Introduction
Lung cancer is one of the most frequently diagnosed
types of cancer and the leading cause of cancer-associated
mortality worldwide (1). Despite
the development and use of multimodality therapies, including
surgery, radiotherapy, conventional chemotherapy and molecular
targeted therapy, the clinical outcome of lung cancer treatment
remains unsatisfactory, with a five-year overall survival rate of
<15% (2). This may be, at least
in part, due to the side-effects associated with currently
available chemotherapeutic drugs and the resistance of advanced
lung cancer (3). Therefore, novel,
effective chemotherapeutic agents are required, particularly those
that are derived from natural products due to their intrinsic
advantages (4–6).
Daphnoretin (Fig. 1)
is an active constituent of Wikstroemia indica C.A. Mey.
which belongs to the Thymelaceae family and is widely distributed
throughout the northwest and southwest regions of China. The root
of this plant is used as a remedy for arthritis, tuberculosis,
syphilis and pertussis (7).
Previous studies have shown that daphnoretin has a number of
biological activities, including antifungal effects (8), as well as the inhibition of various
sites involved in DNA synthesis (9), the activation of protein kinase C in
platelet aggregation (7,10), an antiviral effect on hepatitis B
(11) and respiratory syncytial
virus properties (12).
Furthermore, the anticancer effect of daphnoretin has been reported
in various studies (13–15). Such studies have shown that
daphnoretin exerts its anticancer effects through the inhibition of
cancer cell proliferation, the induction of G2/M-phase
arrest and apoptosis. However, the effect of daphnoretin on human
lung cancer cells has yet to be elucidated.
The present study aimed to investigate the effect of
daphnoretin on the growth of A549 lung cancer cells and the
cellular mechanism involved in daphnoretin-induced apoptosis. The
findings of the present study suggest that daphnoretin may have
potential as an anticancer agent for lung cancer therapy.
Materials and methods
Reagents and chemicals
Daphnoretin was purchased from the National
Institute for the Control of Pharmaceutical and Biological Products
(Beijing, China) and a 1-mmol/l stock solution of daphnoretin was
prepared in dimethyl sulfoxide (DMSO) and stored at −20°C.
Deionized water was used in all of the experiments. Fetal bovine
serum (FBS) was purchased from Solarbio Science and Technology Co.,
Ltd. (Beijing, China), and
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
Hoechst 33342 and DMSO were purchased from Sigma-Aldrich (St.
Louis, MO, USA). An Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) double-staining kit was purchased from
KeyGene Inc. (Nanjing, China). Mouse monoclonal anti-human,
anti-mouse and anti-rat antibodies against Bcl-2-associated X
protein (Bax) (catalogue number, sc-23959), B-cell lymphoma (Bcl)-2
(catalogue number, sc-7382) and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH; catalogue number, sc-365062) were obtained
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The
secondary polyclonal horseradish peroxidase-conjugated goat
anti-mouse and anti-rabbit antibodies were also obtained from Santa
Cruz Biotechnology, Inc. All other reagents were obtained from
Sinopharm Chemical Reagent Co., Ltd. (Shenyang, China).
Cell culture
The A549 human lung cancer cell line was obtained
from the China Center for Type Culture Collection (Wuhan, China)
and maintained in RPMI-1640 supplemented with 10% FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin at 37°C in a humidified
atmosphere of 5% CO2.
MTT assay
The effect of daphnoretin on the proliferation of
A549 cells was measured using MTT assay. In brief, A549 cells were
plated at a density of 1×104 cells per well on 96-well
plates overnight, then treated with various concentrations of
daphnoretin (0, 5, 10, 15 and 20 μmol/l) for 24 and 48 h. A total
of 20 μl MTT solution [2 mg/ml in phosphate-buffered saline (PBS)]
was added to each well and the cells were cultured for 4 h at 37°C.
The medium was then removed and 150 μl DMSO was added to solubilize
the MTT formazan crystals. The plates were then agitated and the
optical density was determined at 570 nm using an ELISA plate
reader (Model 550; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
At least three independent experiments were performed.
Fluorescence microscopy
A549 cells (1×106) were seeded on
six-well plates overnight, then treated with different
concentrations of daphnoretin (0 and 10 μmol/l) for 24 h. The cells
were washed twice with cold PBS, fixed with cold methanol and
acetic acid (3/1, v/v) for 30 min and then stained with Hoechst
33342 (1 mg/ml) for 30 min in the dark. The stained cells were
observed using a fluorescence microscope (magnification, ×400;
Nikon E800; Nikon Corporation, Tokyo, Japan).
Flow cytometric analysis
The apoptotic rates of the A549 cells were
determined using flow cytometric analysis using an Annexin V-FITC
apoptosis kit. In brief, A549 cells (1×106) were seeded
on six-well plates overnight, then treated with various
concentrations of daphnoretin (0, 5, 10 and 15 μmol/l) for 24 h.
Cells (1×106) were then harvested using centrifugation
(326 × g) for 5 min and washed twice with cold PBS. Staining was
performed according to the manufacturer’s instructions (KeyGene
Inc.) and the cells were analyzed using a FACScan flow cytometer
(Becton-Dickinson, San Jose, CA, USA). At least three independent
experiments were performed.
Western blot analysis
The expression of apoptosis-related proteins was
assessed using western blot analysis. In brief, A549 cells
(1×106) were seeded on six-well plates overnight, then
treated with various concentrations of daphnoretin (0, 5, 10 and 15
μmol/l). Following treatment for 24 h, the total proteins were
solubilized and extracted using lysis buffer (20 mM HEPES, pH 7.9,
20% glycerol, 200 mM KCl, 0.5 mM EDTA, 0.5% NP-40, 0.5 mM
dithiothreitol and 1% protease inhibitor cocktail). The protein
concentration was determined using a bicinchoninic acid protein
assay. All samples were separated using SDS-PAGE to determine the
protein expression of Bax, Bcl-2 and GAPDH. Blots were developed
using an enhanced chemiluminescence kit.
Statistical analysis
Statistical analyses were performed using the SPSS
13.0 package (SPSS, Inc., Chicago, IL, USA). All experiments were
performed at least three times. All data are expressed as the mean
± standard deviation. The statistical correlations of the data were
tested for significance using analysis of variance and the
Student’s t-test. P<0.05 and P<0.01 were considered to
indicate statistically significant differences.
Results
Daphnoretin inhibits A549 cell
proliferation
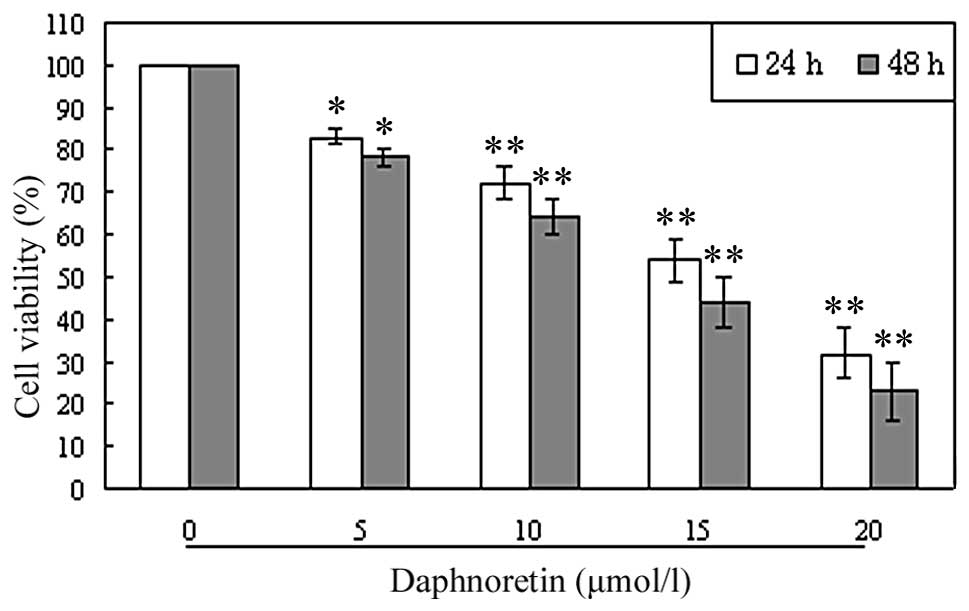
To investigate the growth-inhibiting effect of
daphnoretin, A549 cells were treated with various concentrations of
A549 for 24 and 48 h, and the rate of inhibition was determined
using MTT assay. As shown in Fig.
2, A549 cell growth was observed to be inhibited in a
concentration- and time-dependent manner.
Daphnoretin induces A549 cell
apoptosis
To investigate the apoptosis-inducing effect of
daphnoretin, A549 cells were treated with various concentrations of
daphnoretin. Following treatment with daphnoretin (0 and 10 μmol/l)
for 24 h, cells were analyzed using fluorescent microscopy with
Hoechst 33324 staining. As shown in Fig. 3, chromatin condensation, nuclear
fragmentation and apoptotic bodies were observed in the treated
cells. The results revealed that when exposed to daphnoretin, A549
cells underwent the typical morphological changes that are
associated with apoptosis.
The ratio of apoptotic cells induced by daphnoretin
was measured using flow cytometry. A549 cells were treated with
various concentrations of daphnoretin (0, 5, 10 and 15 μmol/l) for
24 h and analyzed using flow cytometry with Annexin V and PI
staining. As shown in Fig. 4, the
ratio of early and late apoptotic cells was observed to be
significantly increased in the daphnoretin-treated cells compared
with the cells in the control group. The results show that when
treated with daphnoretin for 24 h, the ratio of apoptotic cells
significantly increased in a concentration-dependent manner.
Effect of daphnoretin on Bcl-2 family
gene expression
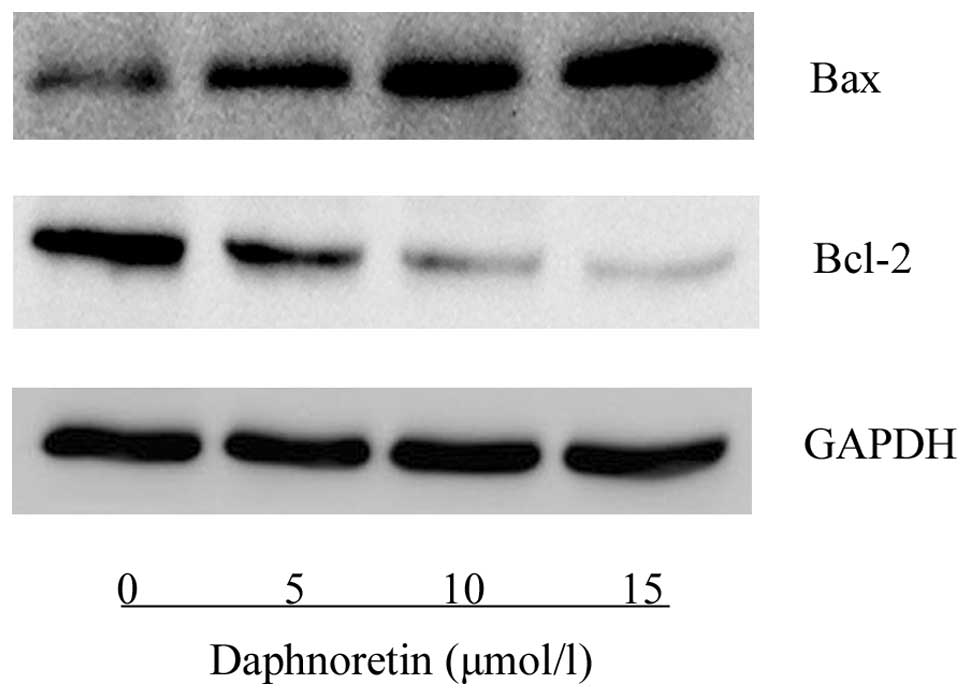
The expression of apoptosis-related proteins was
assessed using western blot analysis. As shown in Fig. 5, daphnoretin treatment induced an
increase in Bax protein expression and a reduction in Bcl-2 protein
expression compared with the control cells. The ratio of Bax to
Bcl-2 was observed to increase in a concentration-dependent
manner.
Discussion
Increasing research attention has been focused on
phytochemicals in the search for novel anticancer agents with
enhanced efficacy to cancer cells and reduced toxicity to normal
cells. Daphnoretin, isolated from Wikstroemia indica C.A.
Mey. (16) as well as Daphne
mezereum L. and Daphne cannabina Wall. (17), have been shown to induce cell cycle
arrest and apoptosis in leukemia, osteosarcoma and HeLa cells
(13–15). These findings suggest that
daphnoretin may have potential as a novel anti-cancer agent.
In the present study, the results demonstrated that
daphnoretin inhibited the growth of A549 lung cancer cells in a
concentration- and time-dependent manner. A549 cells treated with
daphnoretin exhibited typical apoptotic characteristics, including
cytoplasmic shrinkage, plasma membrane blebbing, nuclear chromatin
condensation, chromosomal DNA cleavage and fragmentation of the
cells into membrane-enclosed vesicles or apoptotic bodies. Flow
cytometric analysis revealed that daphnoretin induced A549 cell
apoptosis in a concentration-dependent manner. Moreover, Bax/Bcl-2
were found to be involved in the molecular mechanism of
daphnoretin-induced apoptosis in A549 cells.
Apoptosis is a type of programmed cell death which
occurs through the activation of intrinsic cell suicide pathways
(18). Apoptosis is a hallmark of
anticancer drug-induced cell death (19). Proteins of the Bcl-2 family are key
regulators of the apoptotic pathway (20,21).
The Bcl-2 gene family, which is significantly involved in the
regulation of cell apoptosis, includes anti-apoptotic genes,
including Bcl-2 and Bcl-extra large and pro-apoptotic genes
including, Bax, Bcl2-antagonist/killer, Bcl-2-interacting killer,
BH3 interacting-domain death agonist and Bcl2-associated agonist of
cell death (22,23). Certain traditional Chinese
anticancer drugs have been found to induce cell apoptosis through
targeting the proteins of the Bcl-2 family and the ratio of
Bax/Bcl-2 (24,25). In the present study, western blot
analysis revealed that the daphnoretin-induced apoptosis in the
A549 cells was mediated through the downregulation of Bcl-2 protein
expression and the upregulation of Bax protein expression.
In conclusion, the present study has demonstrated
that daphnoretin is capable of inhibiting proliferation and
promoting apoptosis in A549 lung cancer cells. Furthermore, this
apoptotic response is associated with the regulation of the
expression of the Bcl-2 gene family. These findings indicate that
daphnoretin may have potential as a therapeutic agent for the
management of lung cancer.
Acknowledgements
The present study was supported by the Outstanding
Scientific Fund of Shengjing Hospital (grant no. 201205).
References
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): a population-based study. Lancet
Oncol. 13:790–801. 2012.
|
|
2
|
Erridge SC, Møller H, Price A and Brewster
D: International comparisons of survival from lung cancer: pitfalls
and warnings. Nat Clin Pract Oncol. 4:570–577. 2007.
|
|
3
|
Li S, Bao P, Li Z, et al: Inhibition of
proliferation and apoptosis induced by a
Na+/H+ exchanger-1 (NHE-1) antisense gene on
drug-resistant human small cell lung cancer cells. Oncol Rep.
21:1243–1249. 2009.
|
|
4
|
Hsu SC, Ou CC, Chuang TC, et al: Ganoderma
tsugae extract inhibits expression of epidermal growth factor
receptor and angiogenesis in human epidermoid carcinoma cells: In
vitro and in vivo. Cancer Lett. 281:108–116. 2009.
|
|
5
|
Su CC and Lin YH: Tanshinone IIA
down-regulates the protein expression of ErbB-2 and up-regulates
TNF-alpha in colon cancer cells in vitro and in vivo.
Int J Mol Med. 22:847–851. 2008.
|
|
6
|
Carter BZ, Mak DH, Schober WD, et al:
Triptolide sensitizes AML cells to TRAIL-induced apoptosis via
decrease of XIAP and p53-mediated increase of DR5. Blood.
111:3742–3750. 2008.
|
|
7
|
Ko FN, Chang YL, Kuo YH, Lin YL and Teng
CM: Daphnoretin, a new protein kinase C activator isolated from
Wikstroemia indica C.A. Mey. Biochem J. 295:321–327.
1993.
|
|
8
|
Hu K, Kobayashi H, Dong A, Iwasaki S and
Yao X: Antifungal, antimitotic and anti-HIV-1 agents from the roots
of Wikstroemia indica. Planta Med. 66:564–567. 2000.
|
|
9
|
Hall IH, Tagahara K and Lee KH: Antitumor
agents LIII: The effects of daphnoretin on nucleic acid and protein
synthesis of Ehrlich ascites tumor cells. J Pharm Sci. 71:741–744.
1982.
|
|
10
|
Wang JP, Raung SL, Kuo YH and Teng CM:
Daphnoretin-induced respiratory burst in rat neutrophils is,
probably, mainly through protein kinase C activation. Eur J
Pharmacol. 288:341–348. 1995.
|
|
11
|
Chen HC, Chou CK, Kuo YH and Yeh SF:
Identification of a protein kinase C (PKC) activator, daphnoretin,
that suppresses hepatitis B virus gene expression in human hepatoma
cells. Biochem Pharmacol. 52:1025–1032. 1996.
|
|
12
|
Ho WS, Xue JY, Sun SS, Ooi VE and Li YL:
Antiviral activity of daphnoretin isolated from Wikstroemia
indica. Phytother Res. 24:657–661. 2010.
|
|
13
|
Lee KH, Tagahara K, Suzuki H, et al:
Antitumor agents. 49 tricin, kaempferol-3-O-beta-D-glucopyranoside
and (+)-nortrachelogenin, antileukemic principles from
Wikstroemia indica. J Nat Prod. 44:530–535. 1981.
|
|
14
|
Gu S and He J: Daphnoretin induces cell
cycle arrest and apoptosis in human osteosarcoma (HOS) cells.
Molecules. 17:598–612. 2012.
|
|
15
|
Yang ZY, Kan JT, Cheng ZY, et al:
Daphnoretin-induced apoptosis in HeLa cells: a possible
mitochondria-dependent pathway. Cytotechnology. 66:51–61. 2014.
|
|
16
|
Chen CC, Lin YC, Chen YP and Hsu HYJ: A
study on the constituents of Wikstroemia indica C.A. Mey.
Taiwan Pharm Assoc. 33:28–29. 1981.
|
|
17
|
Majumder PL and Sengupta GCJ: Chemical
Investigation of Daphne cannabina Wall. J Indian Chem Soc.
45:1058–1062. 1968.
|
|
18
|
Xiao R, Ferry AL and Dupont-Versteegden
EE: Cell death-resistance of differentiated myotubes is associated
with enhanced anti-apoptotic mechanisms compared to myoblasts.
Apoptosis. 16:221–234. 2011.
|
|
19
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011.
|
|
20
|
Jendrossek V: The intrinsic apoptosis
pathways as a target in anticancer therapy. Curr Pharm Biotechnol.
13:1426–1438. 2012.
|
|
21
|
Ott M, Norberg E, Zhivotovsky B and
Orrenius S: Mitochondrial targeting of tBid/Bax: a role for the TOM
complex? Cell Death Differ. 16:1075–1082. 2009.
|
|
22
|
Robertson JD and Orrenius S: Molecular
mechanisms of apoptosis induced by cytotoxic chemicals. Crit Rev
Toxicol. 30:609–627. 2000.
|
|
23
|
Tamm I, Schriever F and Dörken B:
Apoptosis: implications of basic research for clinical oncology.
Lancet Oncol. 2:33–42. 2001.
|
|
24
|
Li SG, Wang YY, Ye ZY, et al:
Proliferative and apoptotic effects of andrographolide on the
BGC-823 human gastric cancer cell line. Chin Med J (Engl).
126:3739–3744. 2013.
|
|
25
|
Yu J, Zhou X, He X, Dai M and Zhang Q:
Curcumin induces apoptosis involving bax/bcl-2 in human hepatoma
SMMC-7721 cells. Asian Pac J Cancer Prev. 12:1925–1929. 2011.
|