Introduction
Myxoid liposarcoma (MLS) accounts for ~5% of all
soft tissue sarcomas and 15–20% of liposarcomas in adults (1). This second most common subtype of
liposarcoma usually occurs in the soft tissue of the extremities;
the thigh is affected in more than two-thirds of cases (1–3). MLS is a
disease of young adults and is equally common in males and females
(1). Liposarcoma originates from
primitive mesenchymal cells rather than from mature adipose cells
(2,3).
Usually, symptoms develop slowly as a painless mass within the deep
soft tissue (1,4). One third of the MLS patients develop
distant metastasis, particularly to extrapulmonary location,
including the retroperitoneum, opposite extremity, axilla and bone
(1,5,6).
The current study reports an extremely rare case of
primary dumbbell-shaped epidural MLS of the thoracic spine, which
radiologically mimicked a schwannoma. Written informed consent was
obtained from the patient prior to publication of this case
report.
Case report
A previously healthy 22-year-old woman presented
with a one-month history of back pain, hypalgesia and hypesthesia
below the level of the xiphoid cartilage, and numbness of the lower
extremities. Laboratory findings indicated no abnormalities;
however, a neurological examination revealed muscular weakness and
increased deep tendon reflexes in the bilateral lower
extremities.
Plain radiographs and computed tomography (CT) of
the spine indicated no abnormality. However, magnetic resonance
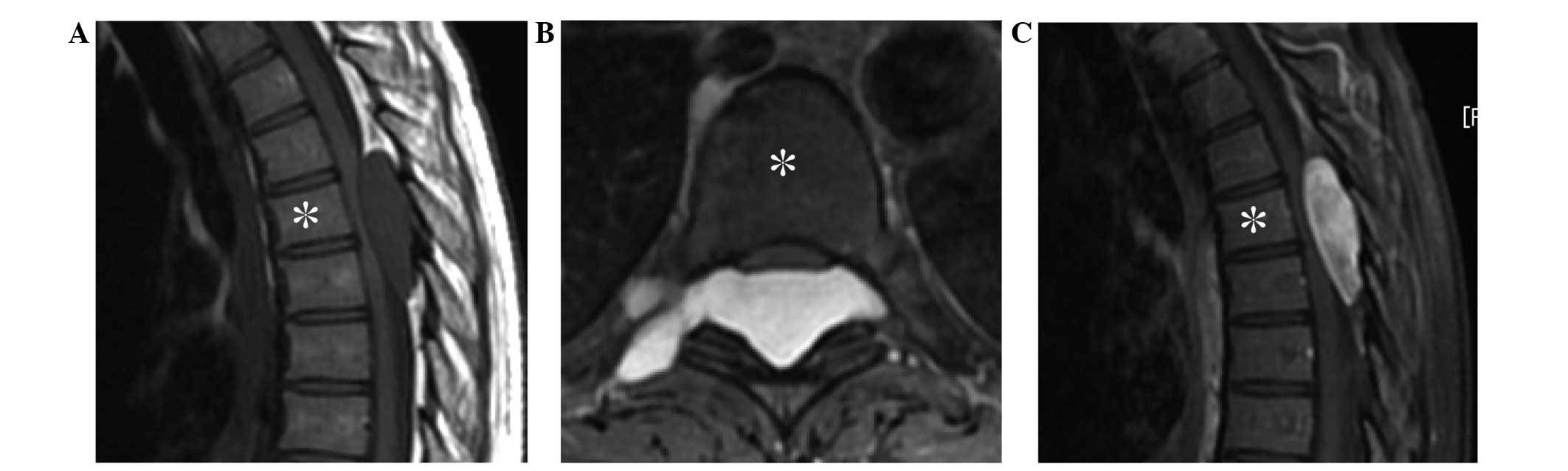
imaging (MRI) revealed an epidural tumor progressing in the right
foramen of the 5th and 6th thoracic vertebrae, measuring
4.6×3.7×1.4 cm, and exhibiting low intensity on T1-weighted imaging
(T1-WI) (Fig. 1A), high intensity on
T2-weighted imaging (T2-WI) (Fig.
1B), and diffuse enhancement on gadolinium-enhanced T1-weighted
fat-suppression imaging (Fig. 1C).
These findings were compatible with schwannoma. As the paralysis of
the patient's lower extremities rapidly progressed and gait
disturbance appeared, surgical treatment was performed. Laminectomy
of the 4th, 5th and 6th thoracic vertebrae was conducted, and the
whitish myxoid tumor was subsequently resected piece by piece.
Following the surgery, the patient's paralysis recovered rapidly
without any disturbance.
Microscopic evaluation of the tumor revealed
proliferation of uniform, mononuclear, short spindle- or
oval-shaped cells arranged in a multi-nodular pattern (Fig. 2A–C). A growth pattern similar to that
of pulmonary edema was also observed (1). Immunohistochemical staining indicated
that the tumor cells were positive for vimentin and S-100 protein
(Fig. 2D), but negative for mouse
double minute 2 homolog and cyclin-dependent kinase 4. A
‘chicken-wire’ pattern of capillary vasculature was clearly visible
following CD34 staining (Fig. 2E).
The MIB1 labeling index was ~6.7%. Furthermore, additional
fluorescence in situ hybridization using a break-apart probe
for the DNA-damage-inducible transcript 3 gene on 12q13 revealed a
rearrangement (Fig. 2F). Finally, the
tumor was diagnosed as an MLS of French Federation of Cancer
Centers Sarcoma Group grade 1, based on the pathological and
genetic findings (7). As additional
whole-body 18F-fludeoxyglucose (FDG) positron emission
tomography (PET)/CT revealed no other tumor, excluding an ovarian
cyst, the epidural tumor of the thoracic spine was diagnosed as
primary.
Adjuvant therapy was performed for any possible
residual tumor remaining following the intralesional resection. As
radiotherapy may cause myelopathy, the patient instead underwent
three courses of adjuvant chemotherapy with doxorubicin (60
mg/m2/course) and ifosfamide (7.5
g/m2/course), uneventfully. At 6 months after the
surgery for the epidural tumor, the patient received an
oophorocystectomy, and pathological diagnosis of an endometrial
cyst was confirmed. The patient has exhibited no symptoms or signs
of local recurrence or metastasis for 18 months
postoperatively.
Discussion
To the best of our knowledge, only five cases of
epidural MLS of the spine have been reported previously in the
English language literature; one case of primary (4) and four cases of metastatic (2,8,9) (Table I).
The present case is the second case of primary epidural MLS of the
spine.
 | Table I.Summary of previously reported cases
of spinal epidural myxoid liposarcoma. |
Table I.
Summary of previously reported cases
of spinal epidural myxoid liposarcoma.
| Author (reference
no.) | Age (years) | Gender | Site |
Primary/metastasis | Treatment | Prognosis |
|---|
| Present case | 22 | F | Thoracic | Primary | Intralesional
resection + CT | NED (18 mo. post
op.) |
| Turanli, et al
(4) | 65 | F | Lumbar | Primary | Marginal
resection | NED (13 mo. post
op.) |
| Kirollos, et
al (2) | 58 | M | Thoracic | Metastasis | Partial resection +
RT | DOD (6 mo. post
op.) |
| Ogose, et al
(8) | 44 | M | Thoracic | Metastasis | Intralesional
resection + RT | DOD (5 mo. post
op.) |
| Ogose, et al
(8) | 57 | F | Thoracic | Metastasis | Marginal
resection | DOD (7 mo. post
op.) |
| Lee, et al
(9) | 39 | F | Cervical | Metastasis | Resection | NA |
As the occurrence of MLS in the epidural space is
exceedingly rare and its MRI findings are very similar to those of
schwannoma, determining a correct preoperative diagnosis without
pathological analysis is considered to be challenging (10,11). MRI
of MLS typically shows lacy or linear amorphous high-intensity foci
of fat within a predominantly low-intensity mass on T1-WI,
intermediate signal intensity foci within a high-intensity mass on
T2-WI, and homogeneous enhancement throughout the mass by
gadolinium (3). Because of the
quantity of fat and myxoid material, the degree of cellularity and
vascularity, and the presence of necrosis, MLS may mimic cystic
tumors (12,13). Similarly, MRIs of schwannomas usually
reveal low to iso intensity on T1-WI, high intensity on T2-WI, and
homogeneous or heterogeneous intensity on gadolinium-enhanced
T1-weighted fat-suppression imaging (14).
Schwannomas occasionally contain cystic degeneration
and, although most (65.2%) are intradural extramedullary lesions,
33.0% and 1.3% are dumbbell tumors and epidural tumors,
respectively (15). Furthermore,
among all dumbbell tumors, schwannomas are the most common (69%),
and Eden Type III (extradural and paravertebral type) is the most
frequent in both all dumbbell tumors (53%) and schwannoma (48%)
(16). Thus, from the localization,
dumbbell-shaped form (Eden type III) and MRI findings of the
present case, the preoperative diagnosis of schwannoma was
considered to be reasonable.
FDG-PET/CT has a critical detection limit in
relation to small lesions, particularly in tumors that exhibit low
18F-FDG uptake, including MLS (17). Therefore, there is the possibility
that other small primary and/or metastatic lesions existed in the
present patient. However, based on the facts that no other tumor,
excluding the endometrial cyst, was revealed on additional
radiological analyses, and that the patient has displayed no
symptoms or signs of local recurrence or metastasis for 18 months,
the tumor was diagnosed as a primary, and not metastatic, epidural
MLS of the thoracic spine.
It is clear that wide resection is the standard
procedure for soft tissue sarcomas, including MLS (4,5,13). However, in the present case, it was
difficult to resect the tumor with an adequate margin due to its
location and the compression of the spinal cord causing progressive
myelopathy, even if the tumor could have been correctly diagnosed
as MLS preoperatively. Although the previously reported case of
primary epidural MLS with marginal resection showed no local
recurrence (4), adjuvant therapy was
considered to be indicated in the current case to prevent further
recurrence and metastasis. MLS responds to chemotherapy and
radiotherapy (5,6,18);
however, adjuvant radiotherapy was not selected in the present case
in order to avoid radiation-induced myelopathy. Instead, adjuvant
chemotherapy with doxorubicin and ifosfamide was selected.
In conclusion, the current study reports a case of
primary epidural, dumbbell-shaped MLS, which radiologically
resembled a schwannoma. Although we were unable to resect the tumor
with an adequate margin, the patient remains disease-free 18 months
after the intralesional tumor resection and subsequent adjuvant
chemotherapy. When radiologically diagnosing spinal epidural
tumors, clinicians should consider the possibility of MLS as well
as schwannoma. Additional treatment with chemotherapy appeared to
be useful in the current case of MLS.
Acknowledgements
This research was supported in part by a
Grant-in-Aid for Young Scientists (B) (no. 25861331) from the Japan
Society for the Promotion of Science.
References
|
1
|
Antonescu CR and Ladanyi M: Myxoid
liposarcoma. WHO Classification of Tumours of Soft Tissue and Bone
(4th). Fletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F:
International Agency for Research on Cancer. (Lyon, France). 39–41.
2013.
|
|
2
|
Kirollos R, Koutsoubelis G, Ross S and Al
Sarraj S: An unusual case of spinal metastasis from a liposarcoma.
Eur J Surg Oncol. 22:303–305. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung MS, Kang HS, Suh JS, Lee JH, Park JM,
Kim JY and Lee HG: Myxoid liposarcoma: Appearance at MR imaging
with histologic correlation. Radiographics. 20:1007–1019. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turanli S, Ozer H, Ozyurekoglu T and
Cakiroglu E: Liposarcoma in the epidural space. Spine.
25:1733–1735. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katz D, Boonsirikamchai P, Choi H, Lazar
AJ, Wang WL, Xiao L, Park MS, Ravi V, Benjamin RS and Araujo DM:
Efficacy of first-line doxorubicin and ifosfamide in myxoid
liposarcoma. Clin Sarcoma Res. 2:22012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung PW, Deheshi BM, Ferguson PC, Wunder
JS, Griffin AM, Catton CN, Bell RS, White LM, Kandel RA and
O'Sullivan B: Radiosensitivity translates into excellent local
control in extremity myxoid liposarcoma: A comparison with other
soft tissue sarcomas. Cancer. 115:3254–3261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coindre JM: Grading and staging of
sarcomas. WHO Classification of Tumours of Soft Tissue and Bone
(4th). Fletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F:
(Lyon, France). International Agency for Research on Cancer. 17–18.
2013.
|
|
8
|
Ogose A, Hotta T, Inoue Y, Sakata S,
Takano R and Yamamura S: Myxoid liposarcoma metastatic to the
thoracic epidural space without bone involvement: Report of two
cases. Jpn J Clin Oncol. 31:447–449. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SY, Kim HJ, Park SY, Park SH and Chung
SK: Myxoid liposarcoma involving the liver, subcutaneous tissue and
epidural space in a polycystic disease patient. Clin Nucl Med.
33:507–509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi H, Kotoura Y, Sakahara H, Hosono
M, Hosono M, Tsaboyama T, Yamamuro T, Endo K and Konishi J:
Schwannoma of the extremities: Comparison of MRI and pentavalent
Technetium-99m-Dimercaptosuccinic acid and Gallium-67-citrate
scintigraphy. J Nucl Med. 35:1174–1178. 1994.PubMed/NCBI
|
|
11
|
Liu Y, Chen X, Wang T and Wang Z: Imaging
observations of a schwannoma of low malignant potential in the
anterior abdominal wall: A case report. Oncol Lett. 8:1159–1162.
2014.PubMed/NCBI
|
|
12
|
Jelinek JS, Kransdorf MJ, Shmookler BM,
Aboulafia AJ and Malawer MM: Liposarcoma of the extremities: MR and
CT findings in the histological subtypes. Radiology. 186:455–459.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sundaram M, Baran G, Merenda G and
McDonald DJ: Myxoid liposarcoma: Magnetic resonance imaging
appearances with clinical and histological correlation. Skeletal
Radiol. 19:359–362. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Verdelhan O, Haegelen C, Carsin-Nicol
B, Riffaud L, Amlashi SF, Brassier G, Carsin M and Morandi P: MR
imaging feature of spinal schwannomas and meningiomas. J
Neuroradiol. 32:42–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirano K, Imagama S, Sato K, Kato F,
Yukawa Y, Yoshihara H, Kamiya M, Deguchi M, Kanemura T, Matsubara
Y, et al: Primary spinal cord tumors: Review of 678 surgically
treated patients in Japan. A multicenter study. Eur Spine J.
21:2019–2026. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozawa H, Kokubun S, Aizawa T, Hoshikawa T
and Kawahara C: Spinal dumbbell tumors: An analysis of a series of
118 cases. J Neurosurg Spine. 7:587–593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brenner W, Eary JF, Hwang W, Vernon C and
Conrad EU: Risk assessment in liposarcoma patients based on FDG PET
imaging. Eur J Nucl Med Mol Imaging. 33:1290–1295. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guadagnolo BA, Zagars GK, Ballo MT, Patel
SR, Lewis VO, Benjamin RS and Pollock RE: Excellent local control
rates and distinctive patterns of failure in myxoid liposarcoma
treated with conservation surgery and radiotherapy. Int J Radiat
Oncol Biol Phys. 70:760–765. 2008. View Article : Google Scholar : PubMed/NCBI
|