Introduction
Wilms' tumors, also known as the embryonic tumors of
the kidneys, are one of the most common malignant, solid
retroperitoneal tumors observed in children, accounting for 5.5% of
all solid tumors in children (1) and
>80% of genitourinary tumors in children <15 years of age.
Although early diagnosis is important for deciding treatment
methods and ensuring satisfactory outcomes, the early symptoms of
Wilms' tumors are not clear. Therefore, the identification of
diagnostic biomarkers is crucial.
Proteomics can rapidly detect protein expression at
different stages of disease occurrence using high-throughput
screening to detect specific protein-labeled molecules. These
highly-expressed, specific proteins are useful in clinical
practice, as they can assist in early diagnosis and may become
targets for disease treatment. In previous years, a number of
methods, including surface-enhanced laser
desorption/ionization-time of flight mass spectrometry
(SELDI-TOF-MS) and matrix-assisted laser desorption/ionization time
of flight mass spectrometry (MALDI-TOF-MS) technologies (2,3), have been
demonstrated to successfully detect specific markers for various
types of cancer, including ovarian, prostate, pancreatic, colon and
breast cancer (4–8).
Post-traumatic stress disorder is complex in nature,
encompassing changes in the neuroendocrine system, and in metabolic
and immune function, together with altered cytokine levels, which
culminate to provoke and accelerate tumor development (9). Since stress-related factors may produce
proteins that interfere with the identification of specific
proteins in the serum of patients with Wilms' tumors, the aim of
the present study was to investigate these post-traumatic
stress-related serum factors. Not only can traumatic stress-related
factors be found to exclude interference for later screening and
identification, the occurrence and development of tumors can also
be understood from the aspect of inflammation. To analyze the
effect of post-traumatic stress-related inflammatory factors on the
development of Wilms' tumors and to identify putative biomarkers,
SELDI-TOF-MS, MALDI-TOF-MS, solid-phase extraction (SPE), SDS-PAGE
and high-performance liquid chromatography-mass spectrometry
(LC-MS) were used in tandem to detect proteins in the sera of
children with Wilms' tumors and compare them with sera isolated
from children with post-traumatic stress disorder and normal
children to identify post-traumatic stress-related factors. Using
this approach, highly-expressed specific proteins were screened and
identified in the sera of children with Wilms' tumors and those
with post-traumatic stress disorder. The specific protein marker of
post-traumatic stress disorder can be distinguished from the
specific marker that we had obtained from the serum of patients
with Wilms' tumors in our previous study (10), and may be used as a reference for
excluding the interfering effects of traumatic factors.
Materials and methods
Collection and treatment of the serum
samples
The study protocol was approved by the ethics review
committee of Zhengzhou University (Zhengzhou, China). Written
informed consent was obtained from each patient or their guardian.
All serum samples used in the current study were provided by the
Department of Pediatric Surgery of The First Affiliated Hospital of
Zhengzhou University and included 61 pre-operative serum samples
from children with Wilms' tumors, of which 28 presented with stage
I tumors, 18 with stage II tumors, 12 with stage III tumors and 3
with stage IV tumors. A total of 34 serum samples were obtained
from children with post-traumatic stress disorder (with sera
collected within 1–3 days of injury sustained during incidents,
including car accidents), and 60 serum samples were obtained from
normal children who underwent routine medical examinations. Whole
blood samples were harvested from the peripheral veins in the
morning prior to breakfast in all participants. After 1–2 h at 4°C,
the samples were centrifuged for 15 min at 3,000 × g. The sera were
collected, placed in numbered tubes and preserved separately at
−80°C.
Screening of differentially-expressed
serum proteins
The serum was gradually thawed in an ice bath for
30–60 min and then centrifuged at 10,000 × g for 5 min at 4°C.
Serum (5 µl) was added to 10 µl U9 serum solution, and was mixed
together at 4°C on a vibrating shaker (600 × g; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 30 min. The serum treated
with U9 was diluted with binding buffer (PeproTech, Rocky Hill, NJ,
USA) to 200 µl, mixed at 4°C on a vibrating shaker (600 × g) for 2
min, and loaded onto a WCX2 protein chip (Ciphergen Biosystems,
Fremont, CA, USA).
Firstly, the standard chip was used with proteins of
known molecular weight to adjust for errors of molecular weight in
the SELDI-TOF-MS system (Ciphergen Biosystems), to reach an error
level of <0.1%. Next, the chip was placed together with the
serum proteins into the mass spectrometer for detection. The
original data were collected using Proteinchip Software version 3.2
(Ciphergen Biosystems) with the laser intensity set to 185, the
detection sensitivity set to 7 and the upper limit of detection set
at 100,000 mass/charge (m/z). The range of optimized collected data
was 2,000–20,000 m/z. Certain factors were excluded based on
previous studies (11,12). To exclude these factors, recombinant
proteins were added to each group to screen for the differential
peak. If the differential peak corresponded to the peak value of a
certain factor to be excluded (the same m/z), this was screened out
and no further purification and identification were performed. The
differential peaks of post-traumatic specific factors were
identified.
Data processing
Correction of the original data was performed using
Biomarker Wizard software version 3.1 (Ciphergen Biosystems) to
homogenize the total ionic strength and molecular weight. The
Biomarker Wizard software and protein chip data analysis system
were used to remove the noise and subtract the baseline with the
discrete wavelet. The peak of each sample was obtained using a
method of finding the local extreme, and peaks with signal-to-noise
ratios of >2 were filtered out. The minimum threshold of the
cluster analysis was set to 10%, and the peaks with <0.3% m/z
were categorized. Wilcoxon rank sum test was performed for the
initially screened m/z peaks to obtain P-values and subsequently
evaluate the ability of each peak in differentiating various
groups. Mass spectrometric data of each sample were further
analyzed using the non-linear support vector machine (SVM)
(13) to screen and identify
differentially-expressed proteins. The radial basis function was
used for SVM, the γ parameter was set to 0.6 and the penalty
function was set to 19.
Statistical analysis
Subsequent to the noise being filtered, the original
mass spectrometric data were treated by cluster analysis, and
Student's t-tests were performed for the mass spectrometric data of
each group. P<0.05 was considered to indiciate a statistically
significant difference. Differentially-expressed proteins between
the groups were identified.
Isolation of serum proteins
Subsequent to the serum proteins being separated
into different groups with organic phase concentrations of 30, 50,
70 and 100% using SPE (Waters, Milford, MA, USA), they were packed
in an Eppendorf® tube (Eppendorf, Hamburg, Germany), marked clearly
and then divided according to hydrophilicity. Each sample was dried
to 10 µl in a vacuum drying device (Thermo Fisher Scientific,
Inc.), subsequent to which, 5 µl sample buffer was added, the
samples were boiled for 15 min and centrifuged at 5,000 × g, and
the supernatant was collected. The sample was next separated by gel
electrophoresis, and the gel was cut into several pieces based on
the corresponding molecular weight axis, placed in different
Eppendorf® tubes, washed, decolorized, dried and incubated with
trypsin for 15 h.
Identification of
differentially-expressed proteins
Proteins (1.5 µl) separated by SPE and
electrophoresis were mixed with 1.5 µl α-cyano-4-hydroxycinnamic
acid (CHCA; Thermo Fisher Scientific, Inc.), placed on the target
plate and dried. Sample calibration was performed using cytochrome
c (molecular weight, 12,361.96; (Thermo Fisher Scientific, Inc.)
plus CHCA and insulin (molecular weight, 5,734.51; (Thermo Fisher
Scientific, Inc.) plus CHCA. The target plate was placed in the
MALDI-TOF-MS device (Bruker Daltonics, Billerica, MA, USA) for
detection. The target protein peak was tracked, and serum samples
with a protein peak m/z of 5,816 were identified.
Identification of specific
proteins
Trypsin-digested serum proteins were identified by
peptide mass fingerprinting using LC-MS followed by Swiss-Prot
database analysis using the Mascot search engine.
MS data processing and database
searching
The MS data processing software used was Launchpad
version 2.4 (Shimadzu, Nakagyo-ku, Kyoto, Japan). A spectrum search
was performed using the Mascot search engine of mouse and human
protein sequences, and the number of maximum allowable restriction
enzyme cutting sites was set to 1. The fixed modification was
cysteine iodoacetyl, and the variable modification was methionine
oxidation. Furthermore, the peptide mass tolerance was set to ±0.3
Da.
Verification of post-traumatic
stress-related protein markers using western blot analysis
Proteins were separated by SDS-PAGE and transferred
to a polyvinylidene fluoride membrane. The membranes were incubated
with primary polyclonal rabbit anti-human thioredoxin 1 (Trx1; cat.
no. bs-0458R; 1:1,000; Beijing Biosynthesis Biotechnology Co.,
Ltd., Beijing, China) or GAPDH (cat. no. bs-0459R; 1:1,000; Beijing
Biosynthesis Biotechnology Co., Ltd.) antibodies for 2 h at 37°C.
The membranes were washed three times with Tris-Buffered Saline and
Tween 20 (Beijing Biosynthesis Biotechnology Co., Ltd.), followed
by incubation with horseradish peroxidase conjugated-goat
anti-rabbit IgG secondary antibodies (cat. no. ZDR-5306; 1:5,000;
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
for 2 h at 37°C. Bands were visualized using enhanced
chemiluminescence followed by image development and fixing. GAPDH
was used as an internal control. The negatives were scanned, and
the gray values of the protein bands in each negative were measured
using the ImagePro plus 6.0 software (Media Cybernetics, Bethesda,
MD, USA). The gray values were divided by group, and SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA) was used to compare the
gray values in the different groups.
Results
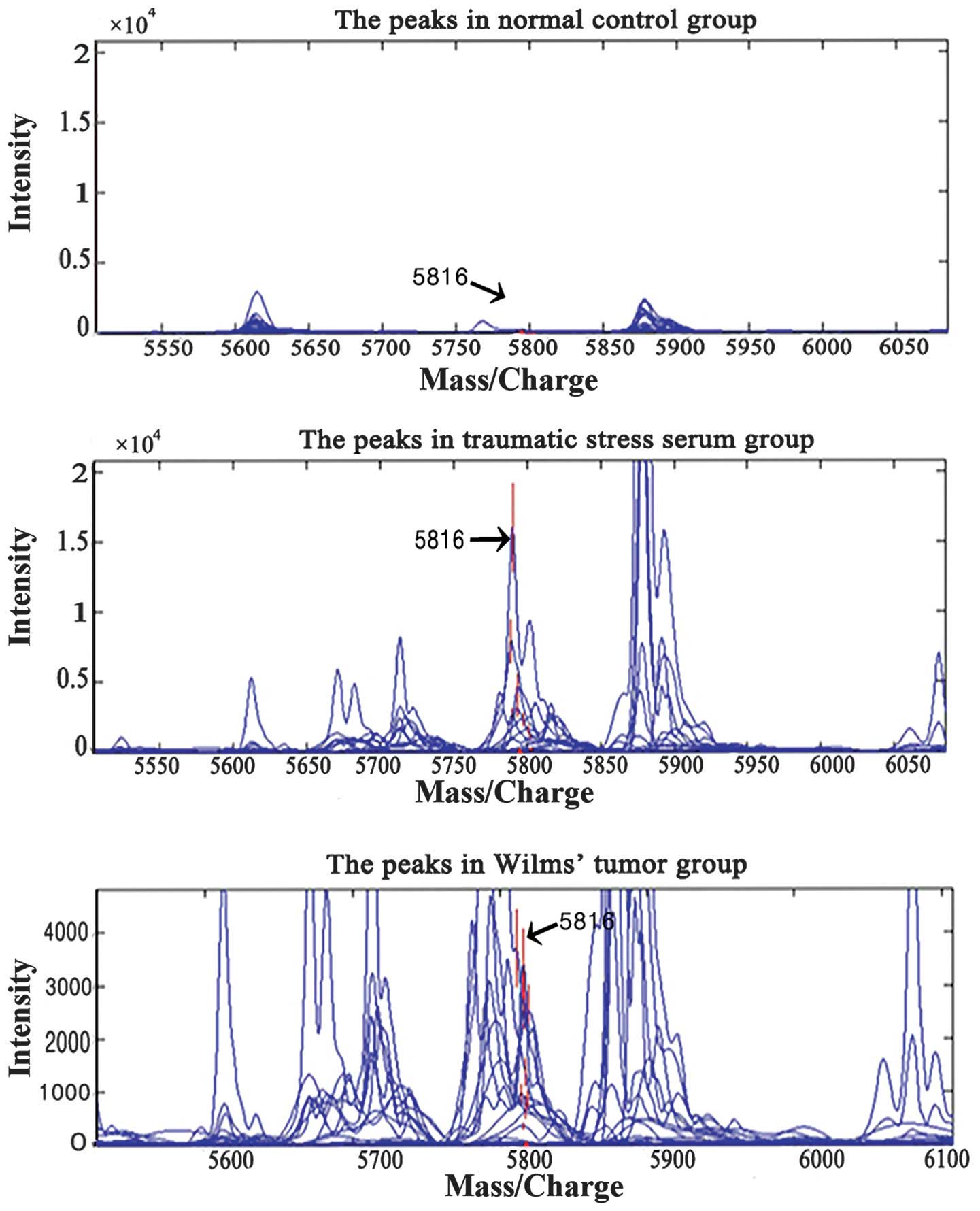
SELDI-TOF-MS analysis
SELDI-TOF-MS was used to screen sera from patients
in the tumor, trauma and normal control groups to identify specific
proteins with an m/z of 5,816. Subsequent to the initial screening
and statistical analysis of the mass spectra data from the tumor
and normal control groups, peaks of 15 m/z were obtained
(P<0.05; Fig. 1). SVM was used to
screen out models with the highest Youden's indices (i.e., the
difference between the true and false positive rates) of the
predictive value. Significantly higher expression of proteins with
an m/z of 5,816 was observed in the tumor group (mean,
2,128.3±137.2; 652.6±134.2 in stage I tumors, 1,152.6±423.1 in
stage II tumors, 1,652.6±523.1 in stage III tumors and
2,252.6±723.1 in stage IV tumors) as compared with children in the
control group (30.4±7.8) (P<0.05; Table I). When the potential markers were
taken as the input values, leave-one-out cross-validation proved
that the specificity of differentiating the combination model of
this marker on the test set was 100.0% and the sensitivity was
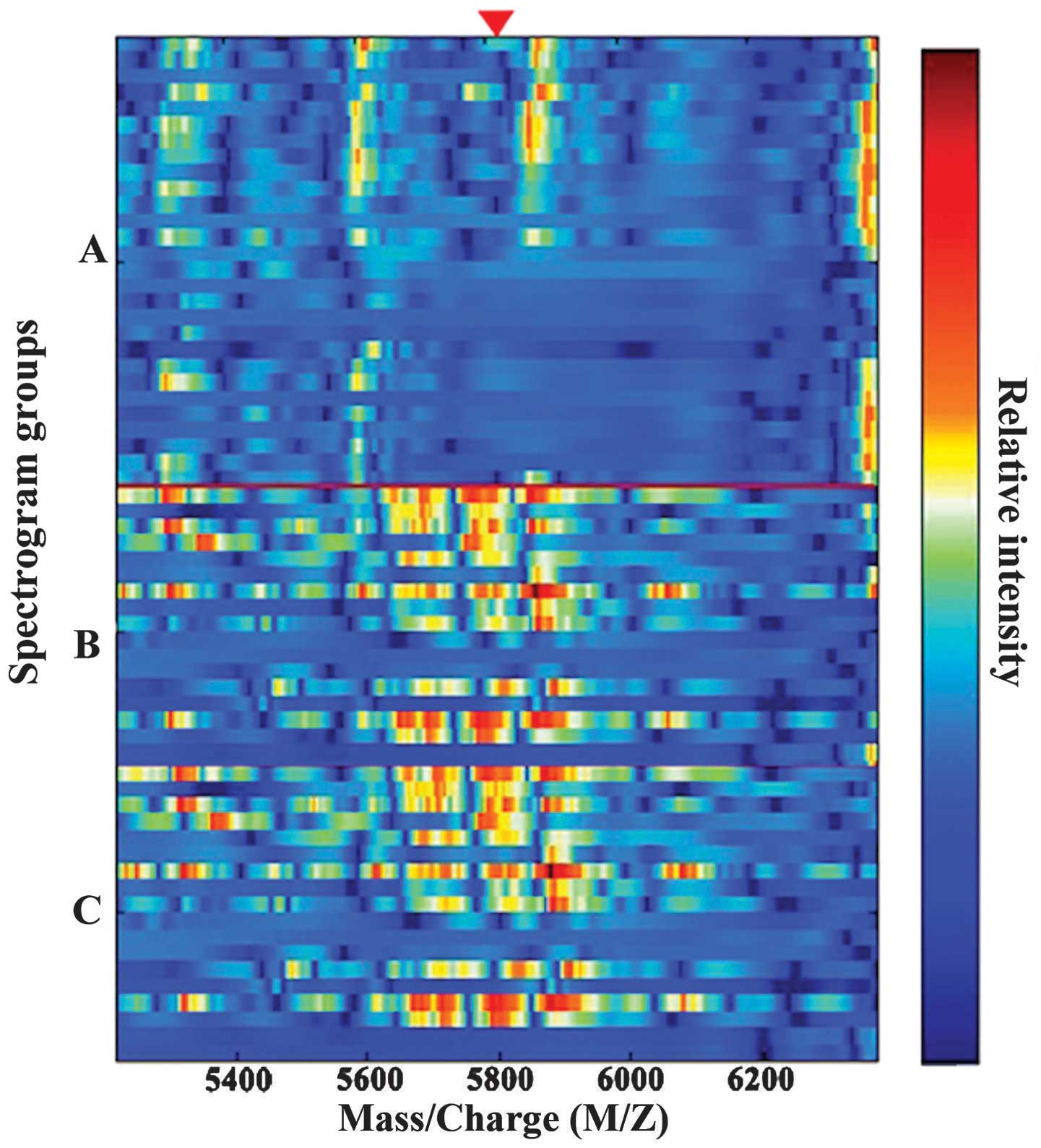
100.0%. Fig. 2 shows the simulated
protein electrophoresis between the tumor, normal and trauma
groups. Significantly greater levels of proteins with an m/z of
5,816 were also noted in the serum of the trauma group
(3,674.6±255.7) as compared with the normal group (30.4±7.8)
(P<0.05; Fig. 1; Table I).
 | Table I.Expression level of proteins with a
mass/charge of 5,816 Da. |
Table I.
Expression level of proteins with a
mass/charge of 5,816 Da.
| Groups | Cases, n | Mean expression
±SD |
|---|
| Trauma | 34 | 3,674.6±255.7 |
| Tumor | 61 | 2,128.3±137.2 |
| Normal | 60 | 30.4±7.8 |
Identification of the putative
biomarker
Subsequent to the target protein being isolated and
purified using SPE and SDS-PAGE, the target peptide produced by
trypsin digestion was identified using high performance LC-MS
(Fig. 3). A Swiss-Prot protein
sequence database query via the Mascot search engine revealed that
the protein with an m/z of 5,816 may be thioredoxin 1 (Trx1). In
addition, the matching rate of the detected peptide amino acid
sequence and the human Trx1 sequence in the database was 46%
(Table II).
 | Table II.Protein fragments with a mass/charge
of 5,816. |
Table II.
Protein fragments with a mass/charge
of 5,816.
| Mass/charge | Protein | Peptide sequence | Rate of coverage,
% | Score |
|---|
| 5,816 | Thioredoxin 1 |
MIKPFFHSLSEKVGEFSGANKE | 46.00 | 65.00a |
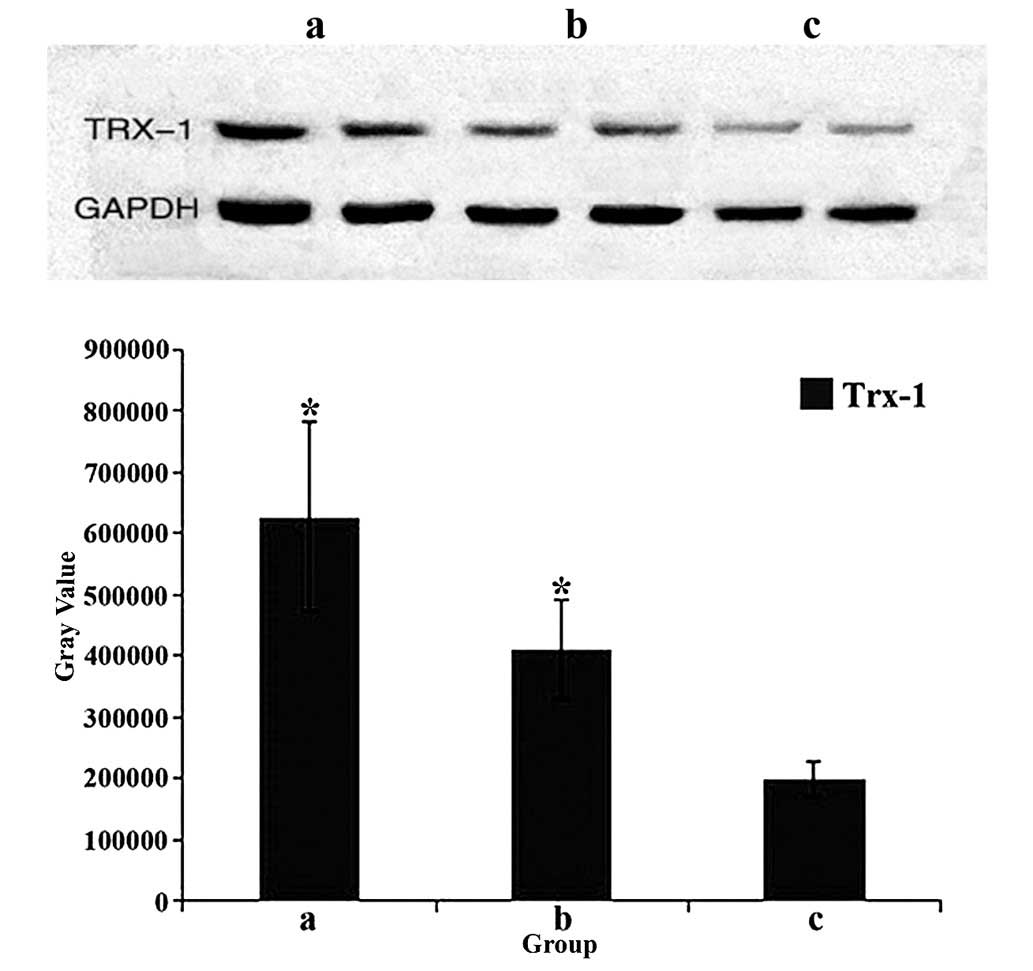
Verification of the target protein was performed
using western blot analysis of 30 randomly selected blood samples
(n=10 per group) to assess Trx1 expression, which was normalized to
GAPDH. For each experiment, two cases from each group were
selected. As shown in Fig. 4, the
highest levels of Trx1 were observed in the trauma group
(P<0.05), and the lowest levels were found in the normal control
group (P<0.05). In addition, significantly higher Trx1 levels
were detected in the tumor group as compared with the control group
(P<0.05).
Discussion
In the current study, SELDI-TOF-MS was used to
screen the sera of patients in the tumor, trauma and control
groups. Protein with an m/z of 5,816 was isolated and purified
using SPE and SDS-PAGE, and was identified as Trx1. Finally, the
presence of significantly increased Trx1 levels in the tumor group
was verified by western blot analysis.
At present, Wilms' tumors cannot be cured
completely, and the treatment is mainly targeted at patients with
mid- to late-stage tumors, and includes surgery and chemotherapy.
The main reason for the poor prognosis of patients with Wilms'
tumors is the delay in early diagnosis and consequently, treatment
initiation. Thus, it is critical to find a simple but accurate
method for the early detection of Wilms' tumors.
Reiche et al (14) reported a positive correlation between
stress and tumor development. Stress affects tumor development
mostly by regulating the neuroendocrine-immune network (15). Stress-induced changes in the circadian
rhythm of the body also play a significant role, and these changes
are mediated by changes in endocrine mediators (e.g.
glucocorticoids, thyrotrophin, growth hormone and luteinizing
hormone), metabolic disorders (changes in body temperature,
proteins and enzymes) and immune dysfunction, to finally induce and
accelerate tumor development and escape of tumor cells from immune
surveillance (9).
In children with Wilms' tumors, tumor compression
and bleeding may induce the release of post-traumatic
stress-related factors, thereby affecting the sensitivity and
accuracy of Wilms' tumor-specific proteins. Furthermore, certain
small molecular weight proteins cannot be easily detected due to
their own characteristics and low concentrations. Therefore, it is
important to identify a factor with a molecular weight similar to
that of the factor obtained in our previous studies, which is
specific to Wilms' tumors alone (10). In the present study, a protein with an
m/z of 5,816 was identified as Trx1, with a coverage of 46%,
suggesting that trauma-related factors exist in the serum of
children with Wilms' tumors. However, their direct or indirect
roles in pediatric tumor initiation or progression should be
verified by further studies.
Trx1, with a molecular weight of 12 kDa, exists in
almost every organism, and exhibits key functions in a number of
important biological activities, including redox signaling; it is
also a critical factor in tumor development (16). Specifically, Trx1 can directly inhibit
the activity of apoptosis signal-regulated kinase-1 to reduce the
rate of tumor cell death and enhance tumor cell growth factor
expression, thereby accelerating tumor cell growth (17,18). Trx1
can also induce the synthesis of vascular endothelial growth factor
to promote angiogenesis and the extension of tumor blood vessels.
In addition, it can antagonize the cancer suppressor protein in the
advanced stage of cancer, promoting tumor metastasis (19). Trx1 is also overexpressed in colon
cancer, significantly accelerating tumor invasion and metastasis
(20).
The current study applied SELDI-TOF-MS technology
along with SVM to screen out post-traumatic stress-related protein
markers with an m/z of 5,816. The specificity and sensitivity of
the identified marker, Trx1, were each 100%, and the intensity of
its expression may be associated with factors such as the
individual differences in the serum samples, experiment process and
errors. Together with inflammatory markers and those biomarkers
described in previous studies (e.g. serum amyloid A and
apolipoprotein CIII) (21,22), Trx1 forms an ideal diagnostic marker,
as its levels were significantly higher in the sera of children
with Wilms' tumor and in those patients with post-traumatic stress
disorder in the present study. The relatively high expression level
in the serum of children with tumors is in accordance with its high
expression level in adult tumors, which suggests that it is a
post-traumatic stress-related serum factor of Wilms' tumors.
Combined with the results of our previous study (10), the current study demonstrated that the
post-traumatic stress-related specific protein that was screened
out was Trx1. The post-traumatic stress-related factor Trx1 was
expressed in the serum of children with Wilms' tumors. The results
shown in this study present the novel idea that enzymes indicative
of oxidative stress may be used as markers for the serological
diagnosis, malignancy grading and prognostic evaluation of Wilms'
tumor, and may form the basis of future studies with larger
cohorts.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81071782).
References
|
1
|
Kaatsch P: Epidemiology of childhood
cancer. Cancer Treat Rev. 36:277–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maurya P, Meleady P, Dowling P and Clynes
M: Proteomic approaches for serum biomarker discovery in cancer.
Anticancer Res. 27:1247–1255. 2007.PubMed/NCBI
|
|
3
|
Diamandis EP: Mass spectrometry as a
diagnostic and a cancer biomarker discovery tool: opportunities and
potential limitations. Mol Cell Proteomics. 3:367–378. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Zhang X, Ge X, Guo H, et al:
Proteomic studies of early-stage and advanced ovarian cancer
patients. Gynecol Oncol. 111:111–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Skytt A, Thysell E, Stattin P, Stenman UH,
Antti H and Wikstrom P: SELDI-TOF MS versus prostate specific
antigen analysis of prospective plasma samples in a nested
case-control study of prostate cancer. Int J Cancer. 121:615–620.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu D, Cao L, Yu J, et al: Diagnosis of
pancreatic adenocarcinoma using protein chip technology.
Pancreatology. 9:127–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hundt S, Haug U and Brenner H: Blood
markers for early detection of colorectal cancer: a systematic
review. Cancer Epidemiol Biomarkers Prev. 16:1935–1953. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gonçalves A, Bertucci F, Birnbaum D and
Borg JP: Proteic profiling SELDI-TOF and breast cancer: clinical
potential applications. Med Sci (Paris). 23:23–26. 2007.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao WG: Mechanism of tumor development
affecting traumatic stress. Yi Xue Zong Shu. 6:830–832. 2012.(In
Chinese).
|
|
10
|
Wang J, Wang L, Zhang D, et al:
Identification of potential serum biomarkers for Wilms tumor after
excluding confounding effects of common systemic inflammatory
factors. Mol Biol Rep. 39:5095–5104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chechlinska M, Kowalewska M and Nowak R:
Systemic inflammation as a confounding factor in cancer biomarker
discovery and validation. Nat Rev Cancer. 10:2–3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hou JM, Zhao X, Tian L, et al:
Immunotherapy of tumors with recombinant adenovirus encoding
macrophage inflammatory protein 3beta induces tumor-specific immune
response in immunocompetent tumor-bearing mice. Acta Pharmacol Sin.
30:355–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y: Active learning with support vector
machine applied to gene expression data for cancer classification.
Chem Inf Comput Sci. 44:1936–1941. 2004. View Article : Google Scholar
|
|
14
|
Reiche EM, Nunes SO and Morimto HK:
Stress, depression, the immune system and cancer. Lancet Oncol.
5:617–625. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shastri A, Bonifati DM and Kishore U:
Innate immunity and neuroinflammation. Mediators Inflamm.
2013:3429312013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kodama A, Watanabe H, Tanaka R, et al: A
human serum albumin-thioredoxin fusion protein prevents
experimental contrast-induced nephropathy. Kidney Int. 83:446–454.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Oliveira Navakoski K, Andermark V, von
Grafenstein S, et al: Butyltin (IV) benzoates: inhibition of
thioredoxin reductase, tumor cell growth inhibition and
interactions with proteins. Chem Med Chem. 8:256–264. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu A, Wangpu X, Han D, et al: TXNDC9
expression in colorectal cancer cells and its influence on
colorectal cancer prognosis. Cancer Invest. 30:721–726. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kakolyris S, Giatromanolaki A, Koukourakis
M, et al: Thioredoxin expression is associated with lymph node
status and prognosis in early operable non-small cell lung cancer.
Clin Cancer Res. 7:3087–3091. 2001.PubMed/NCBI
|
|
20
|
Lincoln DT, Alyatama F, Mohammed FM, et
al: Thioredoxin and thioredoxin reductase expression in thyroid
cancer depends on tumour aggressiveness. Anticancer Res.
30:767–775. 2010.PubMed/NCBI
|
|
21
|
Zhang J, Guo F, Wang L, Zhao W, Zhang D,
Yang H, Yu J, Niu L, Yang F, Zheng S and Wang J: Identification of
apolipoprotein C-I as a potential Wilms' tumor marker after
excluding inflammatory factors. Int J Mol Sci. 15:16186–16195.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Q, Wang J, Dong R, Yang S and Zheng
S: Identification of novel serum biomarkers in child nephroblastoma
using proteomics technology. Mol Biol Rep. 38:631–638. 2011.
View Article : Google Scholar : PubMed/NCBI
|