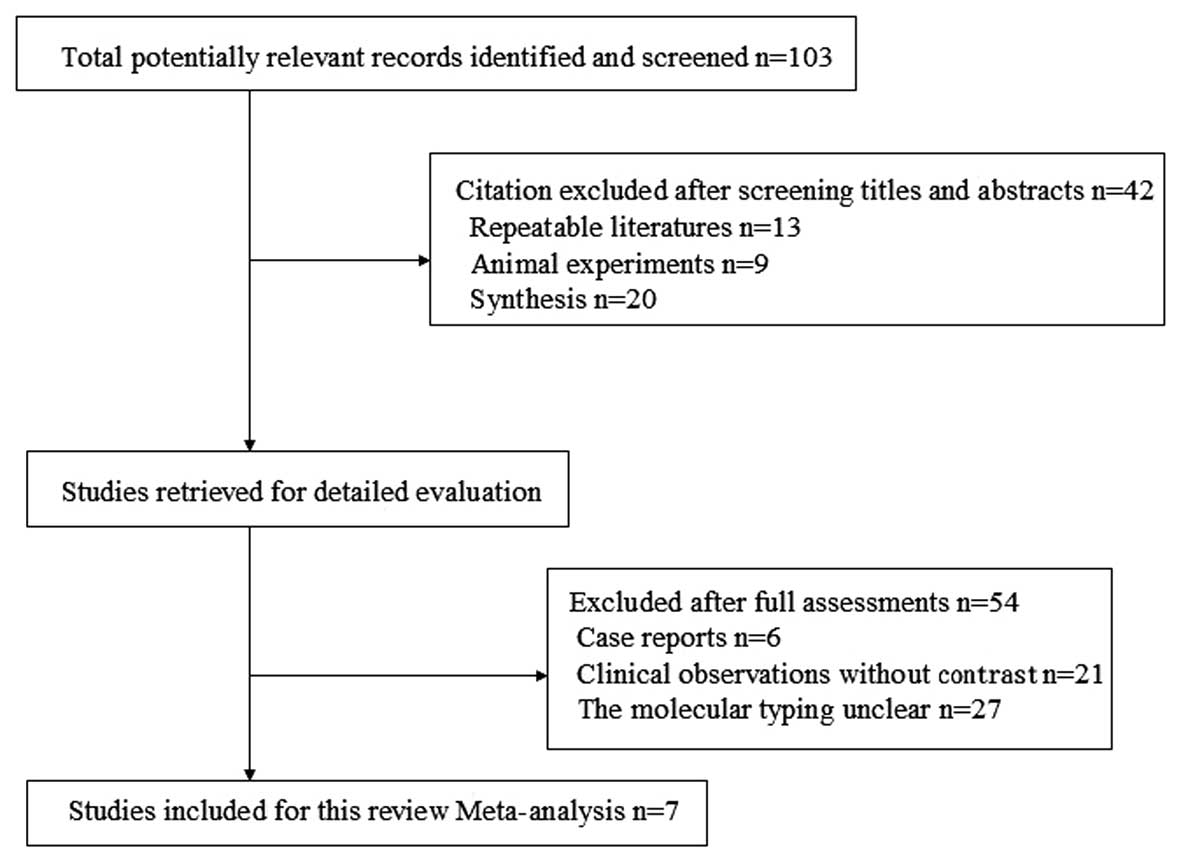
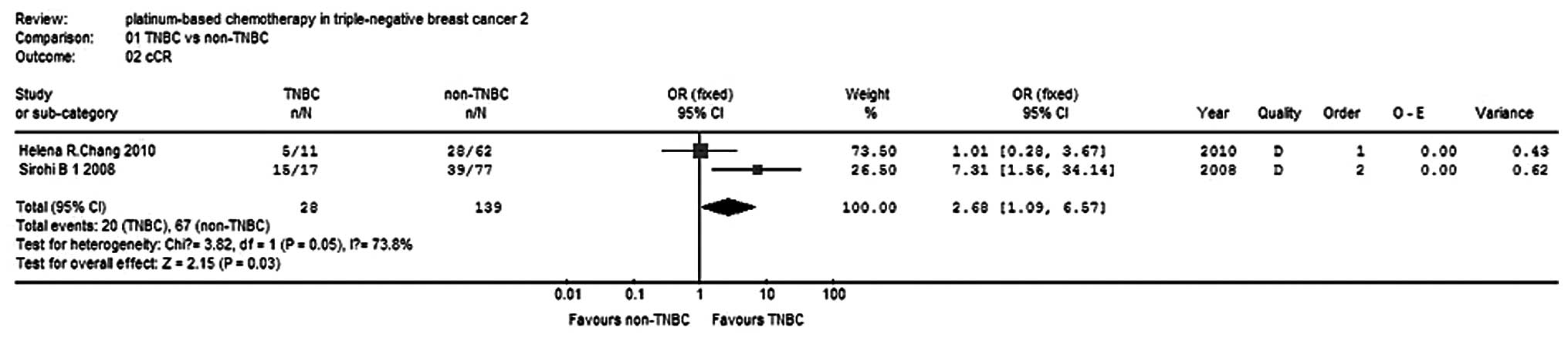
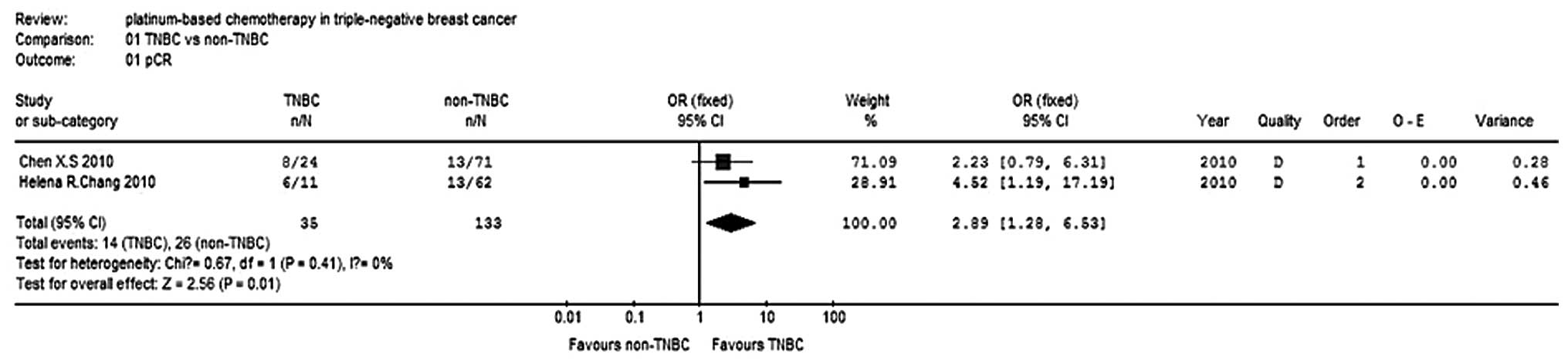
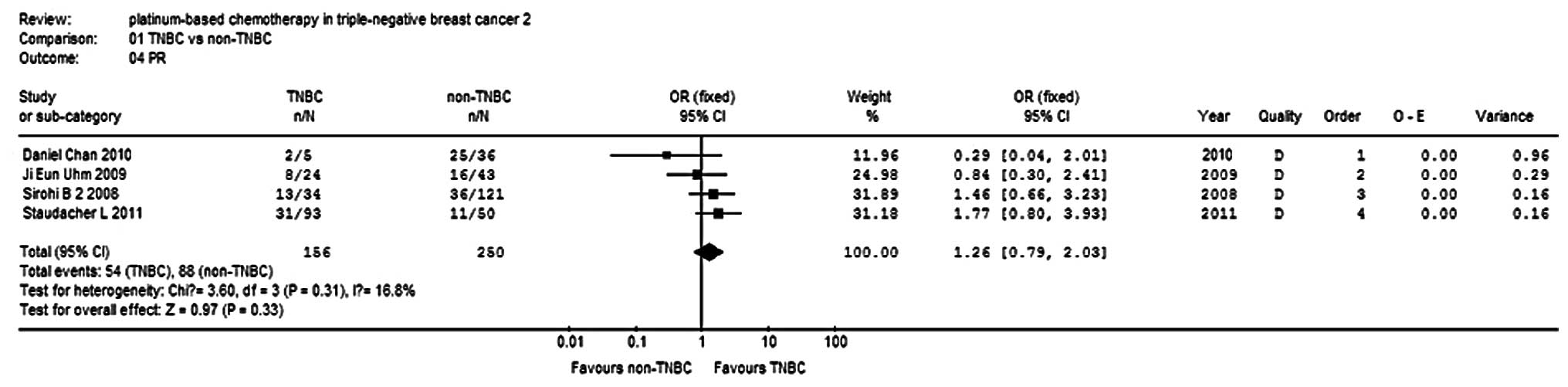
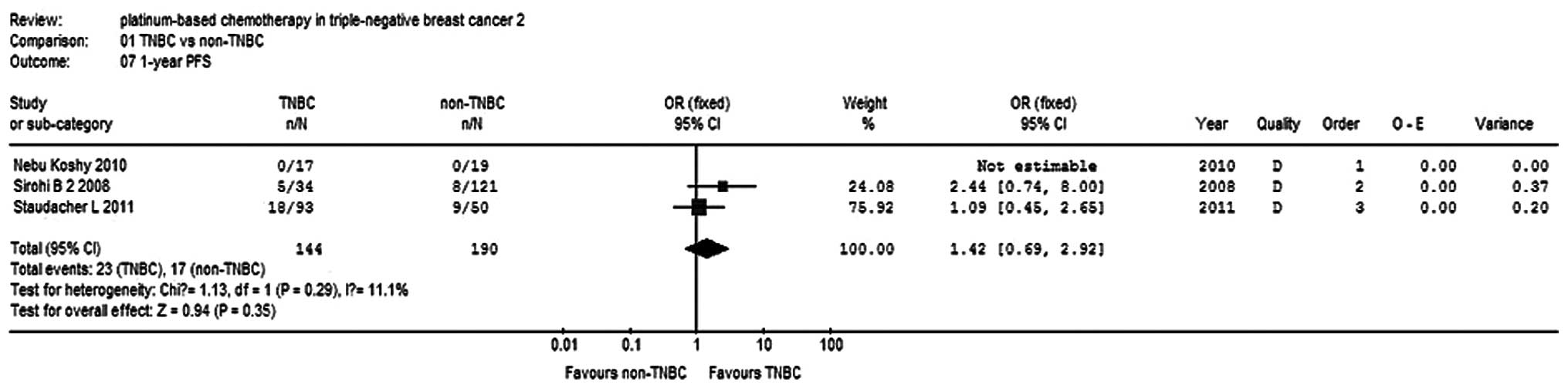
|
1
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, et al: Molecular portraits of human breast
tumours. Nature. 406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turner N, Tutt A and Ashworth A: Hallmarks
of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 4:814–819.
2004.
|
|
3
|
Schneider BP, Winer EP, Foulkes WD, Garber
J, Perou CM, Richardson A, et al: Triple-negative breast cancer:
risk factors to potential targets. Clin Cancer Res. 14:8010–8018.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rouzier R, Perou CM, Symmans WF, Ibrahim
N, Cristofanilli M, Anderson K, et al: Breast cancer molecular
subtypes respond differently to preoperative chemotherapy. Clin
Cancer Res. 11:5678–5685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alli E, Sharma VB, Hartman AR, Lin PS,
McPherson L and Ford JM: Enhanced sensitivity to cisplatin and
gemcitabine in Brca1-deficient murine mammary epithelial cells. BMC
Pharmacol. 11:72011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Silver DP, Richardson AL, Eklund AC, Wang
ZC, Szallasi Z, Li Q, et al: Efficacy of neoadjuvant Cisplatin in
triple-negative breast cancer. J Clin Oncol. 28:1145–1153. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frasci G, D’Aiuto G, Comella P, D’Aiuto M,
Di Bonito M, Ruffolo P, et al: Preoperative weekly cisplatin,
epirubicin, and paclitaxel (PET) improves prognosis in locally
advanced breast cancer patients: an update of the Southern Italy
Cooperative Oncology Group (SICOG) randomised trial 9908. Ann
Oncol. 21:707–716. 2010. View Article : Google Scholar
|
|
8
|
Moher D, Cook DJ, Eastwood S, Olkin I,
Rennie D and Stroup DF: Improving the quality of reports of
meta-analyses of randomised controlled trials: the QUOROM
statement. Quality of Reporting of Meta-analyses. Lancet.
354:1896–1900. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
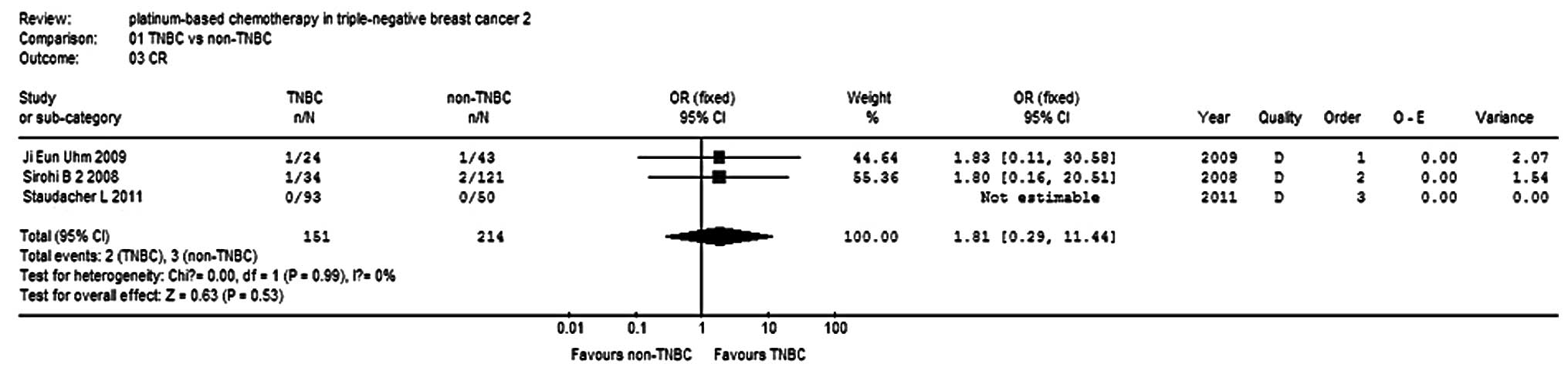
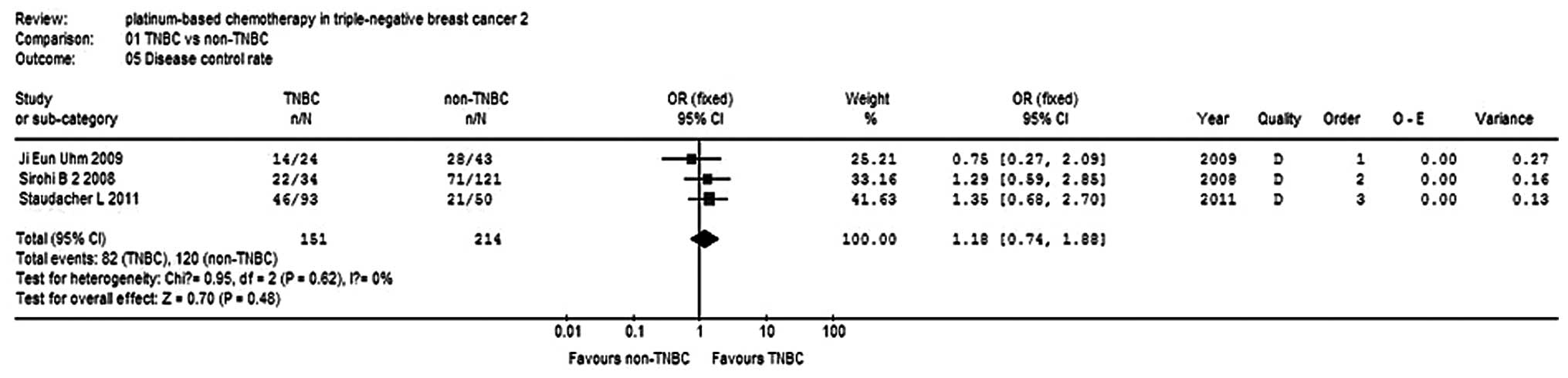
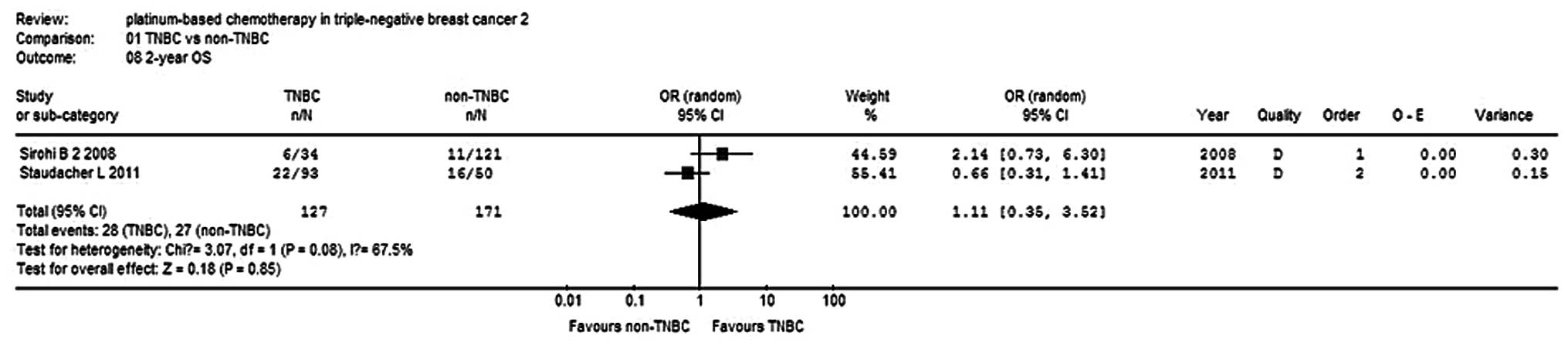
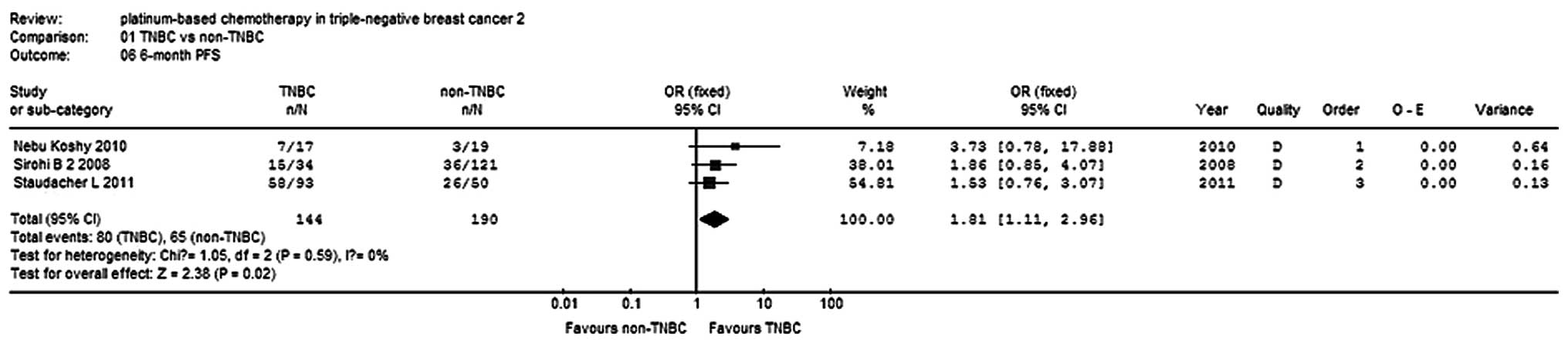
Sirohi B, Arnedos M, Popat S, Ashley S,
Nerurkar A, Walsh G, et al: Platinum-based chemotherapy in
triple-negative breast cancer. Ann Oncol. 19:1847–1852. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang HR, Glaspy J, Allison MA, Kass FC,
Elashoff R, Chung DU and Gornbein J: Differential response of
triple-negative breast cancer to a docetaxel and carboplatin-based
neoadjuvant treatment. Cancer. 116:4227–4237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen XS, Nie XQ, Chen CM, Wu JY, Wu J, Lu
JS, et al: Weekly paclitaxel plus carboplatin is an effective
nonanthracycline-containing regimen as neoadjuvant chemotherapy for
breast cancer. Ann Oncol. 21:961–967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koshy N, Quispe D, Shi R, Mansour R and
Burton GV: Cisplatin-gemcitabine therapy in metastatic breast
cancer: Improved outcome in triple negative breast cancer patients
compared to non-triple negative patients. Breast. 19:246–248. 2010.
View Article : Google Scholar
|
|
13
|
Chan D, Yeo WL, Tiemsim CM, Wong CI, Chuah
B, Soo R, et al: Phase II study of gemcitabine and carboplatin in
metastatic breast cancers with prior exposure to anthracyclines and
taxanes. Invest New Drugs. 28:859–865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uhm JE, Park YH, Yi SY, Cho EY, Choi YL,
Lee SJ, et al: Treatment outcomes and clinicopathologic
characteristics of triple-negative breast cancer patients who
received platinum-containing chemotherapy. Int J Cancer.
124:1457–1462. 2009. View Article : Google Scholar
|
|
15
|
Staudacher L, Cottu PH, Diéras V,
Vincent-Salomon A, Guilhaume MN, Escalup L, et al: Platinum-based
chemotherapy in metastatic triple-negative breast cancer: the
Institut Curie experience. Ann Oncol. 22:848–856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, et al: Response to neoadjuvant therapy and
long-term survival in patients with triple-negative breast cancer.
J Clin Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rouzier R, Pusztai L, Garbay JR, Delaloge
S, Hunt KK, Hortobagyi GN, et al: Development and validation of
nomograms for predicting residual tumor size and the probability of
successful conservative surgery with neoadjuvant chemotherapy for
breast cancer. Cancer. 107:1459–1466. 2006. View Article : Google Scholar : PubMed/NCBI
|