Introduction
To date, heparanase (Hpa) is the only endogenous
endoglycosidase found that degrades the heparan sulfate
proteoglycans (HSPGs) in the extracellular matrix (ECM) and the
basement membrane (BM). Hpa is highly expressed in most mammalian
malignant tumors, and its expression has been significantly linked
to the formation of microvessels and lymphatic vessels, tumor
invasion, metastasis and angiogenesis (1). Previous studies have focused on the
correlation between Hpa expression in cancer tissues and clinical
and pathological features, which demonstrate that increased Hpa
levels are most often associated with increased tumor metastasis,
high microvessel density and reduced patient survival time
following surgery (2–6). However, the antibodies used to
recognize and combine Hpa protein are typically commercial, and the
antigen determinants of such antibodies have not yet been
defined.
Gastric cancer remains one of the most lethal
malignancies in the world. Surgical resection, chemotherapy and
radiotherapy have been the most common forms of management used in
treating such malignancies. Recently, immunotherapy has been
proposed as a new modality for cancer treatment due to its reduced
side effects and easy applicability. It utilizes the immune system
to recognize and specifically eradicate tumor cells and has shown
encouraging results in human clinical trials (7). Tumor-associated antigens (TAAs)
presented by dendritic cells trigger a TAA-specific immune
response, thus they are the crux of antitumor immunotherapy. A
major advance in tumor immunology in the last 20 years was marked
by the verification that CTL or B-cell epitopes rather than
integral TAAs induce immunoreactivity (8). The first group of immunogenic epitopes
in Hpa was identified by Sommerfeldt et al using the
SYFPEITHI algorithm to identify nonapeptides of the Hpa amino acid
sequence (9). In our previous
study, according to the primary structure of Hpa and on the basis
of predicting the Hpa B-cell epitopes via bioinformatics, we
designed and synthesized multiple antigenic peptides (MAPs) using
the eight-branched polypeptide modus (10,11).
We also found that each of the three MAPs was capable of inducing
the production of antibodies (antisera) of high titer (12).
The aim of the present study was to determine the
expression of Hpa MAP presented with explicit epitopes in gastric
cancer and its correlation with clinical and pathological features
of gastric malignancies, using tissue chip technology and
immunohistochemical staining. The antibodies we harnessed were
three self-developed rabbit polyclonal antibodies against Hpa MAP,
as mentioned above, and one commercial polyclonal rabbit antibody
against the 50-8 kDa Hpa heterodimer. In addition, the role of Hpa
MAP in the pathogenesis and aggressiveness of gastric cancer and
its prognostic value were explored.
Materials and methods
Patients and tissue samples
Tissue specimens were obtained from 165 gastric
cancer patients and embedded in paraffin. The clinical data of
these cases were reviewed and analyzed retrospectively. Paired
samples of normal gastric tissue were obtained from 39 patients
during surgery. However, on account of the tissues being worn down
through use of tissue chip technology and immunohistochemical
staining, only 132 samples (35 females and 97 males, aged 17–80
years, average age 58±11 years) of gastric carcinoma tissue and 30
paired normal samples were selected for use in this study. None of
the selected patients had been treated with radiotherapy or
chemotherapy prior to the surgery, and clinical and follow-up data
were available in all cases. All 132 patients were treated at
Department of General Surgery, Zhejiang Provincial People’s
Hospital, China, between January 1998 and December 2004. The
cancers were analyzed for histological type, depth of invasion,
lymphatic involvement and venous infiltration according to the
definitions used in clinical and pathological studies on gastric
cancer. According to the World Health Organization (WHO) gastric
cancer typing criteria of 2002, the lesion diameter was <5 cm in
90 cases and ≥5 cm in the remaining 42 cases. Highly or moderately
differentiated adenocarcinomas were found in 40 cases while poorly
or undifferentiated carcinomas were found in the remaining 92
cases. The number of patients with and without regional lymph node
metastasis, distant metastasis and vessel invasion were 90 (68.2%)
and 42 (31.8%), 25 (18.9%) and 107 (81.1%), and 42 (31.8%) and 90
(68.2%), respectively. According to the gastric cancer TNM staging
criteria revised by the International Union against Cancer (UICC)
and the American Joint Committee on Cancer (AJCC) in 2003, there
were 52 cases in stages I and II, and 80 cases in stages III and
IV. Excluding the cases of mortality, the shortest follow-up time
was 4 years while the longest was 10 years, and the cut-off date
was January 2008.
This study was approved by the ethics committee of
Zhejiang Provincial People’s Hospital. Consent was obtained from
all patients.
Materials and reagents
Polyclonal rabbit antibody against full-length human
Hpa was purchased from InSight Biopharmaceuticals Co., Ltd.,
Israel. Histostain™-Plus kits (SP-9000) and the DAB chromogenic kit
were purchased from Beijing Zhongshan Goldenbridge Biotechnology
Co., Ltd., China. Rabbit polyclonal antibodies to Hpa MAP were
self-designed as described previously (12–14)
and were synthesized and purified by Chinese Peptide Company, Ltd.,
Hangzhou, China. The amino acid sequences of MAP1, MAP2 and MAP3
were KKFKNSTYSRSSVDV (1–15), HCTNTDNPRYKEGDL (279–293) and
STRPGKKVWLGETSS (175–189), respectively, at the large-subunit locus
of Hpa. Three different antibodies against human Hpa were obtained
following the immunization of white-hair-black-eye rabbits with the
three MAPs, respectively. The specificity, immunogenicity and
antitumor activity of the antiserum were evaluated as described
previously (12–14).
Tissue microarray (TMA) construction and
immunohistochemistry
TMA for the gastric cancer tissues and compared
normal tissues was constructed using a semiautomatic tissue arrayer
(Beecher Instruments, Woodland, TX, USA). Areas involving malignant
and normal tissues were marked on hematoxylin and eosin-stained
sections. Cylindrical cores 0.6 mm in diameter were then punched
out of the corresponding paraffin-embedded block and inserted into
a recipient block which was receptor-free and punched with the same
diameter. This procedure was repeated until all the specimens were
implanted into the paraffin block. After successive slicing and
laminating, TMAs were constructed and tissue chips were
obtained.
The labelled streptavidin-biotin technique (SP
method) was used for immunohistochemical staining. The sera of the
MAP1, MAP2, MAP3 and commercial Hpa antibody were used as the
primary antibody. The positive-stained gastric cancer section was
used as the positive control, while normal rabbit serum and
phosphate-buffered saline (PBS) were used as the negative control
instead of the primary antibody. Immunohistochemical staining was
performed following the instruction manual of the kit.
Determination of results
The experimental results were analyzed in a blinded
manner by senior pathologists. The analysis was initially performed
under a low-power lens to select the densely stained areas, then
the pathologists counted 1,000 tumor cells in five randomly
selected areas at ×200 magnification. The scoring used in the
immunohistochemical staining results was based on the coloring of
the cancer cells (0, no staining; 1, light yellow; 2, brown; 3,
dark brown) and on the percentage of positive cells (0, negative;
1, ≤10% positive cells; 2, 11–50% positive cells; 3, ≥51% positive
cells). The intensity of Hpa expression was calculated as the
product of the staining intensity score and the positive cell
percentage score: score 0–2, −; score 3–4, +; score 5–7, ++; and
score 8–9, +++.
Statistical analysis
SPSS 18.0 (SPSS Inc., Chicago, IL, USA) statistical
software was used for data processing. The correlations between the
expression of the four different Hpa antigens (Hpa full-length
protein, MAP1, MAP2 and MAP3) and clinical or pathological
characteristics were evaluated using the Chi-square test. Survival
rates were evaluated by the Kaplan-Meier curve analysis. P<0.05
was considered to indicate a statistically significant result.
Results
Expression of Hpa antigens
Immunohistochemical staining was achieved when the
commercial Hpa antibody and MAP2 antiserum were used as primary
antibodies. The two antibodies stained the gastric cancer cells
equally dark brown, and their positivity rates were similar. The
positive Hpa staining rates using the commercial antibody in
gastric cancer and normal tissue were 60.6% (80/132) and 3.3%
(1/30), respectively, while the rates of positive MAP2 expression
in gastric cancer and normal tissue were 65.2% (86/132) and 3.3%
(1/30), respectively; the positive cell percentage was much higher
in gastric cancer tissue than in normal tissue in both cases
(P<0.05). The two antigens were mainly located in the cell
plasma and cell membranes in cancer tissues, and were restricted to
the glandular epithelium of mucosal cells in normal gastric
tissues. The expression intensity of the antigens in the tumor
tissues (scores: ++ to +++) was much greater than that in the
normal gastric tissues (scores: + to ++). In addition, positive
staining was detected in a few vascular endothelial cells and
smooth muscle cells. Staining using MAP1 and MAP3 antiserum was
negative for both, compared to PBS.
Correlation between Hpa expression and
clinicopathological characteristics
The expression of MAP2 polypeptide was similar to
that of Hpa protein: a markedly higher expression was detected in
the malignant tissues with vessel invasion, serosal involvement,
distant metastasis, poor differentiation and TNM stages III and IV
(P<0.01; Table I). However, no
significant correlation was found between Hpa expression and
patient gender, tumor diameter and histological type.
 | Table ICorrelation between Hpa expression and
clinicopathological characteristics. |
Table I
Correlation between Hpa expression and
clinicopathological characteristics.
| Clinicopathological
characteristics | No. of cases | Expression of
MAP2 | Positivity rate
(%) | Expression of Hpa
protein | Positivity rate
(%) |
|---|
|
|
|---|
| Positive | Negative | Positive | Negative |
|---|
| Gender |
| Male | 97 | 32 | 65 | 67.0 | 35 | 60 | 63.2 |
| Female | 35 | 14 | 21 | 60.0 | 17 | 20 | 54.1 |
| Tumor diameter |
| <5 cm | 90 | 33 | 57 | 63.3 | 35 | 53 | 60.2 |
| ≥5 cm | 42 | 13 | 29 | 69.0 | 17 | 27 | 61.4 |
| Tissue type |
| Intestinal | 95 | 27 | 68 | 71.6 | 34 | 59 | 63.4 |
| Diffuse | 37 | 19 | 18 | 48.6 | 18 | 21 | 53.8 |
| Differentiation |
| Well or
moderate | 40 | 8 | 32 | 80.0 | 9 | 26 | 74.3 |
| Poor or
undifferentiated | 92 | 38 | 54 | 58.7a | 43 | 54 | 55.7a |
| Lymphatic
metastasis |
| Negative | 42 | 24 | 18 | 42.9 | 25 | 16 | 39.0 |
| Positive | 90 | 22 | 68 | 75.6a | 27 | 64 | 70.3a |
| Distant
metastasis |
| Negative | 107 | 46 | 61 | 57.0 | 47 | 60 | 56.1 |
| Positive | 25 | 0 | 25 | 100a | 5 | 20 | 80.0a |
| Vessel invasion |
| Negative | 90 | 41 | 49 | 54.4 | 40 | 49 | 55.1 |
| Positive | 42 | 5 | 37 | 88.1a | 12 | 31 | 72.1a |
| Serosal
infiltration |
| Negative | 39 | 16 | 23 | 59.0 | 17 | 21 | 55.3 |
| Positive | 93 | 30 | 63 | 67.7a | 35 | 59 | 62.8a |
| TNM staging |
| I–II | 52 | 30 | 22 | 42.3 | 29 | 22 | 43.1 |
| III–IV | 80 | 16 | 64 | 80.0a | 23 | 58 | 71.6a |
Correlation between Hpa expression and
prognosis
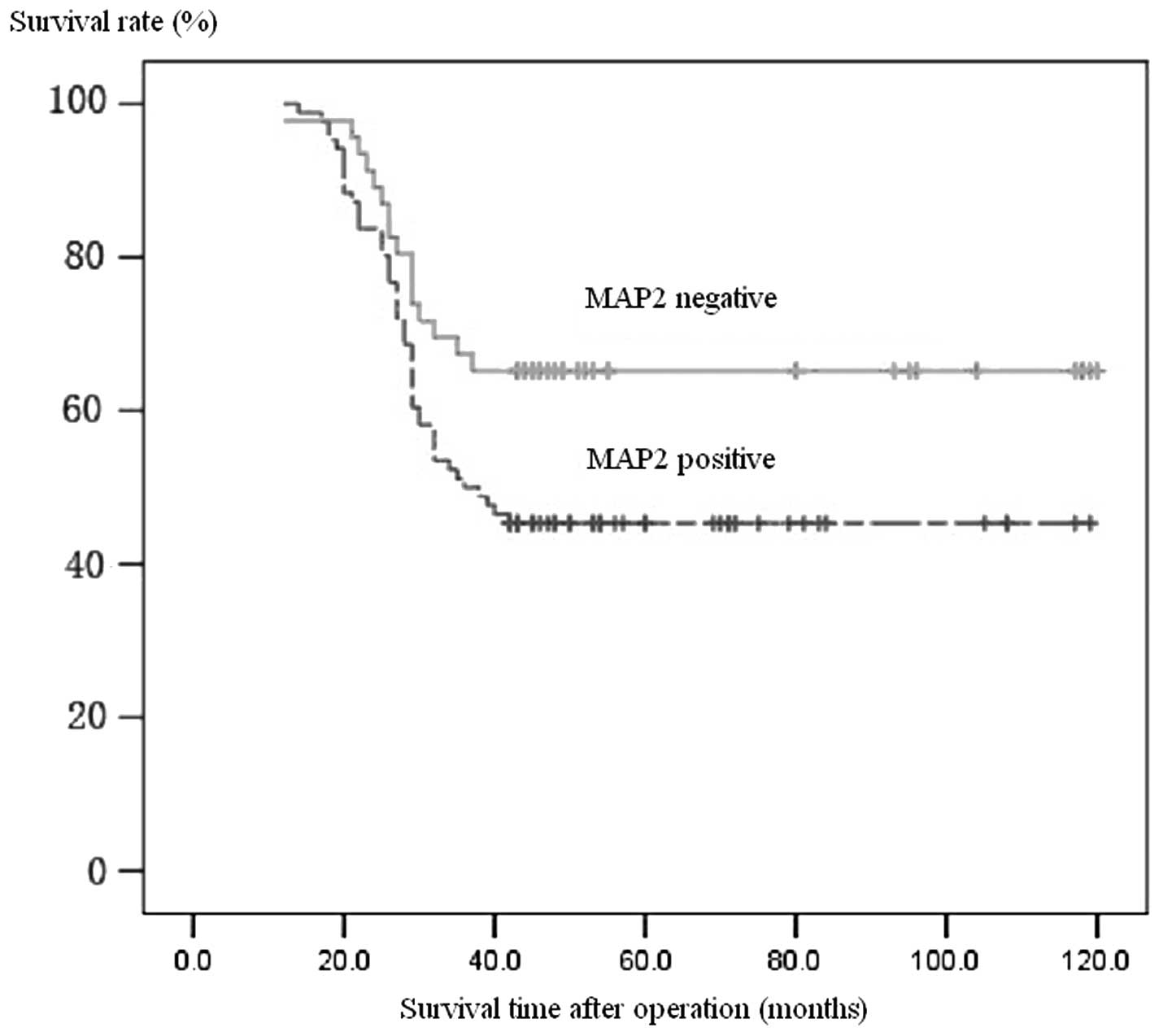
Follow-up data were available for all patients. The
prognosis of patients with positive expression of MAP2 antigen was
far poorer than that of patients with a negative expression, which
was similar to that of Hpa protein using the McNemar matched
Chi-square test (P>0.05). Patients with Hpa expression
demonstrated one- and five-year postoperative survival rates of 80
and 44%, respectively, compared to 92 and 63% in those without Hpa
protein expression. Similarly, patients with positive MAP2 staining
had one- and five-year postoperative survival rates of 84 and 45%,
respectively, compared to 91 and 65% in those with negative MAP2
staining (Fig. 1).
Discussion
Hpa protein is an endo-β-glucuronidase that cleaves
heparan sulfate side chains, presumably at sites of low sulfation,
releasing saccharide products with appreciable size (4–7 kDa) that
still associate with protein ligands and facilitate their
biological potency. Hpa has been detected at relatively low levels
in mammalian lymphoid organs, placenta and platelets and has been
found to be highly expressed in most mammalian malignant tumors,
while it is either not expressed or expressed at extremely low
levels in other normal tissue. In conditions of injury or
inflammation, certain immunocytes (i.e., lymphocytes, macrophages
and neutrophils) secrete Hpa, which has the ability of breaking
down HSPGs and helping cells to migrate, gather at lesions and
fulfill their anti-inflammatory or repairing responsibilities
(1). The activation of Hpa enables
tumor cells to break through the ECM and BM barriers. Furthermore,
Hpa releases multiple cytokine types, including VEGF and bFGF.
These cytokines are crucial in promoting cell movement, enhancing
tumor cell invasion and promoting tumor angiogenesis. They are
therefore considered to be closely associated with invasion,
metastasis and prognosis in multiple malignant tumor types
(12–14).
TMA was proposed in 1998 as a new biochip technology
and has recently been of high interest, particularly in the areas
of human genomes, protein research and drug development. TMA is
envisioned to be available for routine biomedical and diagnostic
applications provided that the ongoing technological developments
are successful in improving sensitivity and specificity, and in
reducing costs. Compared with traditional immunohistochemical
staining, TMA has many advantages including TMA has many
advantages, including lower volume of tissue required, reduced
error rate and greater provision of information to researchers. TMA
is useful for parallel testing of antibody specificities on a broad
range of histological specimens in a single slide, and improves the
reliability and the accuracy of study outcomes by ensuring the
coherence of experimental parameters.
In this study, we found that the Hpa protein and
polypeptide were highly expressed in most advanced-stage gastric
cancer tissues, which were mainly located within the cytoplasm and
cytomembrane. In addition, they were weakly expressed in a few
vascular endothelial cells and smooth muscle cells. However, no
expression of Hpa protein or MAP was detected in normal gastric
mucosal glands. These findings were consistent with previously
published studies (15). According
to the standard scoring used in immunohistochemical staining, the
expression of the three different MAPs in gastric cancer tissues
had clear discrepancies: i) a distinct higher expression of MAP2,
and ii) no expression of MAP1 and MAP3, which was similar to the
findings of our previous study which focused on hepatocellular
carcinoma tissues (12). Such
results may be explained by the different affinity of the three
antibodies against MAP combining with antigenic determinants of
Hpa. As the different epitopes in the Hpa large subunit had
different spatial locations, it can be hypothesized that the
epitopes of MAP2 are dominant, whereas those of MAP1 and MAP3 are
recessive (12).
Certain studies using commercial antibodies against
Hpa whole protein have demonstrated that the expression of Hpa
protein was closely correlated with the invasion and metastasis of
gastrointestinal tumors (16,17).
Our study revealed that the immunohistochemical staining with MAP2
antiserum was similar to that observed using the commercial
antibody, as both had a strong expression in the cell plasma and
cell membrane. Furthermore, a markedly increased expression of Hpa
protein or MAP antigen was detected in cases with lymphatic
metastasis, vessel invasion, distant metastasis, serosal
infiltration, poorly differentiated or undifferentiated cancers and
TNM stage III or IV. The survival curve also showed that the one-
and five-year survival rates of patients without Hpa expression
were significantly higher than those with Hpa expression.
Therefore, Hpa MAP2 antibody is a promising tool in the diagnosis
of gastric cancer phenotypes.
Hpa inhibitors such as polypeptide antibodies block
Hpa, reduce the content of Hpa or lower the enzymatic activity, and
thus prevent the degradation of HS, reduce the release of active
substances and maintain the stability of the ECM. The antitumor
role of Hpa antibodies has been reported in several experimental
documents (18,19). Our previous study also suggested
that Hpa MAP2 antibodies are neutralizing, and are capable of
blocking the hepatocellular cancer cell invasion by inhibiting Hpa
activity (14). However, the
clinical significance of Hpa polypeptides in gastric cancer has not
been fully explored thus far, possibly due to the lack of long-term
follow-up data. In this study, we found for the first time that the
overexpression of Hpa MAP2 is positively correlated with tumor
invasion and metastasis and negatively correlated with prognosis.
Thus, it can be hypothesized that antibodies targeting Hpa MAP2
elicit a potential immunotherapeutic effect in gastric
malignancies, and that downregulation of Hpa MAP2 expression is
likely to improve the prognosis of gastric cancer patients.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (No. 30570816).
References
|
1
|
Kreuger J, Spilmann D, Li JP, et al:
Interactions between heparan sulfate and proteins: the concept of
specificity. J Cell Biol. 174:323–327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barash U, Cohen-Kaplan V, Dowek I, et al:
Proteoglycans in health and disease: new concepts for heparanase
function in tumor progression and metastasis. FEBS J.
277:3890–3903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ilan N, Elkin M and Vlodavsky I:
Regulation, function and clinical significance of heparanase in
cancer metastasis and angiogenesis. Int J Biochem Cell Biol.
38:2018–2039. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Macleod V, Bendre M, et al:
Heparanase promotes the spontaneous metastasis of myeloma cells to
bone. Blood. 105:1303–1309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gohji K, Hirano H, Okamoto M, et al:
Expression of three extracellular matrix degradative enzymes in
bladder cancer. Int J Cancer. 95:295–301. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doweck I, Kaplan-Cohen V, Naroditsky I, et
al: Heparanase localization and expression by head and neck cancer:
correlation with tumor progression and patient survival. Neoplasia.
8:1055–1061. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Borghaei H, Smith MR and Campbell KS:
Immunotherapy of cancer. Eur J Pharmacol. 625:41–54. 2009.
View Article : Google Scholar
|
|
8
|
Rammensee HG, Falk K and Rotzschke O:
Peptides naturally presented by MHC class I molecules. Annu Rev
Immunol. 11:213–244. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sommerfeldt N, Beckhove P, Ge Y, et al:
Heparanase: a new metastasis-associated antigen recognized in
breast cancer patients by spontaneously induced memory T
lymphocytes. Cancer Res. 66:7716–7723. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amexis G and Yong NS: Multiple antigenic
peptides as vaccine platform for the induction of humoral responses
against dengue-2 virus. Viral Immunol. 20:657–663. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haro I and Gómara MJ: Different approaches
to potentiate the immune response induced by a 12-mer synthetic
peptide. Curr Protein Pept Sci. 1:125–137. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang JM, Wang HJ, Du L, et al: Screening
and identification of novel B cell epitopes in human heparanase and
their anti-invasion property for hepatocellular carcinoma. Cancer
Immunol Immunother. 58:1387–1396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du L, Wang HJ, Yang JM, et al: T-helper
epitope peptide improves immunological effects of the B cell
epitopes of human heparanase protein. Chin J Microbiol Immunol.
28:869–872. 2008.
|
|
14
|
Han XM, Wang HJ, Yang JM, et al: Effects
of anti-heparanase antibody on the growth and invasion of HCCLM6
human hepatocellular carcinoma cells. Chin J Oncol. 31:10–14.
2009.PubMed/NCBI
|
|
15
|
Ohtawa Y, Naomoto Y, Shirakawa Y, et al:
The close relationship between heparanase and cyclooxygenase-2
expressions in signet-ring cell carcinoma of the stomach. Hum
Pathol. 37:1145–1152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miao HQ, Liu H, Navarro E, et al:
Development of heparanase inhibitors for anti-cancer therapy. Curr
Med Chem. 13:2101–2111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vlodavsky I, Ilan N, Naggi A, et al:
Heparanase: structure, biological functions, and inhibition by
heparin-derived mimetics of heparan sulfate. Curr Pharm Des.
13:2057–2073. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He X, Brenchley PE, Jayson GC, et al:
Hypoxia increases heparanase-dependent tumor cell invasion, which
can be inhibited by antiheparanase antibodies. Cancer Res.
64:3928–3933. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gingis-Velitski S, Ishai-Michaeli R,
Vlodavsky I, et al: Anti-heparanase monoclonal antibody enhances
heparanase enzymatic activity and facilitates wound healing. FASEB
J. 21:3986–3993. 2007. View Article : Google Scholar
|