Introduction
Colon cancer is the third and fourth most common
type of cancer in females and males worldwide, respectively, with
>550,000 new cases and ~300,000 mortalities reported in 2008.
Over the past 25 years, approximately one million individuals
worldwide have been diagnosed with colon cancer annually (1). The risk factors of colorectal cancer
are varied and include changes in the bacterial community of the
colon (2).
A total of ~100 trillion bacteria colonize the human
gastrointestinal tract and the colon is estimated to be populated
with 1014 types of bacteria (3). It is generally accepted that these
bacteria are not harmful, but extremely beneficial for the
individual (4), and the modulation
of the bacterial community in the colon presents an interesting
approach to improve health. Furthermore, Rafter (5) reported that probiotic intake may
modulate the bacterial community of the colon and reduce colon
cancer incidence.
Lactobacillus is a type of probiotic which colonizes
the human gastrointestinal tract, and reports have indicated that
certain Lactobacillus strains exhibit tumor-suppressing properties
(6). Lactobacillus
delbrueckii is a strain of Lactobacillus used for the
production of yogurt, a study has shown that the yogurt fermented
by L. delbrueckii reduces the formation of colonic aberrant
crypt foci in transgenic rats (7).
However, whether L. delbrueckii inhibits the growth of colon
cancer and the underlying mechanisms of this process remain
unknown.
Therefore, the aim of the present study was to
evaluate whether the supernatants obtained from L.
delbrueckii fermentation (LBF) have the capacity to inhibit the
proliferation and growth of colon cancer cells, as well as to
investigate the underlying mechanisms of this.
Materials and methods
Preparation of LBF solution
L. delbrueckii was obtained from the American
Type Culture Collection [ATCC (Manassas, VA, USA) and fermented in
de Man, Rogosa and Sharpe medium (Sigma-Aldrich, St. Louis, MO,
USA) at 37°C for 24 h. The supernatant fluid was acquired by
centrifugation (3,469 × g for 5 min) and stored at −20°C as LBF
stock solution.
Cell culture of SW620 cells
The human colon cancer SW620 cell line was obtained
from the ATCC and cultured using L-15 medium (Thermo Labsystems,
Milford, MA, USA) containing 2 mmol/l L-glutamine and 2 g/l sodium
bicarbonate, supplemented with antibiotics (100 U/ml penicillin and
100 mg/ml streptomycin) and 10% fetal bovine serum (FBS) purchased
from Gibco-BRL (Carlsbad, CA, USA)]. The cells were maintained at
37°C in a humidified atmosphere of 5% CO2.
Cell viability assay
The growth inhibitory effect of the LBF solution on
SW620 cells was examined using 3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) assay. The SW620
cells (5×105 cells/ml) were seeded into 96-well plates
and incubated for 24 h. Next, 20 μl LBF solution containing various
concentrations of total protein (0, 0.025, 0.038, 0.05, 0.063,
0.076, 0.1, 0.2, 0.25, 0.4, 0.6, 0.625 and 0.75 mg/ml) was added to
each well. The negative control group was treated with
phosphate-buffered saline (PBS; Thermo Labsystems) buffer. Each
concentration of LBF solution was repeated in five wells. Following
24 h of LBF solution treatment, 20 μl MTT solution (5 mg/ml) was
added into each well and incubated for an additional 4 h.
Subsequently, 100 μl dimethyl sulfoxide was added to each well and
the absorbance values of the wells were measured at a wavelength of
492 nm using a Multiskan Ascent plate reader (Thermo
Labsystems).
Cell cycle analysis and Annexin
V/propidium iodide (PI) staining assay
The SW620 cells (3×106 cells/ml) were
seeded into six-well plates and treated with 0.25 mg/ml LBF
solution for 24 h. Following treatment, the cells were harvested
and washed twice with PBS. For the cell cycle analysis, the cells
were fixed in 70% ethanol overnight at 4°C. The fixed cells were
then stained with PI solution (Sigma-Aldrich), which contained
RNase A, for 45 min in the dark and analyzed by flow cytometry. For
the Annexin V/PI staining assay, the cells were stained with
Annexin V and PI solution for 10 min in the dark and analyzed by
flow cytometry (Becton-Dickinson and Company, Franklin Lakes, NJ,
USA). The untreated cells were used as a negative control.
Immunohistochemistry
The SW620 cells (6×104 cells/ml) were
seeded into six-well plates and treated with 0.25 mg/ml LBF
solution for 24 h. The cell monolayer was fixed and treated with
0.5% Triton X-100 (Sigma-Aldrich) for 20 min and 3%
H2O2 for 15 min. Following blocking with 10%
FBS/PBS, primary mouse anti-human caspase 3 polyclonal antibody and
rabbit anti-human Bcl-2 polyclonal antibody (1:100; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) were added and incubated
overnight at 4°C, followed by incubation with the goat anti-mouse
and goat anti-rabbit, polyclonal, secondary antibody (Santa Cruz
Biotechnology, Inc.) at a dilution of 1:200 for 30 min. The
sections were visualized by 3-3′-diaminobenzidine (Roche
Diagnostics GmbH, Mannheim, Germany) and the untreated cells were
used as a negative control.
Western blot analysis
The SW620 cells treated with 0.25 mg/ml LBF solution
for 24 h were collected by centrifugation at 2,220 × g for 5 min at
4°C. The cells were then lysed in radioimmunoprecipitation assay
buffer (Santa Cruz Biotechnology, Inc.) and 50 μg of total protein
was separated on 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis gels (Sigma-Aldrich) for 2 h. Next, the separated
proteins were transferred onto nitrocellulose membranes (Pall
Corporation, Port Washington, NY, USA) by semi-dry apparatus
(Bio-Rad, Hercules, CA, USA) for 1 h, followed by blocking with 5%
non-fat milk for 1 h. Subsequently, the specific primary mouse
anti-human caspase 3 polyclonal (1:1,000), rabbit anti-human Bcl-2
polyclonal (1:1,000) and mouse anti-human β-actin polyclonal
(1:10,000) antibodies were added at optimized dilutions (Santa Cruz
Biotechnology, Inc.) and incubated overnight at 4°C. Following
incubation with the secondary conjugated with HRP goat anti-mouse
and goat anti-rabbit antibodies (1:10,000) for 1 h, the protein
bands were visualized by an enhanced chemiluminescence kit (western
Blotting Luminol reagent; Santa Cruz Biotechnology, Inc.).
Gelatin zymography assay
The SW620 cells treated with 0.25 mg/ml LBF solution
for 24 h and the culture medium were collected by centrifugation at
555 × g for 5 min at 4°C. Next, 20 μl of cell culture medium was
electrophoresed on non-denaturing 10% polyacrylamide gels
containing 0.1% gelatin (Sigma-Aldrich). Following electrophoresis,
the gels were soaked in 2.5% Triton X-100 for 45 min and then
incubated in substrate buffer (50 mM Tris-HCl, pH 7.5; 5 mM
CaCl2; and 0.02% NaN3) for 18 h.
Subsequently, the gels were stained with 0.05% Coomassie brilliant
blue G250 (Sigma-Aldrich) and destained in 10% acetic acid and 20%
methanol.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were performed using SPSS 11.5 software (SPSS,
Inc., Chicago, IL, USA) and one-way analysis of variance with
Bonferroni’s multiple comparison test was used to evaluate the
differences between the different treatment groups. P<0.01 was
considered to indicate a statistically significant difference.
Results
LBF solution inhibits the proliferation
of colon cancer cells
The inhibitory effect of LBF solution on SW620 cells
was detected by MTT assay for 24 h. Compared with the control
group, treatment with LBF solution was found to significantly
inhibit the cellular proliferation of SW620 cells with an
IC50 value of 0.25 mg/ml after 24 h (P<0.001). The
results showed that cells treated with LBF solution exhibit a
markedly reduced proliferation capacity in a
concentration-dependent manner (P<0.001) (Fig. 1).
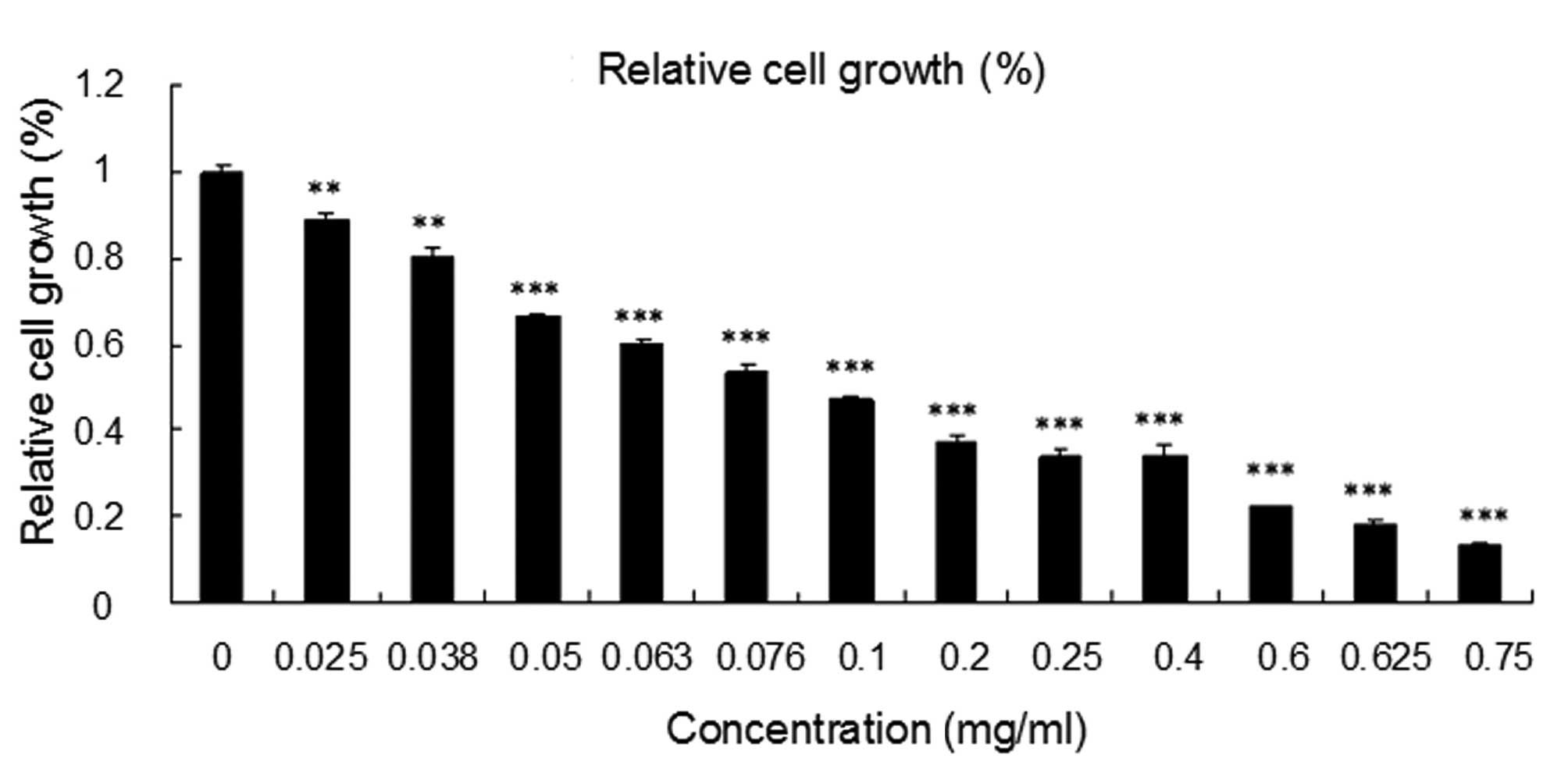 | Figure 1Effect of LBF solution on the
proliferation of colon cancer SW620 cells. The SW620 cells were
treated with various concentrations of LBF solution (0, 0.025,
0.038, 0.05, 0.063, 0.076, 0.1, 0.2, 0.25, 0.4, 0.6, 0.625 and 0.75
mg/ml) for 24 h. Growth is expressed as relative to untreated
control cells. Data are presented as the mean ± SEM, from three
independent experiments. One-way analysis of variance with
Bonferroni’s multiple comparison test was used for statistical
analysis.**P<0.01 and ***P<0.001, vs.
untreated control cells. LBF, Lactobacillus delbrueckii
fermentation. |
Cell cycle arrest and apoptosis induced
by LBF solution treatment
Next, it was investigated whether the LBF solution
inhibits the proliferation of SW620 cells through cell cycle
intervention. The results showed that cells treated with LBF
solution were arrested in the G1 phase and that the ratio of cells
in the G2/M phase was increased compared with that of the control
group (Table I). In addition, the
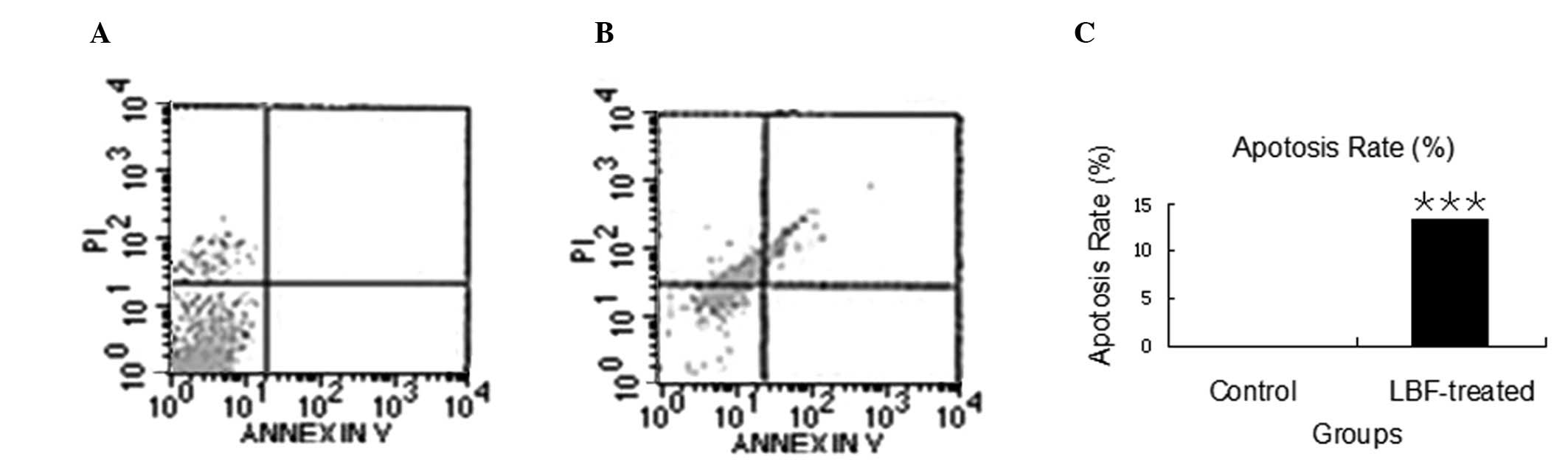
Annexin V assay indicated that the number of apoptotic cells had
increased to 13.26% (P<0.001) in the LBF-treated cells compared
with that in the control group (Fig.
2). These results indicated that the LBF solution treatment
induces cell cycle arrest and apoptosis, which may prevent the
growth of colon cancer cells.
 | Table IEffect of LBF solution on the cell
cycle of SW620 cells. |
Table I
Effect of LBF solution on the cell
cycle of SW620 cells.
| Proportion of cells
in each phase, % |
|---|
|
|
|---|
| Treatment | Sub-G1 | G1 | S | G2-M |
|---|
| Control | 1.97 | 43.90 | 52.48 | 1.65 |
| LBF | 2.04 | 64.72 | 27.85 | 5.39 |
LBF solution induces caspase-dependent
apoptosis
The extrinsic and intrinsic apoptotic pathways have
been well recognized as major mechanisms of cell death in the
majority of cellular systems (8).
Therefore, since the results of the current study have shown that
the LBF solution induces apoptosis, it was next investigated
whether the apoptosis is caspase driven. The expression of caspase
8 was detected in the cells exposed to the LBF solution and the
results showed that the LBF solution did not induce caspase 8
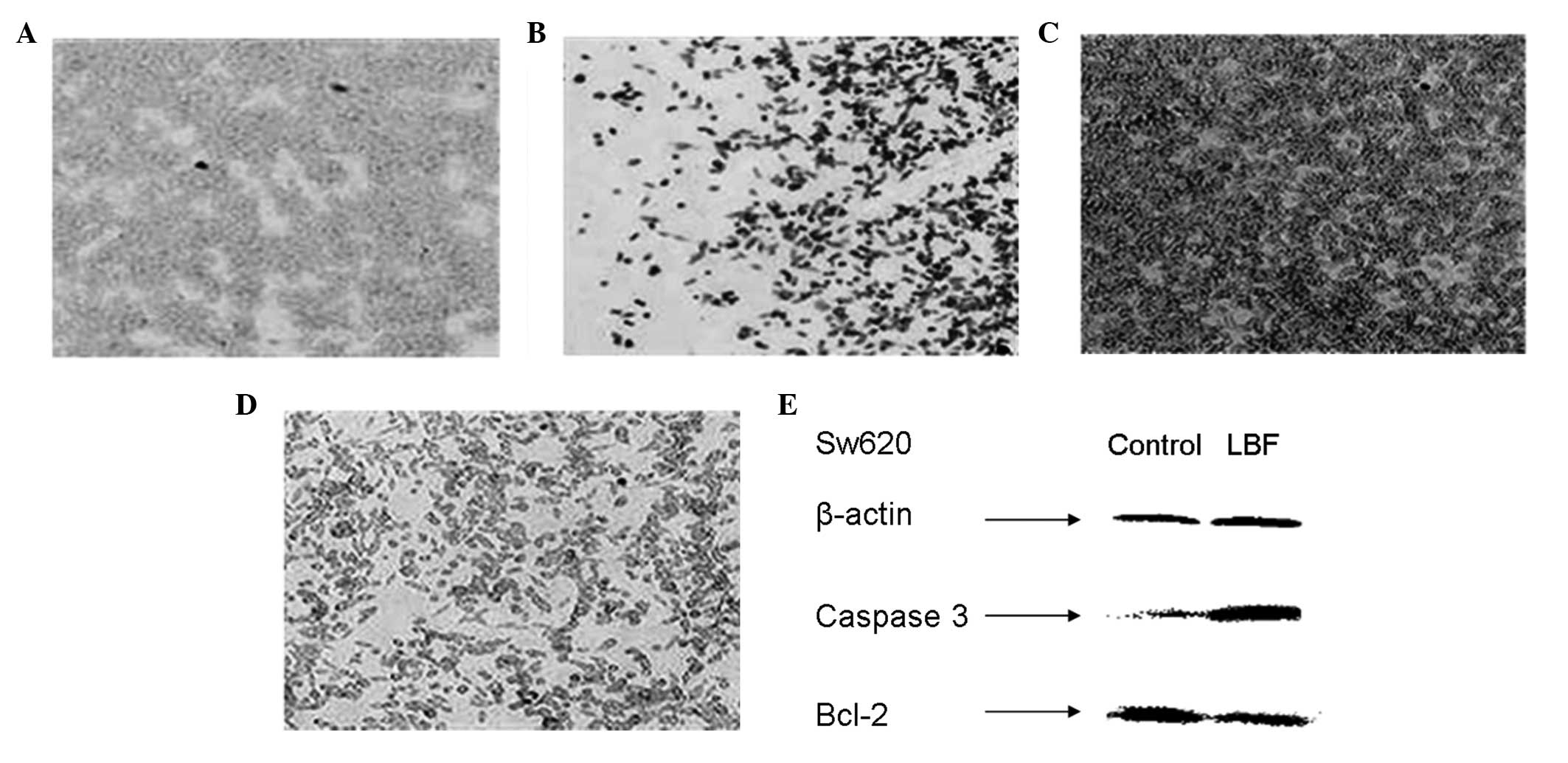
activation (data not shown). However, the caspase 3 expression in
the colon cells treated with LBF solution was found to increase in
the immunohistochemistry assay (Fig. 3A
and B). This observation was confirmed by western blot analysis
(Fig. 3F) and suggested that the
LBF solution induces apoptosis through the caspase 3 intrinsic
apoptotic pathway.
Bcl-2 protects cells from apoptosis by preventing
the release of mitochondrial cytochrome c, therefore, Bcl-2
prevents a caspase 3-dependent proteolytic cascade (9). Furthermore, Bcl-2 expression was
detected in the colon cells exposed to the LBF solution. The
results indicated that LBF solution decreases Bcl-2 expression,
which is likely to contribute to the activation of the caspase 3
intrinsic apoptotic pathway (Fig. 3C, D
and F).
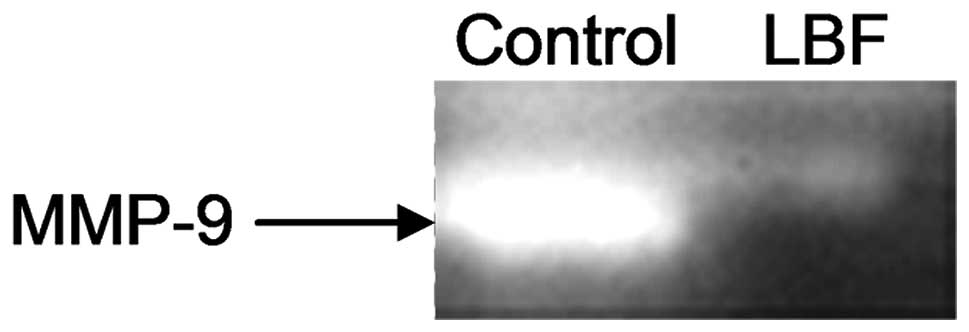
LBF solution inhibits matrix
metalloproteinase (MMP)-9 activity
MMPs are overexpressed in various types of cancer
and have been associated with tumor invasion due to their capacity
to degrade the basement membrane (10). Furthermore, numerous reports have
the shown potent invasive activities of several MMPs, including
MMP-9 (11). To investigate whether
the LBF solution affects the invasive potential of colon cancer
cells, MMP activity was evaluated by gelatin zymography assay. The
results showed that MMP-9 activity was markedly decreased in cells
treated with the LBF solution compared with that of the untreated
sample (Fig. 4). This suggested
that the LBF solution not only inhibits the proliferation of colon
cancer cells, but also antagonizes invasion.
Discussion
Colon cancer is one of the most common types of
cancer in western industrialized countries and environmental
factors are significantly involved in its development (12). Certain epidemiological studies have
shown a decreased incidence of colon cancer in individuals
consuming fermented milk products or yogurt (13). L. delbrueckii is usually used
for yogurt fermentation; however, whether LBF solution may prevent
the growth of colon cancer, and the precise mechanisms underlying
this process, are yet to be thoroughly understood. Therefore, the
aim of the present study was to investigate the effects of LBF
solution on colon cancer SW620 cells in vitro.
The results of the present study indicated that the
LBF solution causes growth inhibition and induces G1 phase arrest
in SW620 cells. Reports have also indicated that the administration
of other Lactobacillus strains, such as L. fermentum or
L. plantarum may inhibit colon cancer formation in mouse
models (14). In addition, studies
have shown that yogurt consisting of milk fermented by the L.
delbrueckii subsp. bulgaricus strain 2038 and Streptococcus
salivarius subsp. thermophilus strain 1131 reduced the number
and size of colon tumors in RasH2 mice (7).
The present study further investigated the
mechanisms of the inhibitory effect of the LBF solution on the
growth of colon cancer cells. The observations revealed that the
LBF solution inhibits the proliferation of SW620 cells by
triggering apoptosis. This insight into the molecular mechanisms of
the solution indicated that the LBF solution may significantly
increase caspase 3 expression with a markedly reduced expression of
Bcl-2, a potent anti-apoptotic protein, in colon cancer SW620
cells. However, the exact compounds in the LBF solution which are
responsible for the apoptotic effects on the colon cancer cells
remain unclear.
Reports on the role and/or effect of probiotics in
the later stages of colon cancer, specifically metastasis, are
limited. However, MMP-9, which degrades the basement collagen
membrane, has been reported to be a particularly important
proteolytic enzyme involved in colon cancer cell invasion (15). The present study revealed that the
LBF solution decreases the MMP-9 protein activity in colon cancer
cells. In addition, a recent study revealed a similar result in the
cell-free supernatant obtained from probiotic L. caser and
L. rhamnosus GG that may decrease MMP-9 activity and inhibit
colon cancer invasion (16).
In conclusion, the results of the present study are
the first to indicate that the fermentation supernatants of the
L. delbrueckii strain may efficiently inhibit proliferation
and induce apoptosis through the caspase 3-dependent pathway in
colon cancer cells. In addition, the fermentation supernatants of
the L. delbrueckii strain were found to decrease MMP-9
activity in SW620 cells, which may prevent the invasion of colon
cancer cells. Unlike chemotherapy, the intake of probiotics are
less likely to cause side effects. Therefore, we hypothesize that
the use of L. delbrueckii, as described in the present
study, for the treatment of colon cancer may be beneficial to
patients.
Acknowledgements
The authors would like to thank Dr Lawrence Owusu
for critically reviewing the study. The study was kindly supported
by a grant from the National Basic Research Program of China (973
project; grant no. 2007CB513006).
Abbreviations:
|
LBF
|
Lactobacillus delbrueckii
fermentation
|
|
FBS
|
fetal bovine serum
|
|
PI
|
propidium iodide
|
|
PBS
|
phosphate-buffered saline
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide
|
|
MMPs
|
matrix metalloproteinases
|
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Watson AJ and Collins PD: Colon cancer: a
civilization disorder. Dig Dis. 29:222–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tjalsma H, Boleij A, Marchesi JR, et al: A
bacterial driver-passenger model for colorectal cancer: beyond the
usual suspects. Nat Rev Microbiol. 10:575–582. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Antonic V, Stojadinovic A, Kester KE, et
al: Significance of infectious agents in colorectal cancer
development. J Cancer. 4:227–240. 2013. View Article : Google Scholar
|
|
5
|
Rafter J: Probiotics and colon cancer.
Best Pract Res Clin Gastroenterol. 17:849–859. 2003. View Article : Google Scholar
|
|
6
|
Rafter J: Lactic acid bacteria and cancer:
mechanistic perspective. Br J Nutr. 88(Suppl 1): S89–S94. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Narushima S, Sakata T, Hioki K, et al:
Inhibitory effect of yogurt on aberrant crypt foci formation in the
rat colon and colorectal tumorigenesis in RasH2 mice. Exp Anim.
59:487–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fulda S and Debatin KM: Targeting
apoptosis pathways in cancer therapy. Curr Cancer Drug Targets.
4:569–576. 2004. View Article : Google Scholar
|
|
9
|
Yang J, Liu X, et al: Prevention of
apoptosis by Bcl-2: release of cytochrome c from mitochondria
blocked. Science. 275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stetler-Stevenson WG and Yu AE: Proteases
in invasion: matrix metalloproteinases. Semin Cancer Biol.
11:143–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Decock J, Thirkettle S, et al: Matrix
metalloproteinases: protective roles in cancer. J Cell Mol Med.
15:1254–1265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanavos P: The rising burden of cancer in
the developing world. Ann Oncol. 17(Suppl 8): viii15–viii23. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wollowski I, Rechkemmer G and Pool-Zobel
BL: Protective role of probiotics and prebiotics in colon cancer.
Am J Clin Nutr. 73(Suppl 2): S451–S455. 2001.PubMed/NCBI
|
|
14
|
Asha and Gayathri D: Synergistic impact of
Lactobacillus fermentum, Lactobacillus plantarum and
vincristine on 1,2-dimethylhydrazine-induced colorectal
carcinogenesis in mice. Exp Ther Med. 3:1049–1054. 2012.
|
|
15
|
Zucker S and Vacirca J: Role of matrix
metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis
Rev. 23:101–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Escamilla J, Lane MA and Maitin V:
Cell-free supernatants from probiotic Lactobacillus casei
and Lactobacillus rhamnosus GG decrease colon cancer cell
invasion in vitro. Nutr Cancer. 64:871–878. 2012.PubMed/NCBI
|