
(33). Metabolically, AMPK inhibits
the expression of certain enzymes involved in hepatic
gluconeogenesis, enhances glucose uptake into muscle and fat cells,
and increases insulin sensitivity in cells, resulting in decreased
insulin levels (33,34). Furthermore, the fact that the
metformin-induced activation of AMPK is mediated by the tumor
suppressor liver kinase B1 (LKB1) suggests the antitumor potential
of metformin (29). AMPK inhibits the
activity of mTOR in the phosphoinositide 3-kinase/Akt/mTOR signal
transduction pathway, which stimulates cellular proliferation
(30,31). AMPK is also known to inhibit cell
cycle progression via the activation of tumor protein p53 (4).
Additional mechanisms of metformin that do not
involve AMPK have been reported. Metformin inhibits cell cycle
progression by decreasing cyclin D1 expression (35), and Cantrell et al (32) identified that telomerase activity was
inhibited by metformin. However, the mechanism of action of
metformin remains to be fully elucidated, for which further studies
are required.
Antitumor effect of metformin in endometrial
cancer
Metformin may be an effective adjuvant for the
treatment of endometrial cancer, based on the observation that type
2 diabetes and obesity are risk factors of endometrial cancer
(5). Epidemiological data has
demonstrated that obese individuals possess a significantly
increased risk of developing endometrial cancer in comparison with
non-obese individuals (risk ratio, 6.25; 95% CI, 3.75–10.42;
P<0.001) (36). An additional risk
factor for endometrial cancer is polycystic ovarian syndrome
(PCOS), in which hyperinsulinemia and hyperandrogenism are the two
central pathological conditions (37). Metformin has a therapeutic effect on
the anovulatory cycle in PCOS (38),
and is expected to reduce the carcinogenic risk in endometrial
cancer (39).
Metformin is additionally likely to have a
significant role in the prevention of endometrial cancer via cell
cycle arrest and induction of apoptosis (32). In preclinical studies, Cantrell et
al (32) observed that metformin
caused G1 arrest at a low dose of 1 mM, and apoptosis via
activation of caspase-3 at a high dose of 2–5 mM in
vitro.
Telomere maintenance by telomerase has a significant
role in tumor growth, and the messenger (m)RNA levels of human
telomerase reverse transcriptase (hTERT) are used as an index for
telomerase activity and cell growth (32). Metformin suppresses the mRNA
expression of hTERT in endometrial cancer cells in a dose-dependent
manner, leading to the inhibition of telomerase activity (35). The suppression of hTERT mRNA may be a
direct effect of metformin or a secondary effect due to cell cycle
arrest, as endometrial, ovarian and cervical cancer cell growth is
additionally inhibited by rapamycin and accompanied by a decrease
in hTERT mRNA (35). A direct
inhibition caused by metformin has been suggested, due to the
observation that rapamycin suppressed hTERT mRNA without cell
growth inhibition or cell cycle arrest in cell lines that were
resistant to rapamycin, indicating that a reduction in hTERT mRNA
is able to occur independently from cell cycle arrest (40).
Progesterone is utilized for the treatment of early
endometrial cancer. However, the therapeutic effect of progesterone
in endometrial cancer cells is insufficient, due to the
downregulation of progesterone receptor (PR) in these cells
(41). Xie et al (41) identified that metformin and
progesterone had a synergistic effect in the treatment of
endometrial cancer. Metformin inhibits the phosphorylation of S6
ribosomal protein (S6RP), increases PR expression and inhibits mTOR
via AMPK phosphorylation, which enhances the efficacy of
medroxyprogesterone acetate (MPA) in the treatment of endometrial
cancer (42).
Ko et al (43)
investigated the efficacy of metformin in 1,495 patients exhibiting
endometrial cancer, including 363 (24%) patients with diabetes.
Patients treated with metformin (54% of diabetic patients in the
study) demonstrated significantly improved recurrence-free survival
(RFS) and overall survival (OS) rates in comparison with patients
who were not administered metformin. RFS in the non-metformin group
was reduced by 1.8-fold (95% CI, 1.1–2.9; P=0.02), while OS was
reduced by 2.3-fold (95% CI, 1.3–4.2; P=0.005). However, there was
no association between metformin treatment and time to recurrence,
indicating that metformin has a survival benefit for mortality, but
does not prolong the time to recurrence, for reasons that remain to
be elucidated (43). Thus, additional
studies are required to confirm if adjuvant therapy with metformin
is effective for patients exhibiting endometrial cancer, regardless
of the complication of diabetes.
Antitumor effect of metformin in breast
cancer
Breast cancer is associated with type 2 diabetes
(36), and a previous epidemiological
study demonstrated that type 2 diabetes increased the risk of
developing breast cancer by 10–20% (44).
Triple-negative (TN) breast cancer refers to breast
cancer cases that do not express the genes for estrogen receptor
(ER), PR and human epidermal growth factor receptor 2 (HER-2)
(45). TN breast cancer develops in
perimenopausal women possessing a high body mass index (BMI) and
overexpression of epidermal growth factor receptor, and has been
identified to be highly sensitive to metformin (45). A previous study revealed that
metformin was able to inhibit cell growth of TN breast cancer at a
similar dose to that utilized for the treatment of type 2 diabetes
by suppressing Ki67, arresting the cell cycle in G1 phase, and
inducing intrinsic and extrinsic apoptosis via caspase-8 and −9
(45). The efficacy of metformin for
the treatment of common subtypes of breast cancer, including
luminal A and B and HER-2+, has additionally been
demonstrated. Colonization and tumor growth were simultaneously
inhibited by metformin, and these effects occurred through a
non-apoptotic mechanism, in which cyclin D1 and E2F transcription
factor 1 (E2F1), which promote the transition from G1 to S phase,
were implicated (46). In addition,
metformin was able to alter tyrosine kinase signaling,
downregulates HER-2 and activates mitogen-activated protein kinase
at an identical dose to that utilized for the treatment of type 2
diabetes (46).
Overexpression of the insulin and insulin-like
growth factor (IGF)-1 receptors is involved in the carcinogenesis
of breast cancer, and breast cancer cell lines such as MCF-7 are
responsive to insulin and IGF-1 (47). The absence of an inhibitory effect of
metformin on cell growth following small interfering RNA inhibition
of AMPK suggested that the effect of metformin on breast cancer
cells occurs via AMPK (48).
The efficacy of metformin has been demonstrated in
diabetic women exhibiting breast cancer in a retrospective study
(49). Of the 155 diabetic patients
included in the study, 68 received metformin and 87 did not, along
with anthracycline-based chemotherapy regimens (49). The pathological complete response rate
was 24% in the metformin-treated group, compared with 8% in the
non-metformin-treated group (P=0.07) (49). Additional phase II and phase III
studies are ongoing. The METEOR study is a phase II randomized
trial of metformin plus letrozole vs. placebo plus letrozole, which
aimed to assess the antitumor effects of metformin in
postmenopausal non-diabetic patients exhibiting ER+
breast cancer (50). An ongoing phase
III clinical trial termed NCIC CTG MA.32, which aimed to study the
effects of metformin on non-diabetic patients with breast cancer,
requires a follow-up period of several years in order to evaluate
the effects of metformin on mortality and define an optimal dose of
metformin for the treatment of early breast cancer (51).
The optimal dose of metformin for the treatment of
breast cancer remains to be elucidated. However, 1,500–2,250 mg/day
metformin was observed to be required in order to reduce tumor size
in xenograft models (50,52), and in the NCIC CTG MA.32 trial, the
metformin group was designed to receive 1,700 mg/day of this drug
(51). These doses are tolerated in
the treatment of type 2 diabetes (8).
Antitumor effect of metformin in ovarian
cancer
The potential pharmacological effects of metformin
in ovarian cancer are of interest. Obesity potentially contributes
to the onset of ovarian cancer, and may additionally be stimulated
by androgens, as in PCOS (36,39).
Hyperandrogenism is caused by hyperinsulinemia, inhibition of IGF
binding protein 1 (IGFBP1) and increased IGF-1 activity (39). Based on the risk reduction for ovarian
cancer exhibited by oral contraceptives with anti-androgen activity
(53) and the effects of metformin on
PCOS, obesity and other tumors, we hypothesize that metformin may
demonstrate efficacy for the treatment of ovarian cancer. Metformin
inhibits tumor growth and induces apoptosis in ovarian cancer cells
in vitro, as reported by Gotlieb et al (54), who identified that metformin inhibited
cell growth in OVCAR-3 and OVCAR-4 cells in a dose-dependent
manner, and administration of metformin in combination with
cisplatin enhanced this pharmacological effect. These effects were
induced by decreased phosphorylation of p70 S6 kinase (p70S6K) and
S6K via AMPK phosphorylation (54).
A number of epidemiological studies have
investigated the effects of metformin in ovarian cancer. In a
case-control study of 1,611 diabetic patients, Bodmer et al
(55) identified that the
carcinogenic risk in the metformin-treated group was significantly
lower (OR, 0.61; 95% CI, 0.30–1.25) than in the
sulfonylurea-treated (OR, 1.26; 95% CI, 0.65–2.44) and
insulin-treated groups (OR, 2.29; 95% CI, 1.13–4.65). In a study
including 1,454 diabetic patients treated with metformin and 2,897
diabetic patients who were not administered metformin for a median
duration of 4.0 years, Home et al (56) observed that none of the patients in
the metformin group developed ovarian cancer, whereas 3 patients in
the non-metformin group did. In an analysis with a median duration
of 5.5 years, 6/3,344 patients treated with metformin and 3/1,103
patients who were not treated with metformin developed ovarian
cancer (56).
In a systematic review of 28 studies, Zhang and Li
(57) identified that metformin
decreased mortality associated with ovarian cancer (relative risk
(RR), 0.44; 95% CI, 0.30–0.64; P<0.001). In an epithelial
ovarian cancer study, the effect of metformin on survival rate was
examined in 61 metformin-treated diabetic patients and 178
non-diabetic controls (58). The
5-year disease-specific survival (DSS) rate in the metformin group
was significantly increased, compared with the control group (67
vs. 47%; P=0.007) (58). Following
adjustment for background factors including BMI, tumor grade,
histology and chemotherapy, metformin remained an independent
predictor of survival (58). In the
same study, the 5-year DSS rate was compared between the
metformin-treated diabetic group and the diabetic control group
(patients on diabetic treatment other than metformin). The 5-year
DSS rate was significantly reduced in the insulin (43%) and
alternative antidiabetic medication group (34%; P=0.004), compared
with the metformin-treated group. There were a limited number of
patients exhibiting diabetes and ovarian cancer, and the small
number of cases (n=61) is a limitation of that study (58). However, the results clearly
demonstrated the overall efficacy of metformin for the treatment of
ovarian cancer (58). As diabetes
itself is known to be a poor prognostic factor for ovarian cancer,
and diabetic patients are also likely to exhibit other poor
prognostic factors, including cardiovascular disease and surgical
history, the therapeutic effects of metformin may be overestimated
when compared with non-metformin treated diabetic patients
(58). Thus, it is a matter of
discussion whether the antitumor effects of metformin should be
compared with diabetic or non-diabetic controls in future
studies.
Antitumor effect of metformin in cervical
cancer
There have been few studies discussing the efficacy
of metformin for the treatment of cervical cancer. However, based
on its effects on tumor inhibition, metformin is likely to inhibit
cervical cancer cell growth (59,60). Xiao
et al (61) investigated the
kinetics of metformin in cervical cancer cells and evaluated LKB1
activity in these cells. The authors observed that metformin
inhibited the growth of the C33A, ME180 and CaSki cervical cancer
cell lines, but exhibited reduced efficacy against HeLa, HT-3 and
MS751 cells (61). Following analysis
of LKB1/AMPK/mTOR signaling, metformin-sensitive cervical cancer
cells were identified to activate AMPK via LKB1 and inhibit mTOR
(61). In contrast, sensitivity to
metformin was lost in LKB1-knockdown cells, whereas in cervical
cancer cells expressing LKB1, metformin induced apoptosis and
autophagy (61). These results
suggest that metformin may be a promising drug for the treatment of
cervical cancer, particularly in tumor cells expressing LKB1, by
increasing LKB1 activity and activating AMPK (61).
Clinical studies of metformin in
gynecological cancer
In antitumor mechanisms in vitro (Fig. 3), metformin arrests the cell cycle in
endometrial cancer cells, decreases hTERT mRNA and inhibits
phosphorylation of S6RP, resulting in inhibition of signaling
downstream of the mTOR pathway (35,42).
Metformin additionally antagonizes IGF-2, enhances expression of PR
and improves the antitumor effect of MPA in cancer cells (41). The antitumor effect of metformin in
breast, cervical and ovarian cancer also involves the inhibition of
mTOR via AMPK activity (48,54,61).
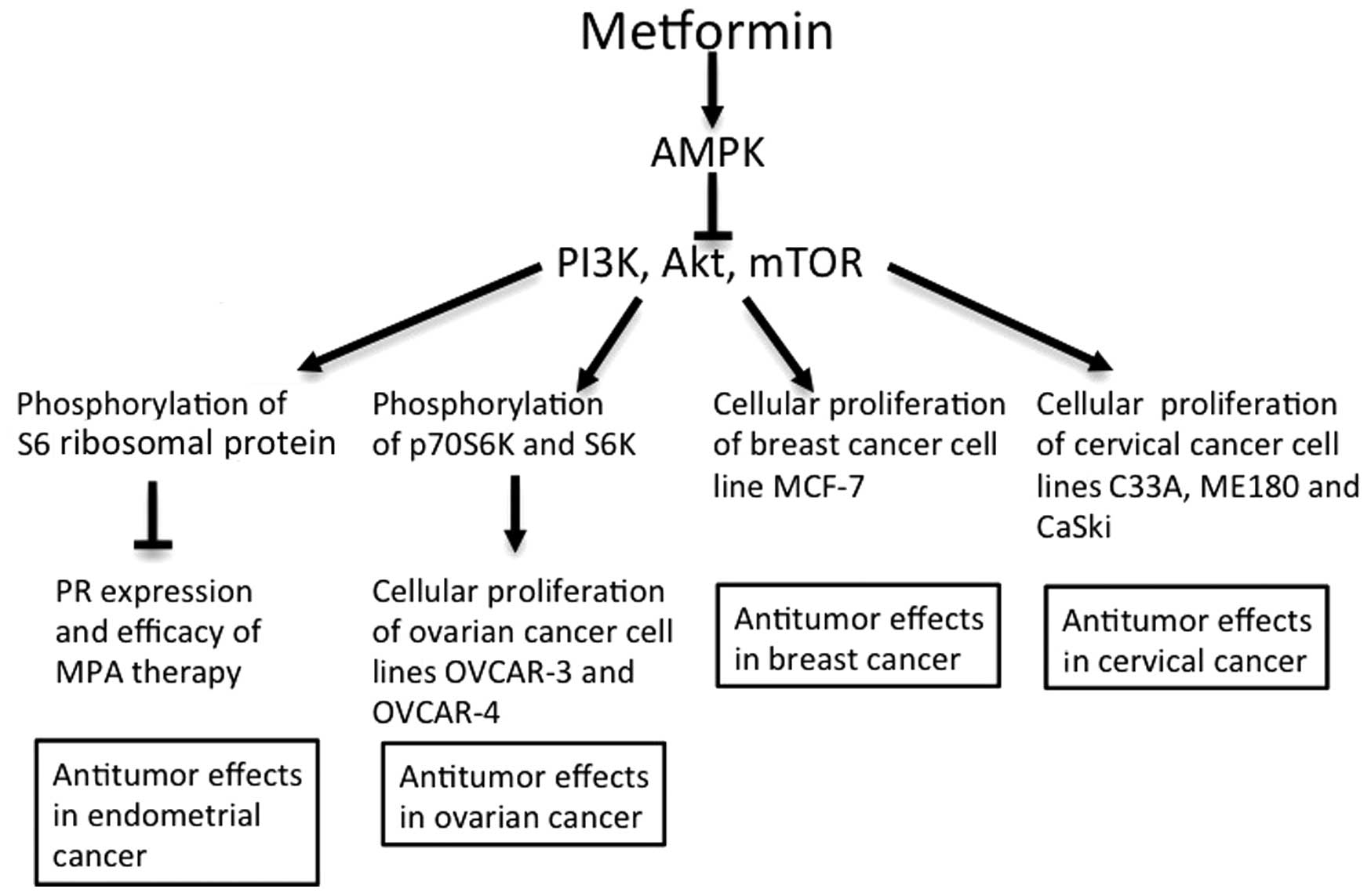 | Figure 3.Antitumor effects of metformin in
gynecological cancer. Antitumor effects of metformin have been
demonstrated in endometrial, ovarian, breast and cervical cancer
in vitro (41,46–48,54,61).
AMPK, adenosine monophosphate-activated protein kinase; PI3K,
phosphoinositide 3-kinase; mTOR, mammalian target of rapamycin; PR,
progesterone receptor; MPA, medroxyprogesterone acetate; S6K, S6
kinase. |
Based on these findings, the efficacy of metformin
for the treatment of gynecological cancer has been examined in
clinical studies, and the results have been analyzed using RR in
systematic reviews (57). Two studies
have demonstrated the efficacy of metformin for the treatment of
endometrial cancer (43,62). In these studies, the RR of treatment
with metformin was 0.49 (95% CI, 0.32–0.73; P=0.001), with no
difference in the results in meta-analysis and no heterogeneity
(I2=0%) (58). A total of
four studies have demonstrated that metformin improves overall
mortality in breast cancer (63–66),
contrarily to three other studies (67–69), which
did not observe any efficacy for this drug. The RR of treatment
with metformin in the aforementioned studies was 0.70 (95% CI,
0.55–0.88; P=0.003) (58). High
heterogeneity was identified in these studies (I2=75%),
but no publication bias. He et al (63) demonstrated that administration of
metformin markedly decreased mortality specific to breast cancer
(63), although their findings did
not correlate with the results of two other studies (67,68). The
RR in these studies was 0.83 (95% CI, 0.63–1.08; P=0.16), and
moderate heterogeneity was apparent (I2=47%) (57). The high I2 values in the
analyses of trials of metformin for breast cancer reflected the
varying results among the studies. In ovarian cancer, an
association between the use of metformin and overall mortality has
been identified in three studies (58,69,70). The
RR in these studies was 0.44 (95% CI, 0.30–0.64; P<0.001), and
there was no heterogeneity (I2=0%) (57). A previous study demonstrated that
metformin improved progression-free survival, with a relapse HR of
0.38 (95% CI, 0.16–0.90; P=0.03) (57). Overall, the results of the above
studies indicate that metformin significantly increases survival in
endometrial, breast and ovarian cancer (Table I). The efficacy of metformin in the
treatment of cervical cancer has not been examined clinically to
date.
 | Table I.Effects of metformin in gynecological
cancer prevention. |
Table I.
Effects of metformin in gynecological
cancer prevention.
| Type of cancer | Relative risk for
all-cause mortality | 95% Confidence
interval | P-value | Heterogeneity,
I2, % |
|---|
| Endometrial | 0.49 | 0.32–0.73 | 0.001 | 0 |
| Breast | 0.70 | 0.55–0.88 | 0.003 | 75 |
| Ovarian | 0.44 | 0.30–0.64 | <0.001 | 0 |
Concurrent antitumor therapies used alongside
metformin, dose adjustment, cancer stage, tumor size and histology
are significant prognostic factors. However, they are not described
in the majority of studies (57). We
hypothesize that failure to adjust for these confounding factors
may cause high heterogeneity. Ethnicity, education level and access
to medical care may additionally influence survival, and these
factors may bias estimates of the efficacy of metformin (57). Future studies should include clinical
trials that consider these factors in order to increase the cohort
size, reduce bias and evaluate the effect of metformin more
accurately. It is additionally important to identify the optimum
dose of metformin based on adverse reactions (57).
Conclusion
Metformin is a first-line drug that is used for the
treatment of type 2 diabetes, and has additionally been identified
to decrease carcinogenic risk and inhibit cancer cell growth
(2,3).
The antitumor mechanism of metformin involves the inhibition of the
mTOR pathway through AMPK activation, as demonstrated in a number
of studies on gynecological cancer (42,48,54,61).
However, additional details of the mechanism responsible for the
antitumor effects of metformin remain to be elucidated. In
endometrial cancer, cell cycle arrest by metformin has been
observed in vitro, and inhibition of telomerase activity may
be an important mechanism to explain the antitumor activity of this
drug (35). Metformin additionally
has an increased antitumor effect when administered in combination
with MPA therapy (41). Clinical
studies of metformin have demonstrated efficacy and safety in
breast, endometrial and ovarian cancer (43,49,50,55–58).
The effect in cervical cancer has not been examined in clinical
studies thus far, although efficacy of metformin in vitro
has been observed (61).
In conclusion, drug repositioning allows rapid
clinical application of a drug with high safety and low cost
(6). For drug repositioning of
metformin, it will be particularly important to understand its
antitumor mechanism, evaluate its adverse reactions and risks in
clinical application, and determine the optimum dose required for
the treatment of gynecological cancer.
Acknowledgements
The authors would like to thank Dr K. Nakagawara
(Keio University School of Medicine, Tokyo, Japan) and Dr K. Aoyama
(Keio University School of Medicine, Tokyo, Japan) for their
helpful assistance. The present study was supported by grants from
the Medical Research Encouragement Prize of The Japan Medical
Association (Tokyo, Japan) and the Keio Gijuku Academic Development
Funds of Keio University (Tokyo, Japan).
References
|
1
|
Nathan DM, Buse JB, Davidson MB, Heine RJ,
Holman RR, Sherwin R and Zinman B: Professional Practice Committee,
American Diabetes Association; European Association for the Study
of Diabetes: Management of hyperglycaemia in type 2 diabetes: A
consensus algorithm for the initiation and adjustment of therapy. A
consensus statement from the American Diabetes Association and the
European Association for the Study of Diabetes. Diabetologia.
49:1711–1721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aljada A and Mousa SA: Metformin and
neoplasia: Implications and indications. Pharmacol Ther.
133:108–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soritau O, Tomuleasa C, Aldea M, Petrushev
B, Susman S, Gheban D, Ioani H, Cosis A, Brie I, Irimie A, et al:
Metformin plus temozolomide-based chemotherapy as adjuvant
treatment for WHO grade III and IV malignant gliomas. J BUON.
16:282–289. 2011.PubMed/NCBI
|
|
4
|
Riedmaier Emami A, Fisel P, Nies AT,
Schaeffeler E and Schwab P: Metformin and cancer: From the old
medicine cabinet to pharmacological pitfalls and prospects. Trends
Pharmacol Sci. 34:126–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campagnoli C, Abbà C, Ambroggio S, Brucato
T and Pasanisi P: Life-style and metformin for the prevention of
endometrial pathology in postmenopausal women. Gynecol Endocrinol.
29:119–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ashburn TT and Thor KB: Drug
repositioning: Identifying and developing new uses for existing
drugs. Nat Rev Drug Discov. 3:673–683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moiseeva O, Deschênes-Simard X, Pollak M
and Ferbeyre G: Metformin, aging and cancer. Aging (Albany NY).
5:330–331. 2013.PubMed/NCBI
|
|
8
|
Nathan DM, Buse JB, Davidson MB,
Ferrannini E, Holman RR, Sherwin R and Zinman B: American Diabetes
Association; European Association for Study of Diabetes: Medical
management of hyperglycemia in type 2 diabetes: A consensus
algorithm for the initiation and adjustment of therapy: A consensus
statement of the American Diabetes Association and the European
Association for the Study of Diabetes. Diabetes Care. 32:193–203.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bailey CJ: Biguanides and NIDDM. Diabetes
Care. 15:755–772. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bailey CJ and Turner RC: Metformin. N Engl
J Med. 334:574–579. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stumvoll M, Nurjhan N, Perriello G, Dailey
G and Gerich JE: Metabolic effects of metformin in
non-insulin-dependent diabetes mellitus. N Engl J Med. 333:550–554.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morgan CL, Mukherjee J, Jenkins-Jones S,
Holden SE and Currie CJ: Association between first-line monotherapy
with sulphonylurea versus metformin and risk of all-cause mortality
and cardiovascular events: A retrospective, observational study.
Diabetes Obes Metab. 16:957–962. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan NN, Brain HP and Feher MD:
Metformin-associated lactic acidosis: A rare or very rare clinical
entity? Diabet Med. 16:273–281. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kilo C: Metformin: A safe and effective
treatment in the management of NIDDM. Mo Med. 94:114–123.
1997.PubMed/NCBI
|
|
15
|
Vasisht KP, Chen SC, Peng Y and Bakris GL:
Limitations of metformin use in patients with kidney disease: Are
they warranted? Diabetes Obes Metab. 12:1079–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horlen C, Malone R, Bryant B, Dennis B,
Carey T, Pignone M and Rothman R: Frequency of inappropriate
metformin prescriptions. JAMA. 287:2504–2505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calabrese AT, Coley KC, DaPos SV, Swanson
D and Rao RH: Evaluation of prescribing practices: Risk of lactic
acidosis with metformin therapy. Arch Intern Med. 162:434–437.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Masoudi FA, Wang Y, Inzucchi SE, Setaro
JF, Havranek EP, Foody JM and Krumholz HM: Metformin and
thiazolidinedione use in Medicare patients with heart failure.
JAMA. 290:81–85. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stang M, Wysowski DK and Butler-Jones D:
Incidence of lactic acidosis in metformin users. Diabetes Care.
22:925–927. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saydah SH, Loria CM, Eberhardt MS and
Brancati FL: Abnormal glucose tolerance and the risk of cancer
death in the United States. Am J Epidemiol. 157:1092–1100. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Michels KB, Solomon CG, Hu FB, Rosner BA,
Hankinson SE, Colditz GA and Manson JE: Nurses' Health Study: Type
2 diabetes and subsequent incidence of breast cancer in the Nurses'
Health Study. Diabetes Care. 26:1752–1758. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Will JC, Galuska DA, Vinicor F and Calle
EE: Colorectal cancer: Another complication of diabetes mellitus?
Am J Epidemiol. 147:816–825. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Everhart J and Wright D: Diabetes mellitus
as a risk factor for pancreatic cancer. A meta-analysis. JAMA.
273:1605–1609. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gapstur SM, Gann PH, Colangelo LA,
Barron-Simpson R, Kopp P, Dyer A and Liu K: Postload plasma glucose
concentration and 27-year prostate cancer mortality (United
States). Cancer Causes Control. 12:763–772. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moore MA, Park CB and Tsuda H:
Implications of the hyperinsulinaemia-diabetes-cancer link for
preventive efforts. Eur J Cancer Prev. 7:89–107. 1998.PubMed/NCBI
|
|
26
|
Evans JM, Donnelly LA, Emslie-Smith AM,
Alessi DR and Morris AD: Metformin and reduced risk of cancer in
diabetic patients. BMJ. 330:1304–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bowker SL, Yasui Y, Veugelers P and
Johnson JA: Glucose-lowering agents and cancer mortality rates in
type 2 diabetes: Assessing effects of time-varying exposure.
Diabetologia. 53:1631–1637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Libby G, Donnelly LA, Donnan PT, Alessi
DR, Morris AD and Evans JM: New users of metformin are at low risk
of incident cancer: A cohort study among people with type 2
diabetes. Diabetes Care. 32:1620–1625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shaw RJ, Lamia KA, Vasquez D, Koo SH,
Bardeesy N, Depinho RA, Montminy M and Cantley LC: The kinase LKB1
mediates glucose homeostasis in liver and therapeutic effects of
metformin. Science. 310:1642–1646. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gwinn DM, Shackelford DB, Egan DF,
Mihaylova MM, Mery A, Vasquez DS, Turk BE and Shaw RJ: AMPK
phosphorylation of raptor mediates a metabolic checkpoint. Mol
Cell. 30:214–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Inoki K, Li Y, Zhu T, Wu J and Guan KL:
TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR
signalling. Nat Cell Biol. 4:648–657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cantrell LA, Zhou C, Mendivil A, Malloy
KM, Gehrig PA and Bae-Jump VL: Metformin is a potent inhibitor of
endometrial cancer cell proliferation - implications for a novel
treatment strategy. Gynecol Oncol. 116:92–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stephenne X, Foretz M, Taleux N, van der
Zon GC, Sokal E, Hue L, Viollet B and Guigas P: Metformin activates
AMP-activated protein kinase in primary human hepatocytes by
decreasing cellular energy status. Diabetologia. 54:3101–3110.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gunton JE, Delhanty PJ, Takahashi S and
Baxter RC: Metformin rapidly increases insulin receptor activation
in human liver and signals preferentially through insulin-receptor
substrate-2. J Clin Endocrinol Metab. 88:1323–1332. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sahra Ben I, Laurent K, Loubat A,
Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le
Marchand-Brustel Y and Bost F: The antidiabetic drug metformin
exerts an antitumoral effect in vitro and in vivo
through a decrease of cyclin D1 level. Oncogene. 27:3576–3586.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Navaratnarajah R, Pillay OC and Hardiman
P: Polycystic ovary syndrome and endometrial cancer. Semin Reprod
Med. 26:62–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dronavalli S and Ehrmann DA: Pharmacologic
therapy of polycystic ovary syndrome. Clin Obstet Gynecol.
50:244–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dumesic DA and Lobo RA: Cancer risk and
PCOS. Steroids. 78:782–785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou C, Gehrig PA, Whang YE and Boggess
JF: Rapamycin inhibits telomerase activity by decreasing the hTERT
mRNA level in endometrial cancer cells. Mol Cancer Ther. 2:789–795.
2003.PubMed/NCBI
|
|
41
|
Xie Y, Wang YL, Yu L, Hu Q, Ji L, Zhang Y
and Liao QP: Metformin promotes progesterone receptor expression
via inhibition of mammalian target of rapamycin (mTOR) in
endometrial cancer cells. J Steroid Biochem Mol Biol. 126:113–120.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsuji K, Kisu I, Banno K, Yanokura M, Ueki
A, Masuda K, Kobayashi Y, Yamagami W, Nomura H, Susumu N and Aoki
D: Metformin: A possible drug for treatment of endometrial cancer.
Open J Obstet Gynecol. 2:1–6. 2012. View Article : Google Scholar
|
|
43
|
Ko EM, Walter P, Jackson A, Clark L,
Franasiak J, Bolac C, Havrilesky LJ, Secord AA, Moore DT, Gehrig PA
and Bae-Jump V: Metformin is associated with improved survival in
endometrial cancer. Gynecol Oncol. 132:438–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wolf I, Sadetzki S, Catane R, Karasik A
and Kaufman B: Diabetes mellitus and breast cancer. Lancet Oncol.
6:103–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu B, Fan Z, Edgerton SM, Deng XS,
Alimova IN, Lind SE and Thor AD: Metformin induces unique
biological and molecular responses in triple negative breast cancer
cells. Cell Cycle. 8:2031–2040. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alimova IN, Liu B, Fan Z, Edgerton SM,
Dillon T, Lind SE and Thor AD: Metformin inhibits breast cancer
cell growth, colony formation and induces cell cycle arrest in
vitro. Cell Cycle. 8:909–915. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sachdev D, Singh R, Fujita-Yamaguchi Y and
Yee D: Down-regulation of insulin receptor by antibodies against
the type I insulin-like growth factor receptor: Implications for
anti-insulin-like growth factor therapy in breast cancer. Cancer
Res. 66:2391–2402. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zakikhani M, Dowling R, Fantus IG,
Sonenberg N and Pollak M: Metformin is an AMP kinase-dependent
growth inhibitor for breast cancer cells. Cancer Res.
66:10269–10273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiralerspong S, Palla SL, Giordano SH,
Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi
GN and Gonzalez-Angulo AM: Metformin and pathologic complete
responses to neoadjuvant chemotherapy in diabetic patients with
breast cancer. J Clin Oncol. 27:3297–3302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim J, Lim W, Kim EK, Kim MK, Paik NS,
Jeong SS, Yoon JH, Park CH, Ahn SH, Kim LS, et al: Phase II
randomized trial of neoadjuvant metformin plus letrozole versus
placebo plus letrozole for estrogen receptor positive
postmenopausal breast cancer (METEOR). BMC Cancer. 14:1702014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Goodwin PJ, Stambolic V, Lemieux J, Chen
BE, Parulekar WR, Gelmon KA, Hershman DL, Hobday TJ, Ligibel JA,
Mayer IA, et al: Evaluation of metformin in early breast cancer: A
modification of the traditional paradigm for clinical testing of
anti-cancer agents. Breast Cancer Res Treat. 126:215–220. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Iliopoulos D, Hirsch HA and Struhl K:
Metformin decreases the dose of chemotherapy for prolonging tumor
remission in mouse xenografts involving multiple cancer cell types.
Cancer Res. 71:3196–3201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ness RB, Grisso JA, Klapper J,
Schlesselman JJ, Silberzweig S, Vergona R, Morgan M and Wheeler JE:
Risk of ovarian cancer in relation to estrogen and progestin dose
and use characteristics of oral contraceptives SHARE Study Group.
Steroid Hormones and Reproductions. Am J Epidemiol. 152:233–241.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gotlieb WH, Saumet J, Beauchamp MC, Gu J,
Lau S, Pollak MN and Bruchim I: In vitro metformin
anti-neoplastic activity in epithelial ovarian cancer. Gynecol
Oncol. 110:246–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bodmer M, Becker C, Meier C, Jick SS and
Meier CR: Use of metformin and the risk of ovarian cancer: A
case-control analysis. Gynecol Oncol. 123:200–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Home PD, Kahn SE, Jones NP, Noronha D,
Beck-Nielsen H and Viberti G: ADOPT Study Group; RECORD Steering
Committee: Experience of malignancies with oral glucose-lowering
drugs in the randomised controlled ADOPT (A Diabetes Outcome
Progression Trial) and RECORD (Rosiglitazone Evaluated for
Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes)
clinical trials. Diabetologia. 53:1838–1845. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang ZJ and Li S: The prognostic value of
metformin for cancer patients with concurrent diabetes: A
systematic review and meta-analysis. Diabetes Obes Metab.
16:707–710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kumar S, Meuter A, Thapa P, Langstraat C,
Giri S, Chien J, Rattan R, Cliby W and Shridhar V: Metformin intake
is associated with better survival in ovarian cancer: A
case-control study. Cancer. 119:555–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pollak M: Metformin and other biguanides
in oncology: Advancing the research agenda. Cancer Prev Res
(Phila). 3:1060–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fogarty S and Hardie DG: Development of
protein kinase activators: AMPK as a target in metabolic disorders
and cancer. Biochim Biophys Acta. 1804:581–591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xiao X, He Q, Lu C, Werle KD, Zhao RX,
Chen J, Davis BC, Cui R, Liang J and Xu ZX: Metformin impairs the
growth of liver kinase B1-intact cervical cancer cells. Gynecol
Oncol. 127:249–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nevadunsky NS, Van Arsdale A, Strickler
HD, Moadel A, Kaur G, Frimer M, Conroy E, Goldberg GL and Einstein
P: Metformin use and endometrial cancer survival. Gynecol Oncol.
132:236–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He X, Esteva FJ, Ensor J, Hortobagyi GN,
Lee MH and Yeung SC: Metformin and thiazolidinediones are
associated with improved breast cancer-specific survival of
diabetic women with HER2+ breast cancer. Ann Oncol.
23:1771–1780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hou G, Zhang S, Zhang X, Wang P, Hao X and
Zhang J: Clinical pathological characteristics and prognostic
analysis of 1,013 breast cancer patients with diabetes. Breast
Cancer Res Treat. 137:807–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Peeters PJ, Bazelier MT, Vestergaard P,
Leufkens HG, Schmidt MK, de Vries F and De Bruin ML: Use of
metformin and survival of diabetic women with breast cancer. Curr
Drug Saf. 8:357–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xiao Y, Zhang S, Hou G, Zhang X, Hao X and
Zhang J: Clinical pathological characteristics and prognostic
analysis of diabetic women with luminal subtype breast cancer.
Tumour Biol. 35:2035–2045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lega IC, Austin PC, Gruneir A, Goodwin PJ,
Rochon PA and Lipscombe LL: Association between metformin therapy
and mortality after breast cancer: A population-based study.
Diabetes Care. 36:3018–3026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bayraktar S, Hernadez-Aya LF, Lei X,
Meric-Bernstam F, Litton JK, Hsu L, Hortobagyi GN and
Gonzalez-Angulo AM: Effect of metformin on survival outcomes in
diabetic patients with triple receptor-negative breast cancer.
Cancer. 118:1202–1211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Currie CJ, Poole CD, Jenkins-Jones S, Gale
EA, Johnson JA and Morgan CL: Mortality after incident cancer in
people with and without type 2 diabetes: Impact of metformin on
survival. Diabetes Care. 35:299–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Romero IL, McCormick A, McEwen KA, Park S,
Karrison T, Yamada SD, Pannain S and Lengyel E: Relationship of
type II diabetes and metformin use to ovarian cancer progression,
survival, and chemosensitivity. Obstet Gynecol. 119:61–67. 2012.
View Article : Google Scholar : PubMed/NCBI
|