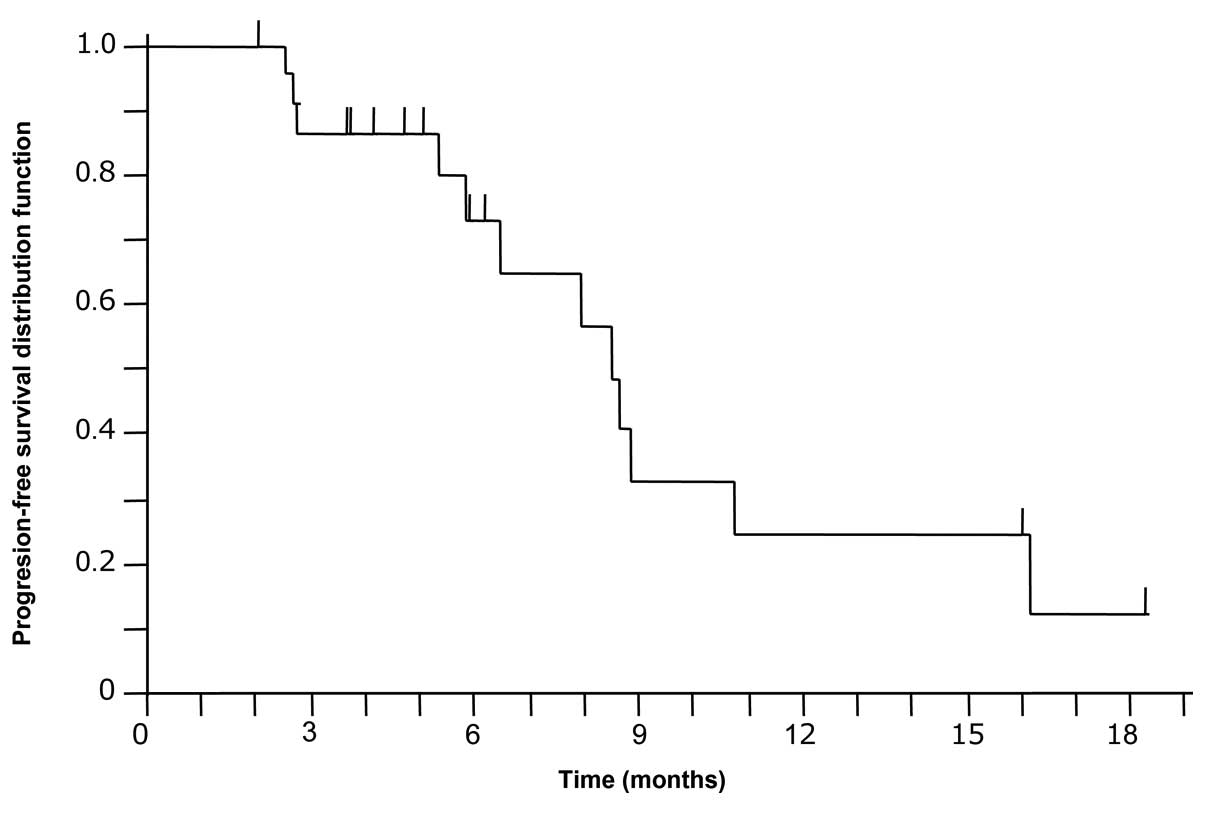
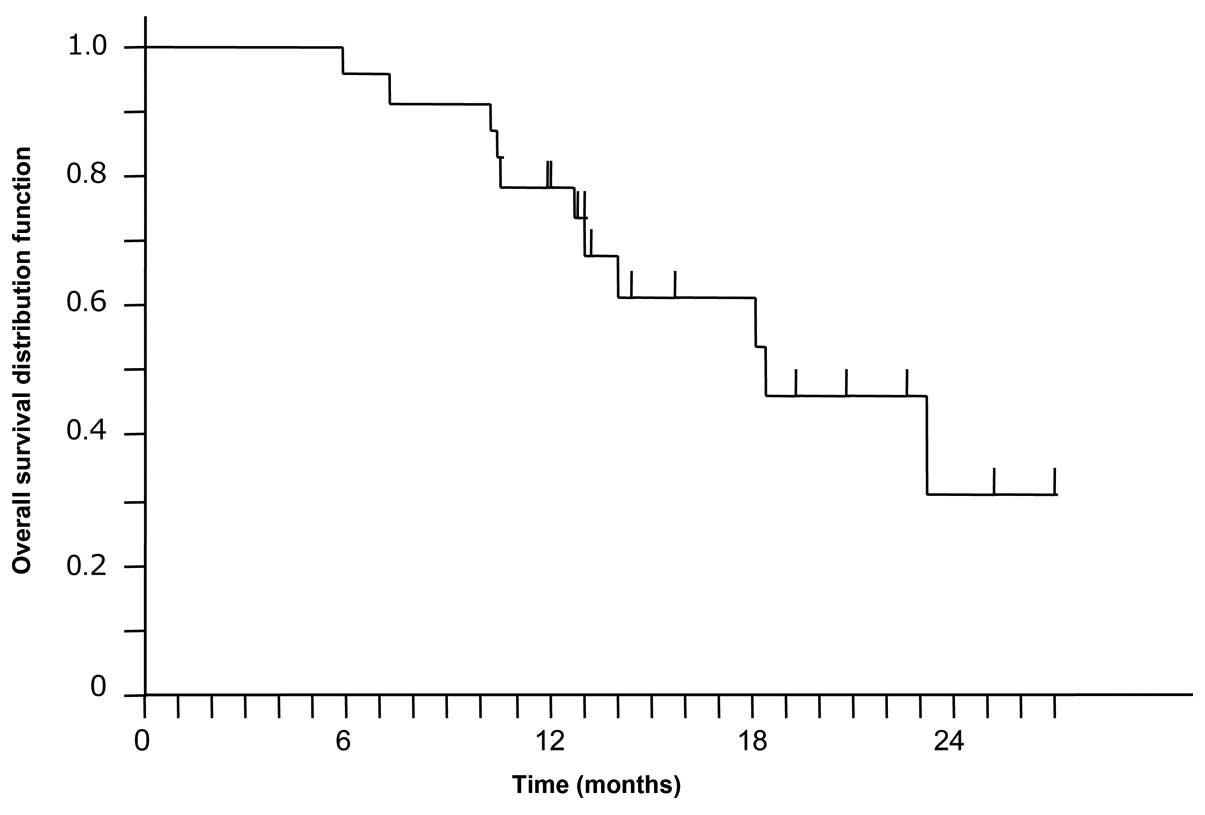
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108.
2005.
|
|
2
|
Langer CJ, Besse B, Gualberto A, et al:
The evolving role of histology in the management of advanced
non-small-cell lung cancer. J Clin Oncol. 28:5311–5320. 2010.
|
|
3
|
No authors listed. Chemotherapy in
non-small cell lung cancer: a meta-analysis using updated data on
individual patients from 52 randomised clinical trials. Non-small
Cell Lung Cancer Collaborative Group. BMJ. 311:899–909. 1995.
|
|
4
|
NSCLC Meta-Analyses Collaborative Group.
Chemotherapy in addition to supportive care improves survival in
advanced non-small-cell lung cancer: a systematic review and
meta-analysis of individual patient data from 16 randomized
controlled trials. J Clin Oncol. 26:4617–4625. 2008.
|
|
5
|
Spiro SG, Rudd RM, Souhami RL, et al: Big
Lung Trial participants: Chemotherapy versus supportive care in
advanced non-small cell lung cancer: improved survival without
detriment to quality of life. Thorax. 59:828–836. 2004.
|
|
6
|
Scagliotti GV, Parikh P, von Pawel J, et
al: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008.
|
|
7
|
Paz-Ares L, de Marinis F, Dediu M, et al:
Maintenance therapy with pemetrexed plus best supportive care
versus placebo plus best supportive care after induction therapy
with pemetrexed plus cisplatin for advanced non-squamous
non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3,
randomised controlled trial. Lancet Oncol. 13:247–255. 2012.
|
|
8
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004.
|
|
9
|
Gazdar AF: Personalized medicine and
inhibition of EGFR signaling in lung cancer. N Engl J Med.
361:1018–1020. 2009.
|
|
10
|
Presta LG, Chen H, O’Connor SJ, et al:
Humanization of an anti-vascular endothelial growth factor
monoclonal antibody for the therapy of solid tumors and other
disorders. Cancer Res. 57:4593–4599. 1997.
|
|
11
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005.
|
|
12
|
O’Byrne KJ, Koukourakis MI, Giatromanolaki
A, et al: Vascular endothelial growth factor, platelet-derived
endothelial cell growth factor and angiogenesis in non-small-cell
lung cancer. Br J Cancer. 82:1427–1432. 2000.
|
|
13
|
Bremnes RM, Camps C and Sirera R:
Angiogenesis in non-small cell lung cancer: the prognostic impact
of neoangiogenesis and the cytokines VEGF and bFGF in tumours and
blood. Lung Cancer. 51:143–158. 2006.
|
|
14
|
Kim KJ, Li B, Winer J, Armanini M, et al:
Inhibition of vascular endothelial growth factor-induced
angiogenesis suppresses tumour growth in vivo. Nature. 362:841–844.
1993.
|
|
15
|
Willett CG, Boucher Y, di Tomaso E, et al:
Direct evidence that the VEGF-specific antibody bevacizumab has
antivascular effects in human rectal cancer. Nat Med. 10:145–147.
2004.
|
|
16
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006.
|
|
17
|
Reck M, von Pawel J, Zatloukal P, et al:
Phase III trial of cisplatin plus gemcitabine with either placebo
or bevacizumab as first-line therapy for nonsquamous non-small-cell
lung cancer: AVAil. J Clin Oncol. 27:1227–1234. 2009.
|
|
18
|
Niho S, Kunitoh H, Nokihara H, et al:
JO19907 Study Group: Randomized phase II study of first-line
carboplatin-paclitaxel with or without bevacizumab in Japanese
patients with advanced non-squamous non-small-cell lung cancer.
Lung Cancer. 76:362–367. 2012.
|
|
19
|
Patel JD, Hensing TA, Rademaker A, et al:
Phase II study of pemetrexed and carboplatin plus bevacizumab with
maintenance pemetrexed and bevacizumab as first-line therapy for
nonsquamous non-small-cell lung cancer. J Clin Oncol. 27:3284–3289.
2009.
|
|
20
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
|
|
21
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982.
|
|
22
|
National Cancer Institute. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
Accessed February 15, 2010
|
|
23
|
Ohe Y, Ohashi Y, Kubota K, et al:
Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323.
2007.
|
|
24
|
Schiller JH, Harrington D, Belani CP, et
al: Eastern Cooperative Oncology Group: Comparison of four
chemotherapy regimens for advanced non-small-cell lung cancer. N
Engl J Med. 346:92–98. 2002.
|
|
25
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990.
|
|
26
|
Ferrara N: The role of vascular
endothelial growth factor in pathological angiogenesis. Breast
Cancer Res Treat. 36:127–137. 1995.
|
|
27
|
Patel JD, Socinski MA, Garon EB, et al:
PointBreak: a randomized phase III study of pemetrexed plus
carboplatin and bevacizumab followed by maintenance pemetrexed and
bevacizumab versus paclitaxel plus carboplatin and bevacizumab
followed by maintenance bevacizumab in patients with stage IIIB or
IV nonsquamous non-small-cell lung cancer. J Clin Oncol.
31:4349–4357. 2013.
|