Introduction
Anogenital warts (condyloma acuminatum or venereal
warts) are a common sexually transmitted disease among females and
males (1,2). The causal role of human
papillomaviruses (HPV) in anogenital wart formation has been firmly
established biologically and epidemiologically (3,4).
Genital HPV infections are transmitted primarily through sexual
contact, with a lifetime risk of 50–80% (5). The highest rate of genital HPV
infection has been identifed in adults between 18 and 28 years of
age (6,7). The immune system effectively repels
the majority of HPV infections and is associated with marked
localised cell mediated immune responses. However, approximately
10% of individuals develop a persistent infection, with risk of
developing benign proliferative lesions, high-grade precursors and
eventually invasive carcinomas (8).
HPVs are classified into high- or low-risk types depending on
oncogenic potential. Low-risk types 6 and 11 are isolated in
approximately 90% of genital wart cases (3). The most common clinical treatment is
conservative, with local chemical or physical destruction and
immunological therapy (9). In more
extreme cases conservative therapy is insufficient and surgical
excision is required.
Giant condyloma acuminata (GCA; Buschke-Löwenstein
tumour) is an extremely rare clinical form of genital warts,
characterised by aggressive down growth into underlying dermal
structures (10,11). A complex histological pattern may
exist with areas of benign condyloma intermixed with foci of
atypical epithelial cells or well differentiated squamous cell
carcinoma. GCA is mainly localised to the genital region, however,
in rare cases the tumour is localised to distinct histological
zones of the anorectal region. Due to infiltration of the
underlying tissue, fistulae and abscesses may be observed. GCA is
resistant to chemotherapy or radiotherapy and usually requires
local radical resection for curative treatment. The study was
approved by the ethics committee of University Medical School,
Zagreb. Written informed consent was obtained from the patient.
Case report
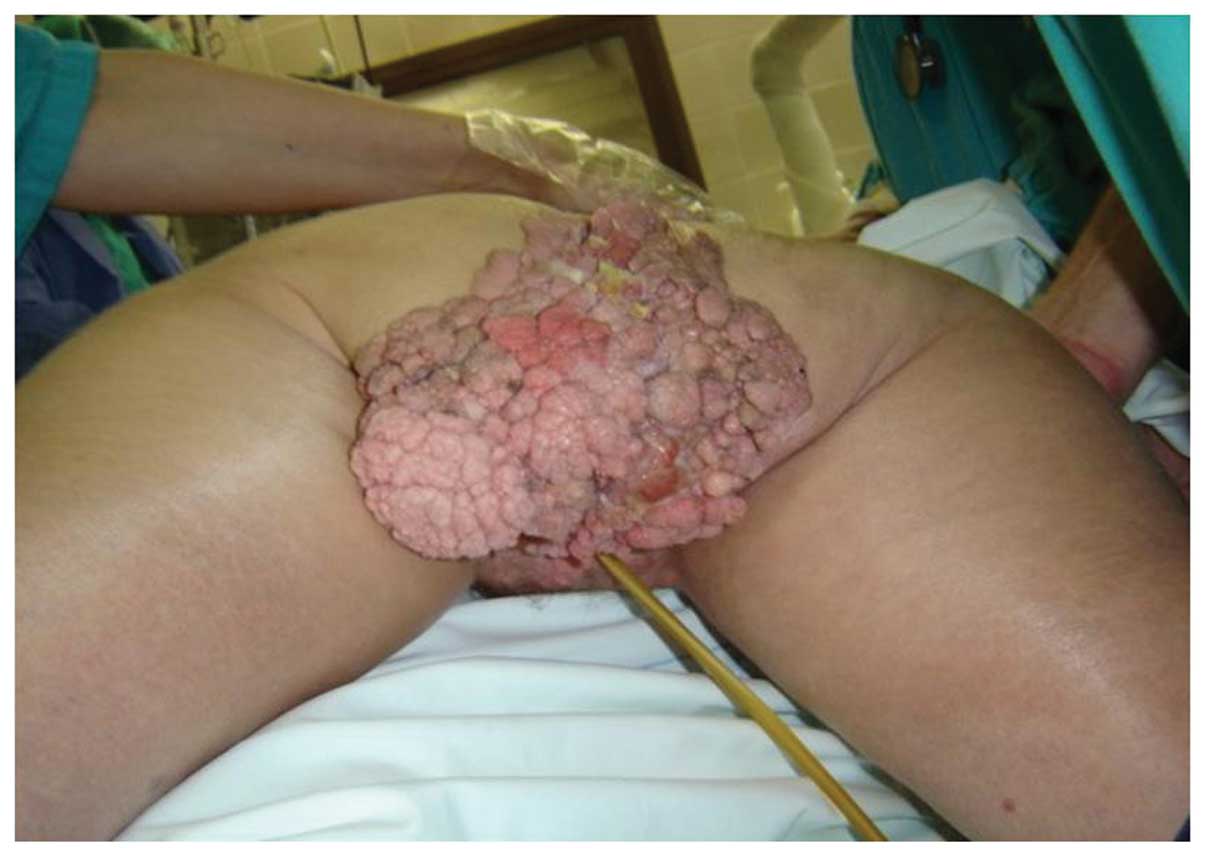
A 55-year-old female presented with cauliflower-like
growth over the anogenital and sacral region. The growth had been
diagnosed previously as condyloma acuminatum which was resistant to
conservative therapy (Fig. 1). The
patient’s medical history was as follows: at 17 years old the
patient was diagnosed with infective mononucleosis and 6 months
later with viral pneumonia. In 1979, the patient suffered from
pyelonephritis caused by E. coli, with subsequent unilateral
permanent kidney lesion. Multiple condyloma were diagnosed for the
first time during the patient’s first pregnancy in 1970, at the
perineal surface and were surgically removed in the same year,
following delivery. Four years following excision recurrence was
identified and was treated successfully with albothyl solution for
two months.
During the first trimester of the patient’s second
pregnancy (1978) warts appeared for the third time with altered
clinical presentation; spread across the entire anogenital region
(perineum, anal orifice and labia majora) and became multilayered
and painful. Despite repeated albothyl therapy, growth continued.
Prior to labour the warts were removed by electro-cauterisation and
the whole surface was treated locally with interferon (IFN)
ointment. During the following year there were no visible warts.
Between 1980 and 1981, due to recurrence, the patient underwent two
additional surgical procedures followed by IFN treatment. Positive
response to treatment lasted for 6–8 months and was followed by
five excision procedures under local anaesthesia between 1983 and
1984. All condyloma were removed. The severity of the disease
increased the following year and was successfully treated with IFN
ointment over one year. In 1986, tumour size increased again. The
patient recieved local IFN therapy, however, treatment response was
inadequate as the tumour was reduced in size by 50%. Condyloma size
remained constant until 2003 when the patient entered menopause.
Podophyllin treatment was administered, however, the side-effects
included bilateral inguinal lymphadenopathy and marked pain. At
this point the patient was admitted to our unit.
Between 2005 and 2008 the patient underwent five
surgical procedures. The procedures were performed by
gynaecological and plastic surgeons due to the size and location of
the tumour. The first surgery was a loop colostomy on the sigmoid
colon performed by an abdominal surgeon. The following procedures
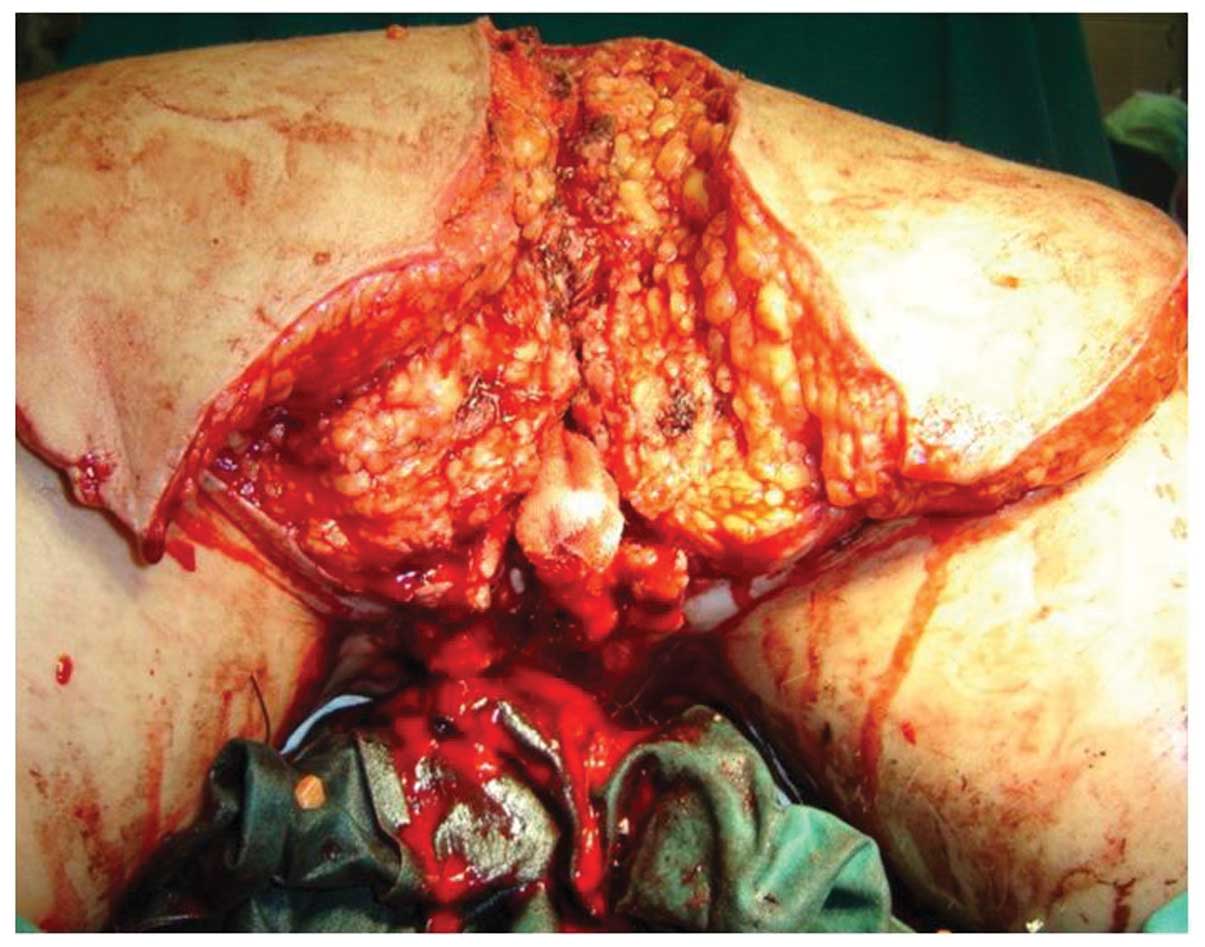
were performed by a plastic and reconstructive surgeon over four
surgical periods; radical excision of the vulvar, perineal, anal
and sacral condyloma with the preservation of urethral orifice,
vagina and anus (Fig. 2).
Reconstruction of the dorsal defect was formed using two large
fascio-cutaneal flaps based on the superior gluteal artery. The
remaining defect of the vulva was reconstructed by local
transpositional fascio-cutaneal flaps from the medial side of the
upper thighs. Following the third surgery, a postoperative
infection developed with partial dehiscence of the local
transpositional flaps from the upper thighs, therefore, necrectomy
was performed, covering the residual with a split thickness skin
graft from the right upper thigh. Seven months following, excision
of the cicatrices was performed due to development of contractures
of the perineal skin and the defects split thickness skin graft was
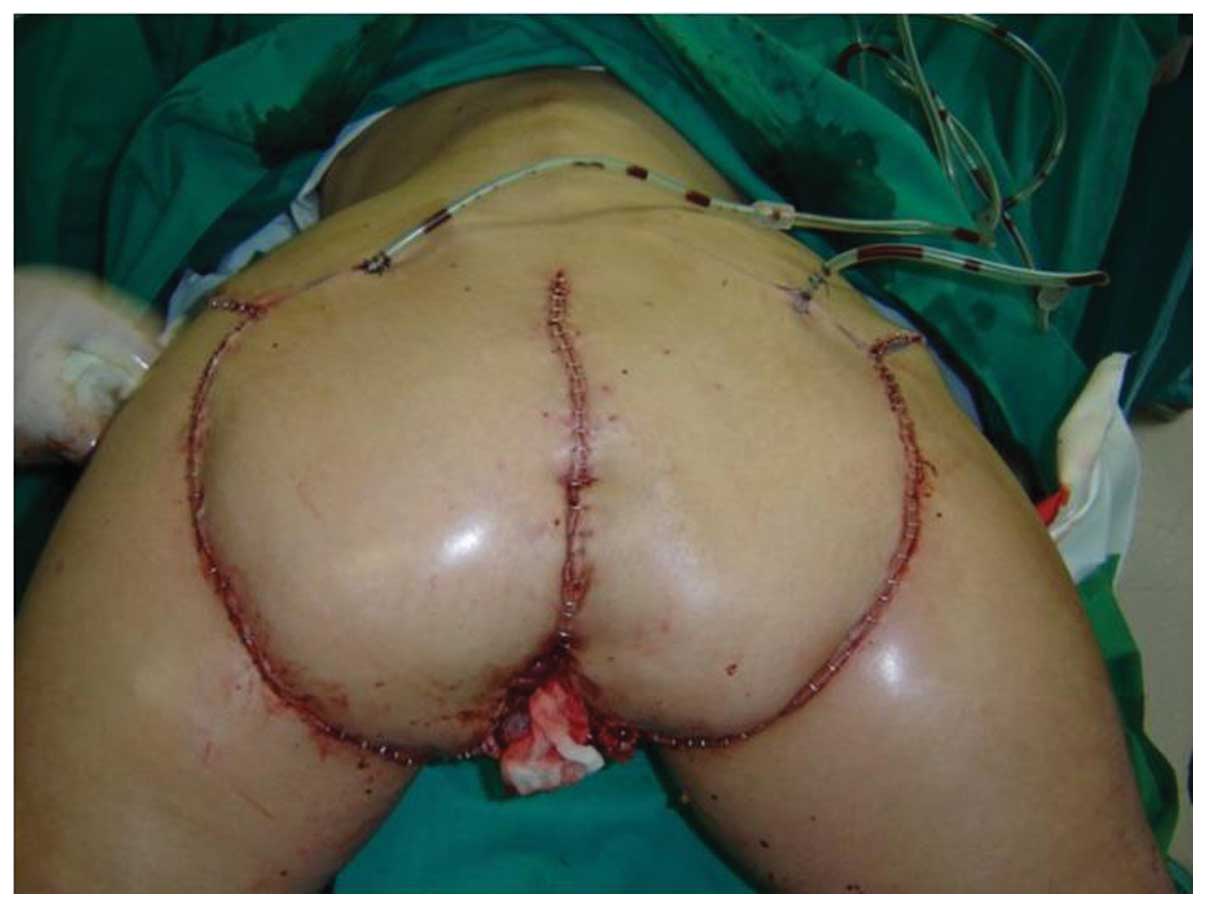
utilised for covering. The final surgery was the occlusion of the
colostomy performed by the abdominal surgeon (Fig. 3).
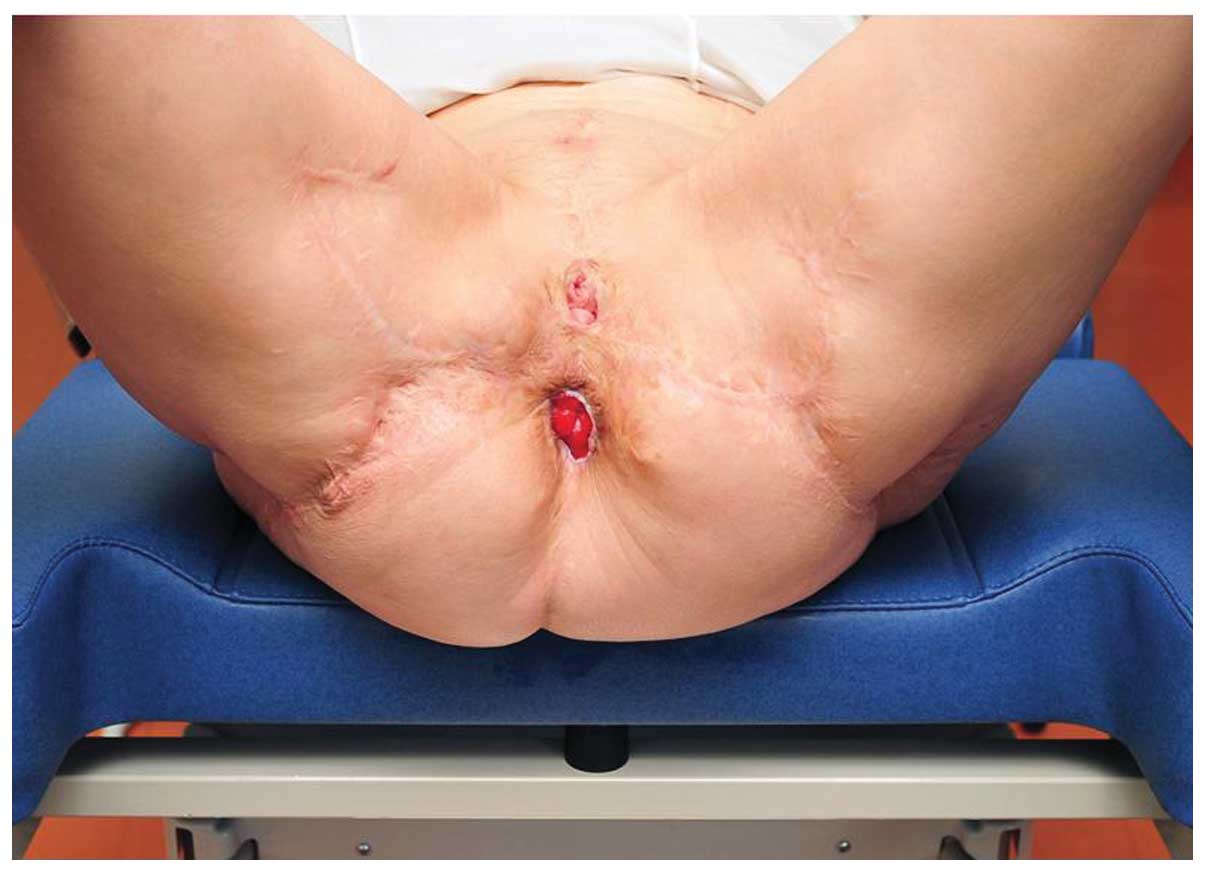
Following 6 months, local status was a preserved
urethral orifice and vaginal introitus with a small rectal mucosa
prolapse (Fig. 4).
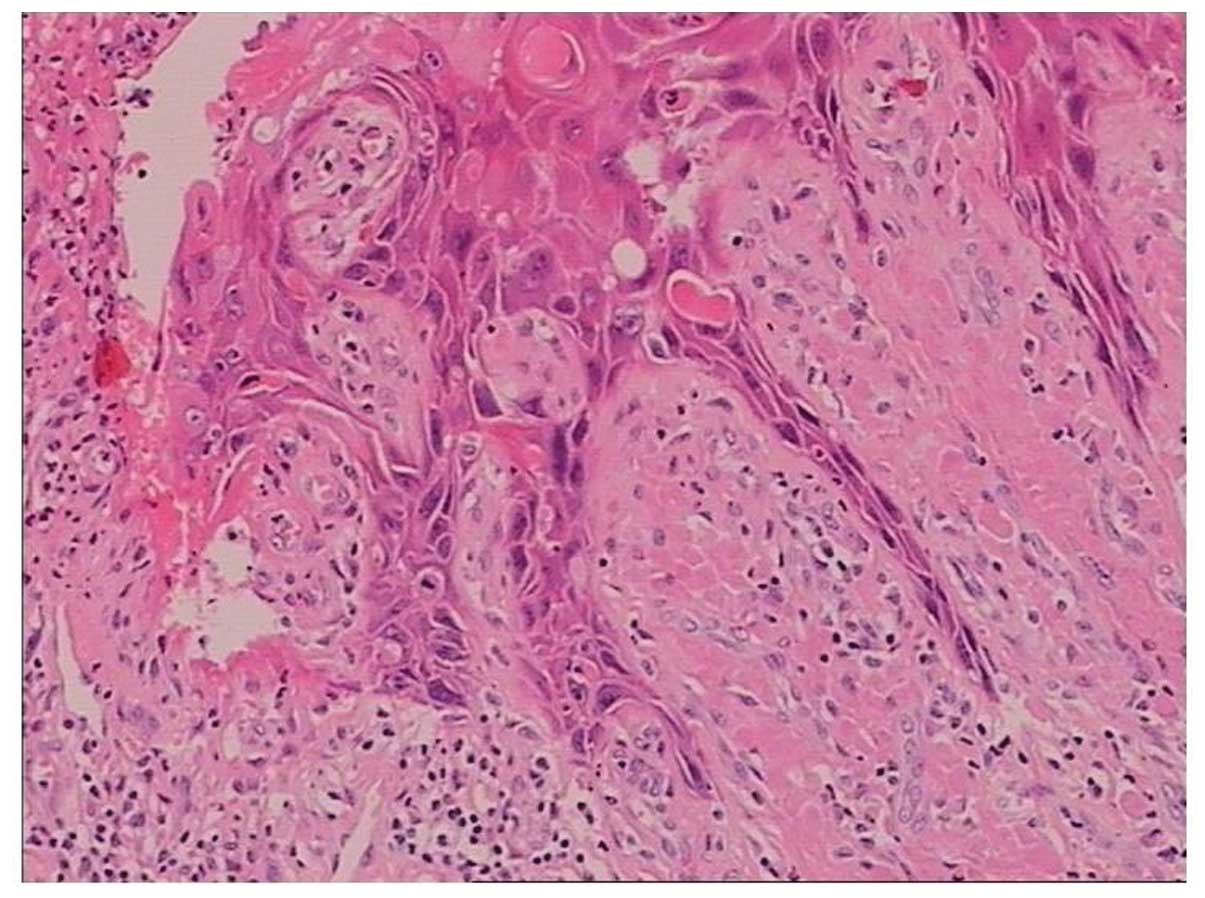
In the present case, the giant condyloma appeared to
exhibit characteristics of a normal condyloma acuminatum and a
superficial planocellular carcinoma. The majority of the material
received for pathology analysis was condyloma accuminatum with
prominent acanthosis, dyskeratosis, hyper-keratosis and prominent
granular layer. In superficial epithelial cells typical perinuclear
cytoplasmic ‘halos’ and pyknotic or slightly enlarged nuclei were
observed and bi-nuclear cells were present. In specific areas
invasive superficial squamous carcinoma was identified with
invasion of the underlaying dermis with small clusters of cells
accompanied with prominent mononuclear inflammatory infiltrate.
Immunohistochemical analysis with p16 monoclonal antibody (clone
E6H4 against p16 protein; CINtec Histology; Roche Diagnostics GmbH,
Mannheim, Germany) revealed positivity in tumour cells. Additional
analysis using Digene Hybrid Capture 2 (Qiagen, Hilden, Germany)
was performed and the presence of HPV genotype 6 and 11 was
confirmed (Fig. 5).
Discussion
Anogenital warts are the most common outcome of HPV
genital infection. Therapeutics against this sexually transmitted
disease are currently associated with low efficacy, due to a 30–70%
recurrence rate identified six months following therapy
administration (9). In rare cases,
anogenital warts develop into extremely large tumour masses leading
to deterioration of patient quality of life. An identified
underlying histopathology of specific cases of giant condyloma is
superficial planocellular carcinoma. Patients with high
susceptibility to local development and fast progression (in growth
and malignancy) and the highest rate of recurrence often exhibit
various types of immunodeficiency. In addition, immunodeficiency
leads to difficulties in evaluation of optimal therapeutic
management. However, patients with no marked immunodeficiency and
treatment-resistent genital warts have been identified.
Furthermore, condyloma lesions occasionally form large exophytic
masses, interfering with intercourse, normal urination, defecation
or vaginal delivery.
Commonly, GCA develops as cauliflower-like masses
and the tumours exhibit histological features of
pseudo-epitheliomatous profileration and local invasion. In the
absence of metastases, they are termed Buschke-Löwenstein tumours.
Due to the aggressive local development of these masses they belong
to the verrucous carcinoma group, although a malignant histological
alteration in the form of micro-invasive carcinoma or
well-differentiated epidermoid keratinising carcinoma has been
reported. Due to the high frequency of local recurrence, radical
surgical excision is the current treatment of choice as topical
preparations and chemotherapy are generally considered ineffective
(12). The method selected for
reconstruction is crucial, particularly in neglected cases similar
to the present case study. Local tissue availability and the
patient’s condition and attitude towards the development of the
disease are major factors for reconstruction with local
fascio-cutaneous flaps. Five years following surgury the present
patient is disease-free with no recurrence.
References
|
1.
|
Brentjens MH, Yeung-Yue KA, Lee PC and
Tyring SK: Human papillomavirus: a review. Dermatol Clin.
20:315–331. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Fleischer AB Jr, Parrish CA, Glenn R and
Feldman SR: Condylomata acuminata (genital warts): patient
demographics and treating physicians. Sex Transm Dis. 28:643–647.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Aubin F, Prétet JL, Jacquard AC, Saunier
M, Carcopino X, Jaroud F, Pradat P, Soubeyrand B, Leocmach Y,
Mougin C and Riethmuller D; EDiTH Study Group: Human papillomavirus
genotype distribution in external acuminata condylomata: a large
French national study (EDiTH IV). Clin Infect Dis. 47:610–615.
2008. View
Article : Google Scholar
|
|
4.
|
Trottier H and Burchell AN: Epidemiology
of mucosal human papillomavirus infection and associated diseases.
Public Health Genomics. 12:291–307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Muñoz N, Bosch FX, de Sanjosé S, Herrero
R, Castellsagué X, Shah KV, Snijders PJ and Meijer CJ;
International Agency for Research on Cancer Multicenter Cervical
Cancer Study Group: Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003.
|
|
6.
|
Koutsky L: Epidemiology of genital human
papillomavirus infection. Am J Med. 102:3–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Mao C, Hughes JP, Kiviat N, Kuypers J, Lee
SK, Adam DE and Koutsky LA: Clinical findings among young women
with genital human papillomavirus infection. Am J Obstet Gynecol.
188:677–684. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Stanley M: Immune responses to human
papillomavirus. Vaccine. 24(Suppl 1): S16–S22. 2006. View Article : Google Scholar
|
|
9.
|
Jablonska S: Traditional therapies for the
treatment of condylomata acuminata (genital warts). Australas J
Dermatol. 39(Suppl 1): S2–S4. 1998.
|
|
10.
|
Frei W: About carcinoma similar pointed
condyloma on the penis. Arch Derm Syph. 160:109–114. 1930.(In
German).
|
|
11.
|
von Krogh G, Lacey CJ, Gross G, Barrasso R
and Schneider A: European course on HPV associated pathology:
guidelines for primary care physicians for the diagnosis and
management of anogenital warts. Sex Transm Infect. 76:162–168.
2000.PubMed/NCBI
|
|
12.
|
Balik E, Eren T and Bugra D: A surgical
approach to anogenital Buschke Loewenstein tumours (giant condyloma
acuminata). Acta Chir Belg. 109:612–616. 2009.PubMed/NCBI
|