Introduction
An aneurysmal bone cyst (ABC) is a benign, locally
aggressive and expansile tumor that typically occurs in the long
bones or vertebral bodies of children and young adults.
Radiographically, a lytic lesion and expansion are the basic
characteristics of ABC, which usually has well-defined margins.
Histologically, the tumor contains blood-filled cystic spaces
separated by fibrous septa containing osteoclast-type giant cells,
fibroblasts and reactive woven bone (1,2).
Previously, ABC was believed to occur exclusively in
bone (3), but in recent years a few
cases of soft tissue ABC (STABC) have been reported (1,4–7). STABC
is a recently recognized extremely rare tumor with fewer than 20
well- documented cases in the literature (7), as shown in Table I. We report the imaging and
pathological findings of a STABC in a 10-year-old girl and discuss
its differential diagnosis.
 | Table IReported cases of soft-tissue
aneurysmal bone cyst. |
Table I
Reported cases of soft-tissue
aneurysmal bone cyst.
| Case | Age (years) | Site | Author and year |
|---|
| 1 | 32 | Thigh | Salm, 1972 |
| 2 | 45 | Abdominal wall | Salm, 1972 |
| 3 | 15 | Groin | Amir, 1992 |
| 4 | 7 | Left common
carotid | Petrik, 1993 |
| 5 | 20 | Infraspinous
region | Rodriguez-Peralto,
1994 |
| 6 | 57 | Left upper arm | Lopez-Barea,
1996 |
| 7 | 29 | Left retroclavicular
region | Shannon, 1997 |
| 8 | 8 | Shoulder | Dal Cin, 2000 |
| 9 | 51 | Pelvis | Samura, 2000 |
| 10 | 8 | Right shoulder | Nielsen, 2002 |
| 11 | 29 | Right groin | Nielsen, 2002 |
| 12 | 37 | Upper arm | Nielsen, 2002 |
| 13 | 28 | Left deltoid | Nielsen, 2002 |
| 14 | 30 | Thigh | Nielsen, 2002 |
| 15 | 21 | Right gluteus
medius | Wang, 2004 |
| 16 | 12 | Left thigh | Ajilogba, 2005 |
| 17 | 10 | Left thigh | Ellison, 2007 |
| 18 | 26 | Right thigh | Pietschmann,
2011 |
| 19 | 38 | Left upper arm | Pietschmann,
2011 |
Patient and methods
The study was carried out according to the
principles of the Declaration of Helsinki; informed consent was
obtained and Shanghai Ninth People's Hospital Ethics Committee
approved the study. The patient images contained in this article
were captured by a hospital-based photographer at Shanghai Ninth
People's Hospital, Shanghai Jiao Tong University School of Medicine
(8). Permission to use these images
in this study has been obtained from the parents of the girl who
participated in this study.
A 10-year-old girl presented with a one-month
history of pain in her left shoulder. She was admitted to hospital
as the pain had increased over the previous 10 days, affecting the
mobility of her left shoulder. Her parents denied any trauma to the
area. Physical examination revealed a 6×5-cm painful lump in the
posterior aspect of the left shoulder; the lump was solid, smooth
and non-pulsatile. Shoulder mobility was limited; initiative
outreach was <80°, extension was <30°, and anteflexion was
<30°. There were no signs of inflammation, and the white cell
count was 5.8×109 cells/l. Serum electrolytes were
normal.
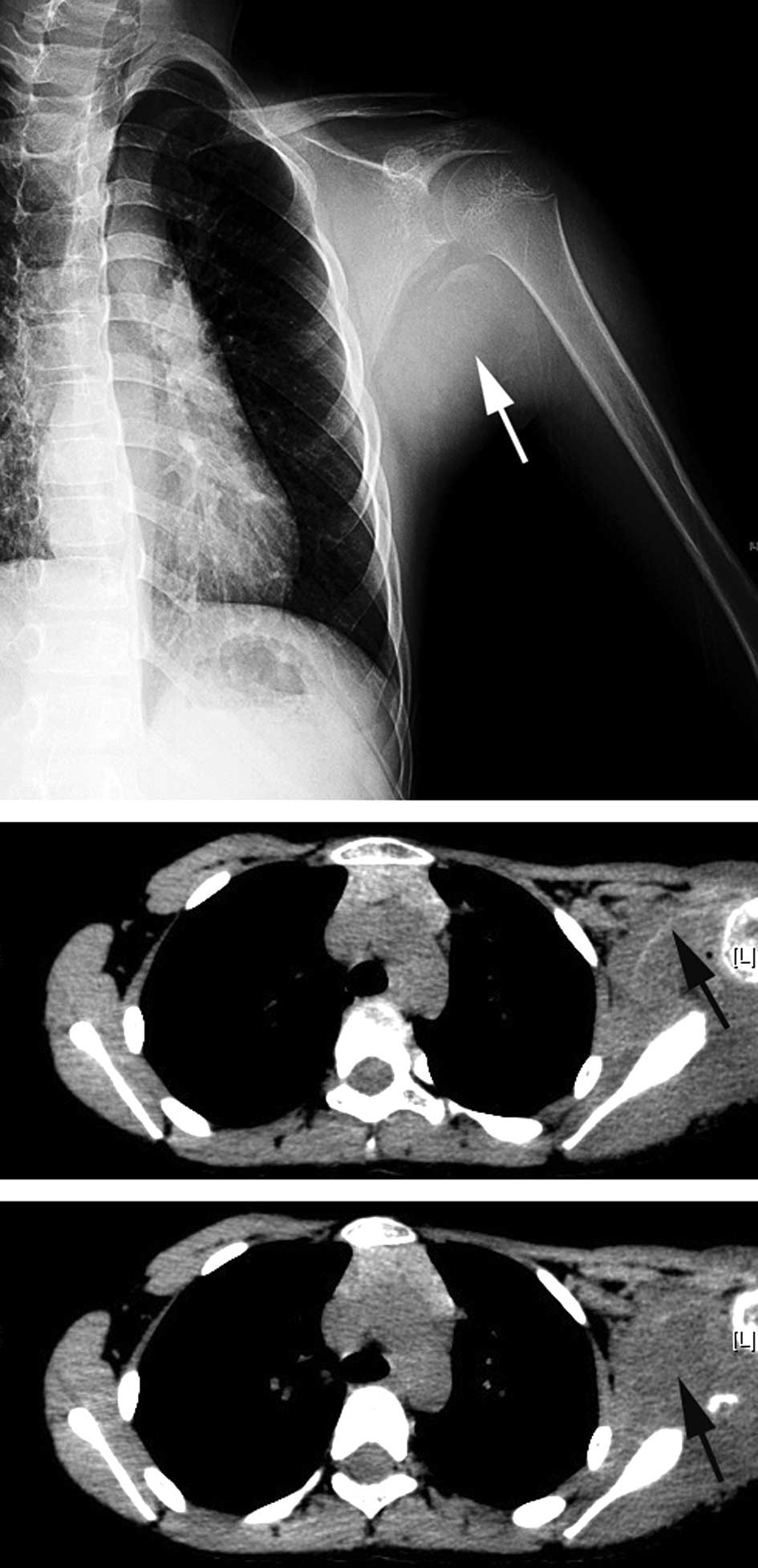
Radiography indicated a round soft tissue lesion
with a well-defined calcification margin located at the posterior
aspect of the left shoulder, next to the scapula and proximal
humerus. The adjacent bony structures were not involved, and there
was no significant periosteal reaction on these bones (Fig. 1A). Computed tomography (CT) revealed
an abnormality of the soft tissue in the left shoulder with an
arcuated thin rim with an ambiguous density, suggesting
calcification (Fig. 1B). The
intralesional density was slightly uneven (Fig. 1C).
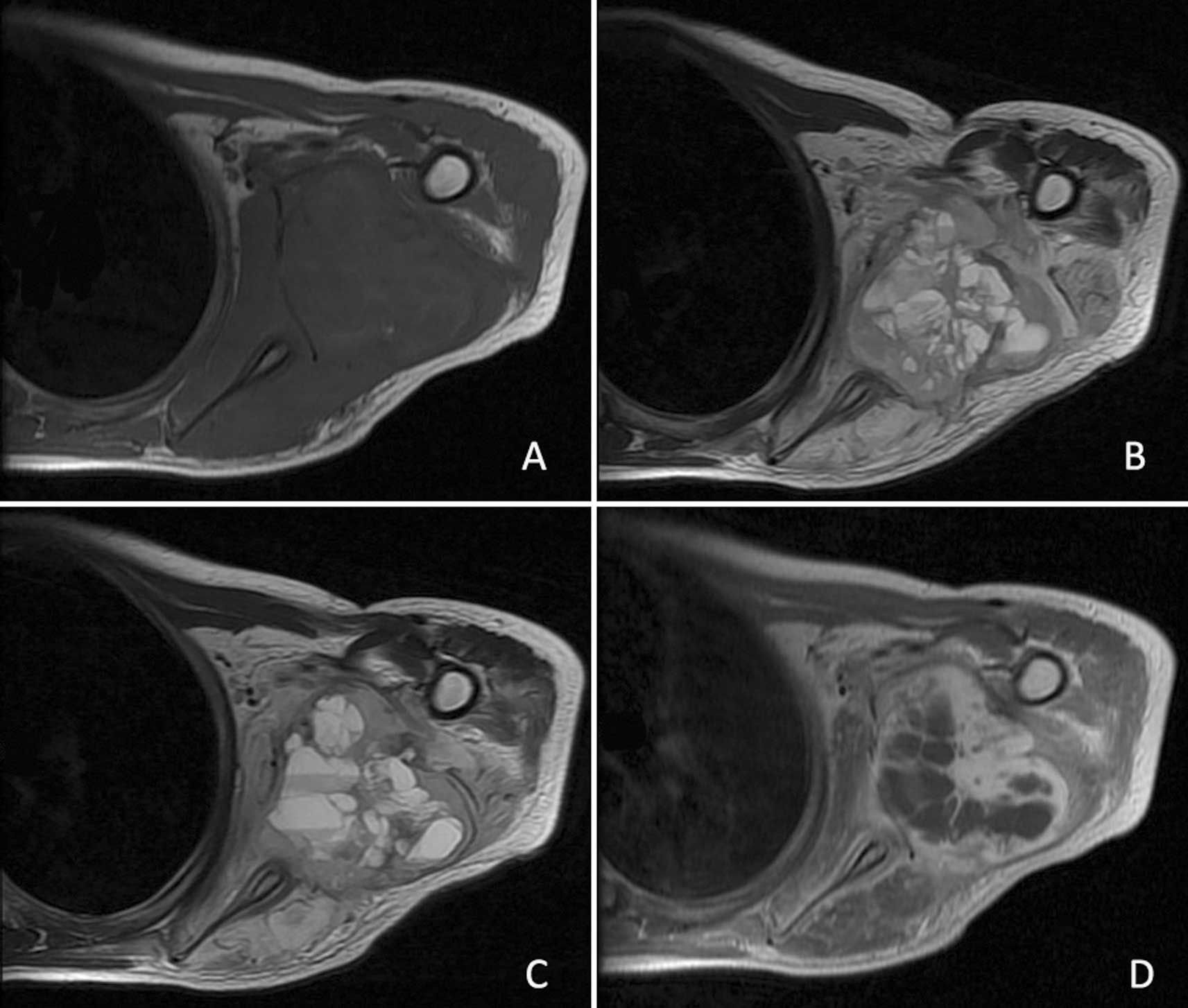
Magnetic resonance imaging (MRI) revealed a
rectangular 5.9×4.6-cm soft tissue lesion in the posterior aspect
of the left shoulder with an uneven intralesional signal. On
T1-weighted MRI, the lesion showed an overall signal intensity (SI)
similar to that of the surrounding normal muscles. The SI of the
intralesional septa appeared a little higher than that of the
separated areas (Fig. 2A). By
contrast, the mass showed a predominantly high SI with a
well-defined margin on T2-weighted MRI; the SI of the intralesional
septa had a low to intermediate SI, while the separated areas were
of high SI (Fig. 2B). Fluid-fluid
levels were also observed in our patient (Fig. 2C). Following administration of
gadopentetate dimeglumine, the lesion manifested peripheral and
intralesional septa enhancement, while the separated areas were not
significantly enhanced (Fig. 2D).
MRI further demonstrated that the scapula and proximal humerus were
not involved and the sclerotin was normal.
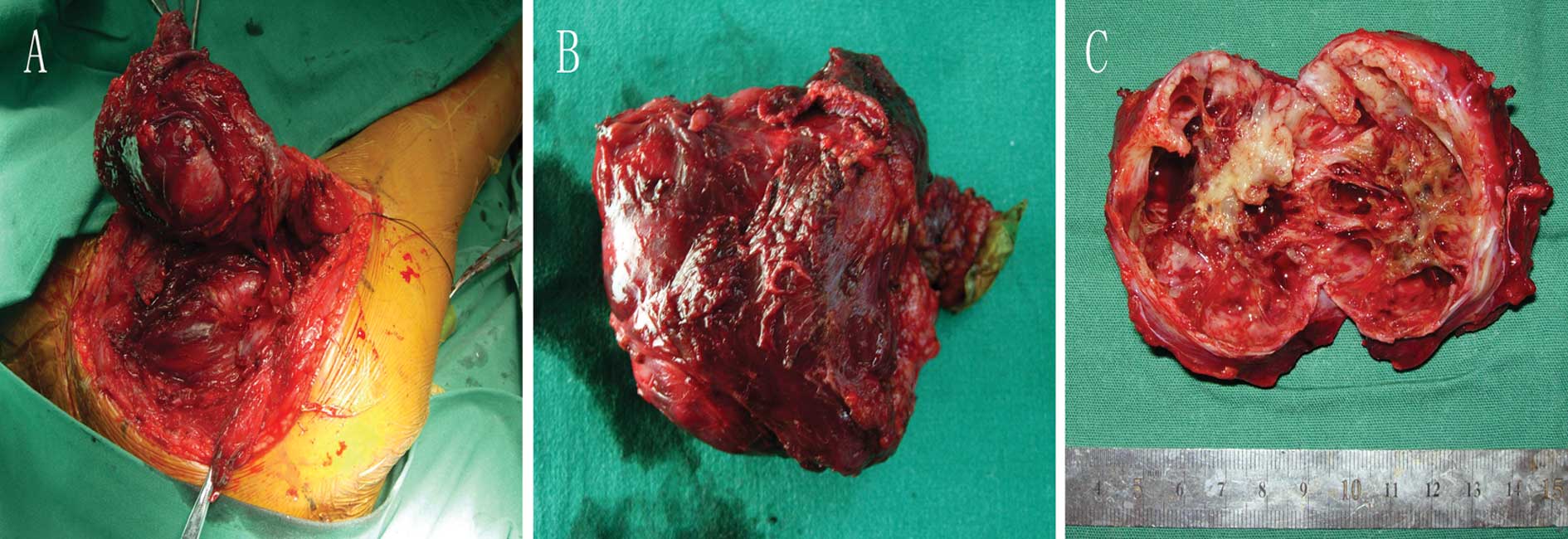
The mass was subsequently completely excised under
general anesthesia. Intraoperatively, we found that there was no
relationship between the tumor and the adjacent bones and the mass
was mainly located between the infraspinatus muscle, teres major
muscle and tere muscle (Fig. 3A).
On gross inspection, the specimen was a rectangular 8.8×5.7×4.6-cm
soft tissue mass (Fig. 3B), the
exterior of which consisted of a rim formed partly of fibrous
tissue with areas of a thin eggshell-like layer of bone (Fig. 3C). The center of the lesion
consisted of irregular blood-filled cavities separated by septa of
varying thickness (Fig. 3C).
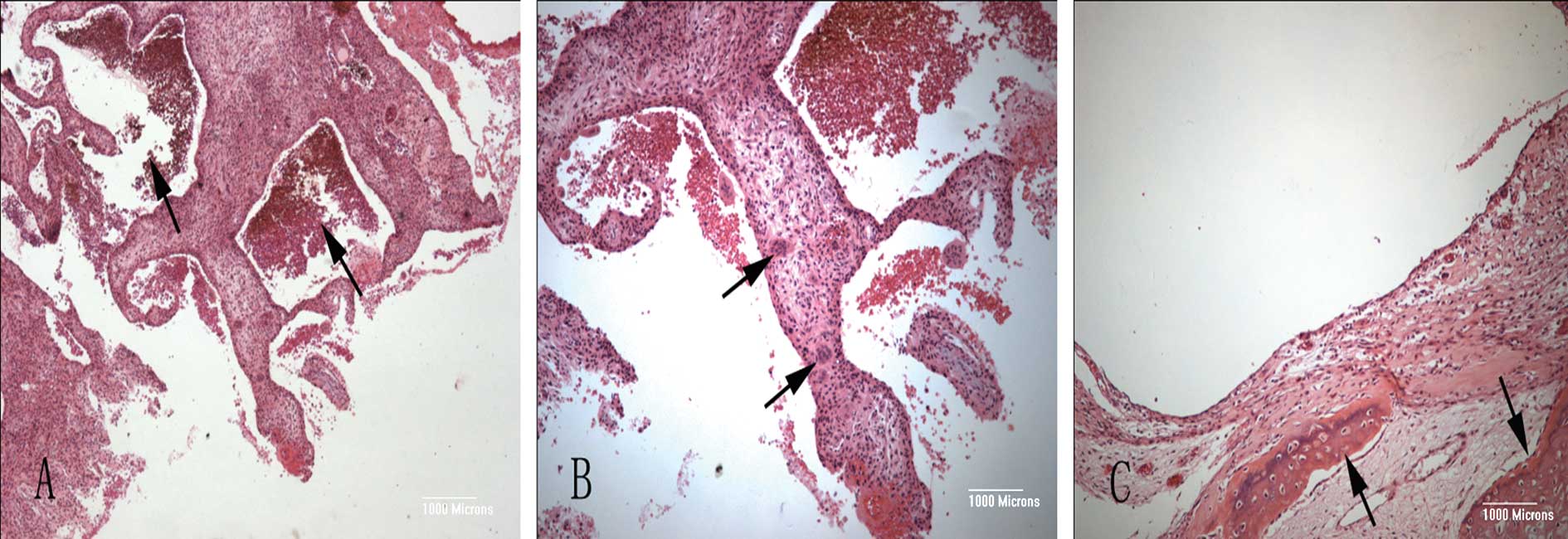
Microscopically, the lesion was composed of cystic blood-filled
spaces. The cyst walls contained fibroblasts, inflammatory cells,
multinucleated giant cells and reactive woven bones (Fig. 4).
Discussion
ABC is a type of benign lesion that may occur in any
bone, but rarely in soft tissue. It occurs predominantly during the
first two decades of life. An ABC is composed of numerous irregular
cysts filled with blood and separated by connective tissue septa
containing fibroblasts, osteoclast-type giant cells and reactive
bone (1,2). It usually appears on radiographs as an
eccentric lytic and expansile lesion with a well-defined margin
within bones (1).
ABC was first described by Jaffe and Lichtenstein in
1942 (9). Its development has been
widely regarded as a reactive process since then. However, it is
recognized that there is no previous lesion in the majority of
cases of this disease. Panoutsakopoulos et al (10) demonstrated the presence of a
chromosomal translocation t(16;17)(q22;p13) in two osseous cases,
suggesting a neoplastic process. These initial findings were later
confirmed by several subsequent cytogenetic studies (11,12).
Oliveira et al (13)
reported that the t(16;17)(q22;p13) translocation fuses the
promoter region of the osteoblast cadherin 11 gene (CHD11) on
chromosome 16q22 to the entire coding sequence of the ubiquitin
protease USP6 gene. Ye et al (14) showed that the overexpression of USP6
in pre-osteoblastic MC3T3 cells is sufficient to drive the
formation of tumors that reproduced the molecular and histological
features of ABC. These authors also suggested that USP6 may play a
direct role in establishing a degradative and vascularized
microenvironment. Five partner genes known to upregulate USP6
expression in ABC have been identified: CDH11(16Q22), ZNF9(3Q21),
OMD(9Q22), COL1A1(17Q21) and TRAP(1P34) (15). In addition, Geiersbach et al
(16) reported an ABC case with an
SS18 rearrangement, which has not previously been described. These
findings clearly demonstrate that ABC is a neoplastic disease.
STABC is extremely rare. The number of published
STABC cases does not exceed 20, with only a few epidemiological and
histological reports (7). Of these
cases, only 5 are in the pediatric age group, as shown in Table I. Our patient is a 10-year-old girl
with a lesion in the posterior aspect of the left shoulder. STABC
shows a rather similar appearance to its osseous counterpart on
radiography and MRI (4). In our
case, plain radiographs revealed a rectangular lesion with a fuzzy
image of non-uniform density in the center, when observed
carefully. Although the peripheral rim was not as clear as
described in previous cases, the specific features of ABC on MRI,
including an expansile lesion surrounded by a thin low- signal rim,
increased signal with augmented T1-weighting and a lobulated
contour with fluid-fluid levels were all clearly visible in our
patient. The MRI presentation of our case may be described as
relatively typical.
The differential diagnosis of STABC mainly includes
giant cell tumor of soft tissue, giant cell tumor of the tendon
sheath, extraskeletal telangiectatic osteosarcoma (EOS) and
myositis ossificans.
Giant cell tumors are often associated with ABCs,
since ABCs often contain a large number of giant cells. These two
diseases are easily confused, and as a result it is occasionally
difficult to distinguish ABC from a giant cell tumor, especially
when a giant cell tumor exhibits bleeding, necrosis and cystic
changes. However, giant cell tumors usually occur in adults over
the age of 20, whereas ABCs are often found in the first two
decades of life. However, the results noted in Table I show that the age of patients with
STABC is not fixed. Histologically, compared with giant cell tumor
of soft tissue or giant cell tumor of the tendon sheath, STABC
often contains more reactive bone-like tissue and woven bone with a
well-defined peripheral rim, but fewer giant cells.
Differentiation between STABC and EOS is essential
as they require completely different management and have different
outcomes. EOS most commonly affects individuals older than 30 and
is rarely encountered during the first two decades of life
(17). EOS differs from STABC in
that it usually has an ill-defined rim on X-rays and is more prone
to becoming malignant. Microscopically, anaplastic tumor cells and
atypical mitosis may be found in EOS, which do not exist in
STABC.
Although radiography and CT may reveal certain
similarities between STABC and myositis ossificans, i.e., a
radiolucent lesion with a thin rim of ossification at the
periphery, the presence of septa within the lesion in STABC may
differentiate it from myositis ossificans (4). Myositis ossificans usually contain a
solid inner component, whereas STABC does not show the presence of
any solid parts (except for the septa). Nevertheless, STABC may
also be secondary to myositis ossificans. In this case, cystic
spaces filled with blood and connective tissue septa are also the
main points of differentiation.
In conclusion, STABC is an extremely rare type of
benign soft tissue tumor, with no more than 20 cases having been
reported in the English language literature. It is occasionally
secondary to lesions such as giant cell tumor, myositis ossificans
and conventional osteosarcoma, therefore caution should be used in
the diagnosis of STABC. If STABC is correctly diagnosed, surgical
treatment should be implemented with complete excision considered
the most appropriate treatment.
Acknowledgements
This study was supported by the National High
Technology Research and Development Program of China (863
program)(grant no. 2009AA03Z311), the National Natural Science
Foundation of China (grant no. 81071472) and the Key Disciplines of
Shanghai Municipal Education Commission (grant no. J50206).
References
|
1
|
Nielsen GP, Fletcher CD, Smith MA, Rybak L
and Rosenberg AE: Soft tissue aneurysmal bone cyst: a
clinicopathologic study of five cases. Am J Surg Pathol. 26:64–69.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodriguez-Peralto JL, Lopez-Barea F,
Sanchez-Herrera S and Atienza M: Primary aneurysmal cyst of soft
tissues (extraosseous aneurysmal cyst). Am J Surg Pathol.
18:632–636. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang XL, Gielen JL, Salgado R, Delrue F
and De Schepper AM: Soft tissue aneurysmal bone cyst. Skeletal
Radiol. 33:477–480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ajilogba KA, Kaur H, Duncan R, McFarlane
JH and Watt AJ: Extraosseous aneurysmal bone cyst in a 12-year-old
girl. Pediatr Radiol. 35:1240–1242. 2005.PubMed/NCBI
|
|
5
|
Ellison DA, Sawyer JR, Parham DM and
Nicholas R Jr: Soft-tissue aneurysmal bone cyst: report of a case
with t(5;17) (q33;p13). Pediatr Dev Pathol. 10:46–49. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pietschmann MF, Oliveira AM, Chou MM, et
al: Aneurysmal bone cysts of soft tissue represent true neoplasms:
a report of two cases. J Bone Joint Surg Am. 93:e452011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van de Luijtgaarden AC, Veth RP, Slootweg
PJ, et al: Metastatic potential of an aneurysmal bone cyst.
Virchows Arch. 455:455–459. 2009.PubMed/NCBI
|
|
8
|
Fan JY, Wang YF, Han B, Ji YR, Song HD and
Fan XQ: FOXL2 mutations in Chinese families with Blepharophimosis
syndrome (BPES). Transl Res. 157:48–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jaffe HL and Lichtenstein L: Solitary
unicameral bone cyst: with emphasis on the roentgen picture, the
pathologic appearance and the pathogenesis. Arch Surg.
44:1004–1025. 1942. View Article : Google Scholar
|
|
10
|
Panoutsakopoulos G, Pandis N, Kyriazoglou
I, Gustafson P, Mertens F and Mandahl N: Recurrent
t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes
Cancer. 26:265–266. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sciot R, Dorfman H, Brys P, et al:
Cytogenetic-morphologic correlations in aneurysmal bone cyst, giant
cell tumor of bone and combined lesions. A report from the CHAMP
study group. Mod Pathol. 13:1206–1210. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dal Cin P, Kozakewich HP, Goumnerova L,
Mankin HJ, Rosenberg AE and Fletcher JA: Variant translocations
involving 16q22 and 17p13 in solid variant and extraosseous forms
of aneurysmal bone cyst. Genes Chromosomes Cancer. 28:233–234.
2000.PubMed/NCBI
|
|
13
|
Oliveira AM, Perez-Atayde AR, Inwards CY,
et al: USP6 and CDH11 oncogenes identify the neoplastic cell in
primary aneurysmal bone cysts and are absent in so-called secondary
aneurysmal bone cysts. Am J Pathol. 165:1773–1780. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye Y, Pringle LM, Lau AW, et al:
TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces
matrix metalloproteinase production via activation of NF-kappaB.
Oncogene. 29:3619–3629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oliveira AM, Perez-Atayde AR, Dal Cin P,
et al: Aneurysmal bone cyst variant translocations upregulate USP6
transcription by promoter swapping with the ZNF9, COL1A1, TRAP150,
and OMD genes. Oncogene. 24:3419–3426. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geiersbach K, Rector LS, Sederberg M, et
al: Unknown partner for USP6 and unusual SS18 rearrangement
detected by fluorescence in situ hybridization in a solid
aneurysmal bone cyst. Cancer Genet. 204:195–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee KH, Joo JK, Kim DY, Lee JS, Choi C and
Lee JH: Mesenteric extraskeletal osteosarcoma with telangiectatic
features: a case report. BMC Cancer. 7:822007. View Article : Google Scholar : PubMed/NCBI
|