Introduction
Worldwide, lung cancer is the main cause of cancer
mortality. Despite the aggressive treatment methods that have been
adopted in the past decades, including radiotherapy, chemotherapy
and surgery, the five-year survival rate of lung cancer patients
remains low.
As one of the novel therapeutic approaches to
cancer, oncolytic viral treatments possess great potential. In
recent years, it has been found that a variety of viruses possess
tumor-oncolytic effects (1).
Newcastle disease virus (NDV) is among these oncolytic viruses and
has been regarded as a promising oncolytic agent that has been used
in experimental clinical therapy for >40 years (2).
NDV has previously been reported to replicate in and
kill tumor cells, and since then, researchers have been trying to
apply NDV to tumor oncolytic treatments (3). Several clinical trials have proved
that NDV is a safe and effective therapeutic agent. The antitumor
effect of NDV has been demonstrated in various types of cancers and
has shown a significant inhibitory effect on tumor cell growth
(4). Human and animal studies have
revealed that, subsequent to restructuring, NDV could also be a
promising vaccine carrier (5–7).
Recombinant NDV strains can be generated using reverse genetics,
which results in improved oncolytic and immunoregulatory
properties. Rabies virus glycoprotein (RVG), has been revealed to
induce the production of neutralizing antibodies and thereby afford
complete protection against the challenge of RV (5,8).
The recombinant NDV (rl-RVG) virus applied in the
present study, provided by Harbin Veterinary Research Institute
(Harbin, Heilongjiang, China), was created with the avirulent NDV
LaSota strain and rabies virus glycoprotein (RVG) gene. Human lung
adenocarcinoma A549 cell tumor-bearing mice were infected with
rL-RVG to study the impact on the proliferation of A549 cell
xenograft tumors and the mechanism behind its effect.
Materials and methods
Materials
rl-RVG and NDV were provided by Harbin Veterinary
Research Institute (Harbin, China). The lung adenocarcinoma A549
cell line, which was purchased from the Cell Culture Center of the
Basic Institute of Medical Sciences, Peking Union Medical College
(Beijing, China), was reserved for the present experiment.
Four-week-old female BALB/c nude mice, were provided by the
Comparative Medicine Center of Yangzhou University (Yangzhou,
China). Cell culture reagents were acquired from Gibco (Life
Technologies, Carlsbad, CA, USA). All polymerase chain reaction
(PCR) primers were synthesized by Shanghai Jierui Biotech Co., Ltd.
(Shanghai, China). TRIzol reagent was acquired from Fermentas
(Burlington, ON, Canada). The PCR master mix was acquired from
CWbio Co., Ltd. (Beijing, China). The terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling (TUNEL) assay kit was
acquired from Kaiji Biotech (Nanjing, China). Anti-mouse monoclonal
RVG antibodies were purchased from EarthOx (1:500; Millbrae, CA,
USA) and anti-chicken monoclonal NDV antibodies were provided by
MedImmune, LLC (1:500; Gaithersburg, MD, USA). The polyclonal
horseradish peroxidase (HRP)-conjugated goat anti-rabbit and goat
anti-mouse antibodies were purchased from Beijing CoWin Biotech,
Co., Ltd. (Beijing, China). The HRP-AffiniPure rabbit anti-chicken
monoclonal antibody was purchased from EarthOx Life Science
(1:10,000; Millbrae). Mouse anti-cluster of differentiation (CD)49b
monoclonal antibody was acquired from BioLegend (San Diego, CA,
USA).
Tumor-bearing mouse model
construction
Dulbecco’s modified Eagle’s medium supplemented with
10% fetal bovine serum was used to culture the lung adenocarcinoma
A549 cells. The cells were incubated at 37°C under a humidified
atmosphere of 95% air and 5% CO2. At 90% confluence, the
A549 cells were reaped and resuspended in phosphate-buffered saline
(PBS) at 8.0×106 cells/ml. The tumor-bearing mouse model
was constructed by subcutaneous injection of 2.0×106
cells along the left oxter of four-week-old female BALB/c mice. A
tumor between 5 and 20 mm in diameter was assessed in each mouse
after ~10 days. All tumor-bearing mice were divided randomly into
three groups, the PBS, NDV and rl-RVG groups.
Animal experiment
When the tumors reached 5–20 mm in diameter, the
tumors of each mouse were injected with 300 μl PBS in the control
PBS group, 6.3×108 pfu NDV in the NDV group and
6.3×108 plaque forming units rl-RVG in the rl-RVG group.
Viral transfection was performed twice a week for three weeks. The
volume of the tumor was measured at zero, seven, 14 and 21 days.
After 21 days, all mice were anesthetized and sacrificed, and the
tumor, lung and spleen tissues were split by blunt dissection.
Certain tissues were fixed in 4% paraformaldehyde and others were
stored at −80°C for subsequent analysis. This study was approved by
the Laboratory Animal Management Committee of Jiangsu University
(Zhenjiang, China).
Growth curve of tumor and inhibition
rates
When the diameter of the tumor had reached 5–20 mm,
Vernier calipers were used to measure the short (a) and long (b)
diameters of the tumor every seven days. A growth curve of the
tumor volume was drawn according to the formula: Volume =
a2 × b × 0.52. The tumor inhibition rates were counted
based on the last measurement of tumor volume according to the
following formula: Tumor inhibition rate = (average tumor volume in
PBS group − average tumor volume in treatment group) / mean tumor
volume in PBS group × 100.
Morphological analysis
The tumor, spleen and lung tissues were fixed in 4%
paraformaldehyde, embedded in paraffin, sliced into 5-μm thick
sections and stained following a hematoxylin and eosin staining
procedure. Under an optical microscope (Eclipse TS100; Nikon
Corporation, Tokyo, Japan), pathological changes were detected in
the tissue sections and images were captured for documentation.
Reverse transcription (RT)-PCR
analysis
PCR was used to determine the expression of the RVG
and NDV genes. The total RNA was extracted from the sections of
tumor tissue using TRIzol reagent (Thermo Fisher Scientific,
Waltham, MA, USA). The cDNA was synthesized using Oligo (dT)
primers (Takara Bio, Inc, Otsu, Japan) and reverse transcriptase
(Takara Bio, Inc.). The primers for the NDV
hemagglutinin-neuraminidase (HN) gene were
5′-CTGGACGGTTTGGTGGGAA-3′ and 5′-TAATGCGACTGCGGGATGTG-3′. The
primers for the glycoprotein (G) gene were
5′-AGCCGATGCTCACTACAAG-3′ and 5′-CTGGAGGAGGGATGATTGC-3′. The PCR
protocol was as follows: An initial denaturation at 94°C for 5 min,
then denaturation at 94°C for 30 sec, annealing at 53°C (RVG) or
55°C (NDV) for 30 sec, and extension at 72°C for 30 sec, for 30
cycles. An incubation step was executed in the final extension at
72°C for 10 min. Electrophoresis of the PCR products was performed
in agarose gel, and the results were visualized using ethidium
bromide. The bands were analyzed with Quantity One software
(Bio-Rad, Hercules, CA, USA).
Western blotting
Western blotting was used to confirm the expression
of the RVG and NDV proteins. Tumor tissue (1 g) was cut into
sections and homogenized on ice. Following rapid centrifugation at
12,00 × g, the supernatant was discarded and the pellet was
resuspended with 1,000 μl pre-cooled radioimmunoprecipitation assay
lysis buffer (3 μl sodium orthovanadate, 3 μl phenylmethyl sulfonyl
fluoride and 3 μl protease inhibitor cocktail; KangChen Bio-tech,
Inc., Shanghai, China). The mixture was homogenized and lysed for
60 min on ice. Following centrifugation at 12,000 × g for 15 min at
4°C, the supernatant was transferred into a 1.5 ml tube and mixed
with an equal volume of loading buffer (2X) and β-mercaptoethanol
(1:20). The extracted protein was separated on a 10% SDS-PAGE gel,
and the NDV and RVG protein expression levels were determined
through use of the indicated primary antibodies (1:500) for 1–1.5
h. Following two washes with PBS, HRP-conjugated secondary
immunoglobulin (Ig)G antibodies (1:10,000) were used, then samples
were washed with PBS twice. GAPDH was used as a negative control
and internal reference.
Immunohistochemistry test
The tumor tissues were fixed with 4%
paraformaldehyde, embedded in paraffin and sliced into 5-μm thick
sections. Sections were then placed in dimethylbenzene and a
gradient of ethanol (100, 95, 90, 80 and 70%), for 5 min each, for
dewaxing, followed by blocking with serum. The samples were
incubated with primary antibodies for RVG and NDV (1:200) for three
hours each at 37°C and then washed with PBS three times. All
sections were incubated with the HRP-conjugated secondary IgG
antibodies (1:10,000) for 30 min at 37°C. Subsequent to being
washed three times with PBS, the samples were stained with
3,3-diaminobenzidine, kept at room temperature without light for 10
min and then stained with hematoxylin. The samples were then
dehydrated using an ethanol gradient (70, 80, 90, 95 and 100%),
then rinsed in xylene for 10 min twice. The sections were observed
and images were captured under an optical microscope.
Apoptosis assay
A TUNEL assay kit was used for the analysis of
apoptosis according to the manufacturer’s instructions. The
sections were analyzed and the images captured under an optical
microscope. The apoptosis index was calculated as follows: The
number of apoptotic cells / (the number of apoptosis cells + the
number of non-apoptotic cells) × 100.
Flow cytometry
The number of CD3−/CD49+
natural killer (NK) cells was detected using flow cytometry.
Splenocyte suspensions were prepared from the spleens of the
sacrificed mice. Splenocytes (100 μl aliquots) were labeled with
0.5 μl CD49b-fluorescein isothiocyanate and 2.5 μl hamster
CD3e-phycoerythrin, respectively. The cells were analyzed with a
FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA) using the CellQuest (BD Biosciences) and WinMDI 2.9 (Scripps
Research Institute, La Jolla, CA, USA) software.
Statistical analysis
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was
used to analyze all data and the results were reported as the mean
± standard deviation. One-way analysis of variance was used to
analyze the statistical significance, followed by post-hoc
comparisons to compare the differences between multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Growth curve of tumor and inhibition
rates
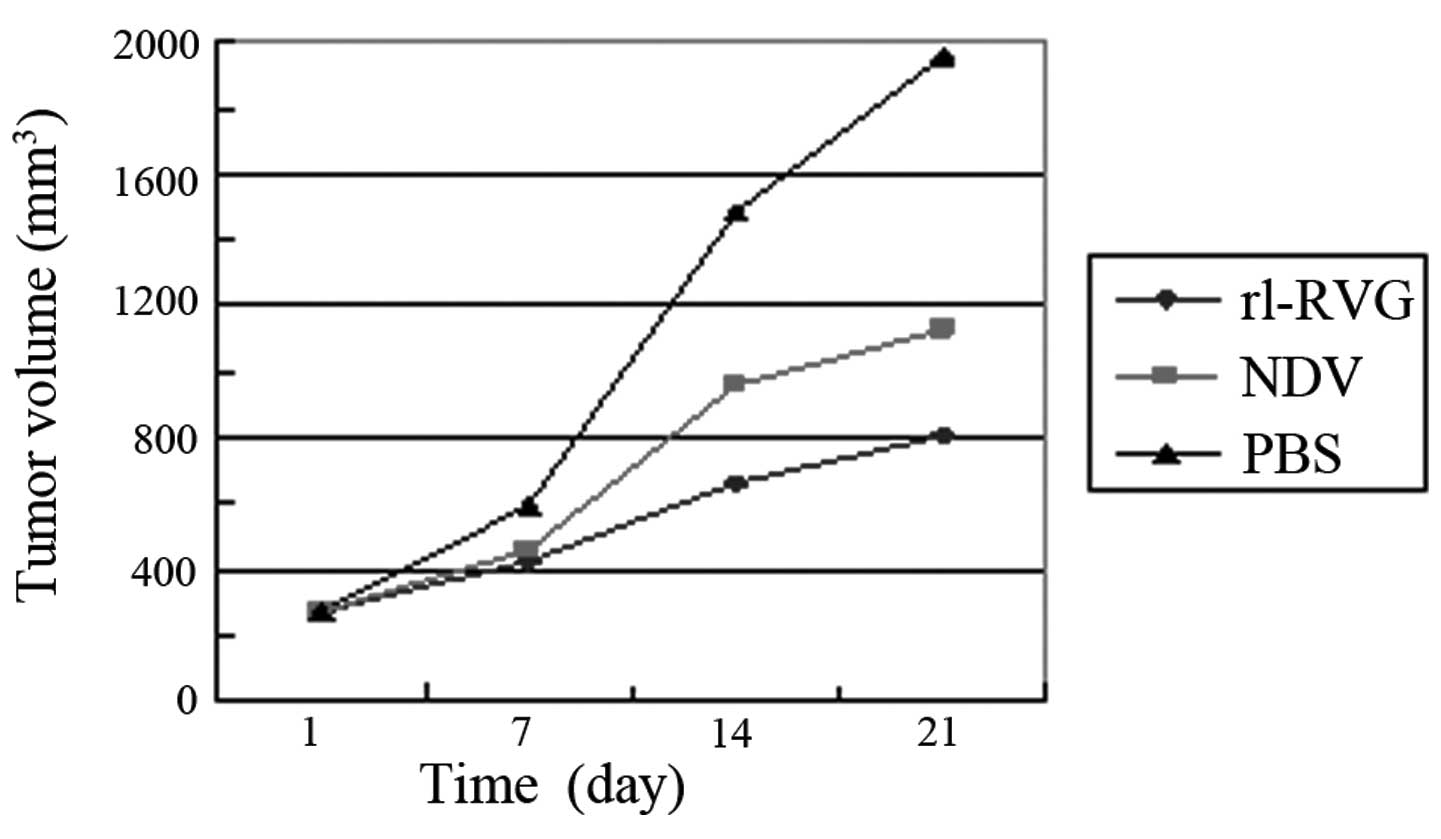
The tumor volume in the PBS group continued to
increase throughout the 21-day period. In the rl-RVG and NDV
groups, the volumes were markedly smaller at day 21 compared with
those detected in the PBS group (P<0.01), and the tumor volumes
in the rl-RVG group were smaller compared with the NDV group
(P<0.05). The tumor inhibition rates were calculated based on
the following formula: Volume = a2 × b × 0.52. The tumor
inhibition rate of rl-RVG was revealed to be 58.9%, which meant
that the growth of the tumor in the rl-RVG group was inhibited by
58.9% compared with the PBS group. The tumor inhibition rate of NDV
was 42.5%, meaning that the growth of the tumor in the NDV group
was inhibited by 42.5% compared with the PBS group (Fig. 1; Table
I).
 | Table IComparison of the tumor volume in nude
mice. |
Table I
Comparison of the tumor volume in nude
mice.
| Time, days | rl-RVG | NDV | PBS | F-value | P-value |
|---|
| 1 | 274.67±114.20 | 274.08±127.05 | 271.78±110.79 | 0.001 | 0.999 |
| 7 | 444.18±111.22 | 453.65±164.94 | 591.45±244.50 | 0.846 | 0.458 |
| 14 | 657.56±176.74 | 958.26±274.09 |
1483.21±446.75a | 7.752 | 0.009 |
| 21 | 804.96±176.74 |
1127.88±274.09c |
1960.92±446.758b | 26.044 | <0.001 |
Morphological and histopathological
analysis
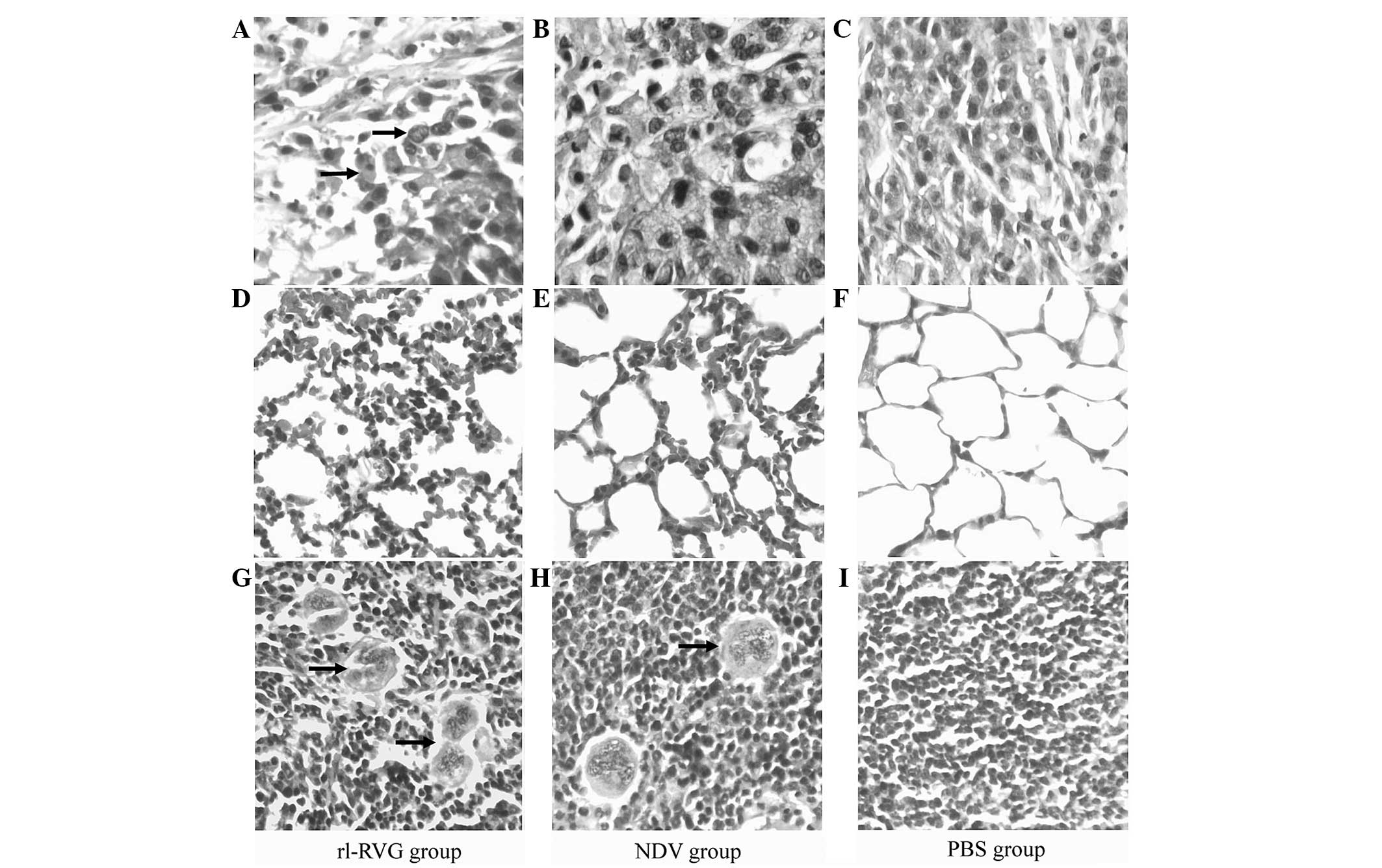
The effect of the various treatments on the
morphology of the tissue samples from the tumor, lung and spleen
were compared. The results revealed that tumor cells from the
rl-RVG group underwent the most destructive necrosis, while
necrosis was not observed in the PBS group.
In the lung tissue from the rl-RVG and NDV groups,
there was a severe inflammatory reaction, while there was no
inflammation in the lung tissue from the PBS group. The
inflammatory reaction was more severe in the rl-RVG group compared
with the NDV group.
The spleen tissues exhibited a significant increase
in size in the rl-RVG and NDV groups of mice compared with the PBS
group, and the spleen tissues were larger in the rl-RVG group
compared with those in the NDV group. In the spleen, rl-RVG
transfection induced more aggregation of multinucleated giant cells
(Fig. 2).
Expression of the G and NDV genes
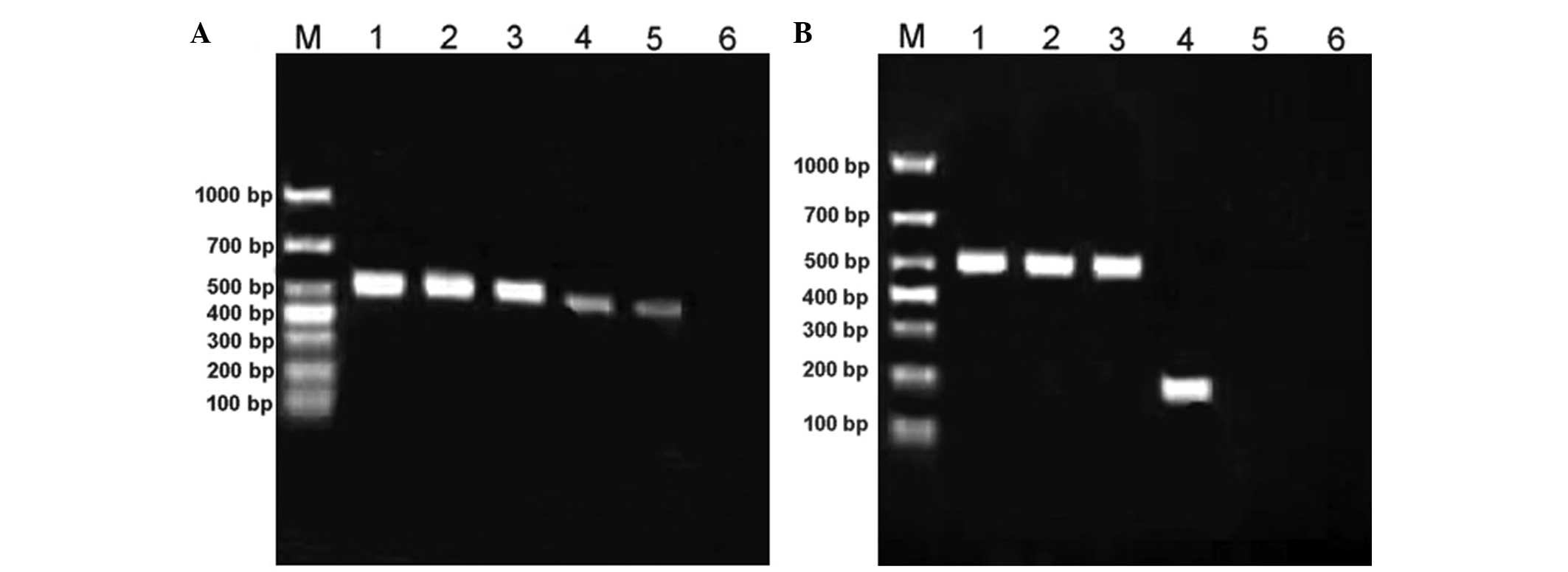
RT-PCR was used to detect RVG and NDV gene
expression in the tumor tissue. The results revealed clear RVG gene
expression (~176 bp) following transfection with rl-RVG. In the PBS
and NDV groups, the RVG was not expressed. The NDV gene (~462 bp)
was expressed in the rl-RVG and NDV groups, but was not expressed
in the PBS group (Fig. 3).
Expression of the G and NDV proteins
Western blotting
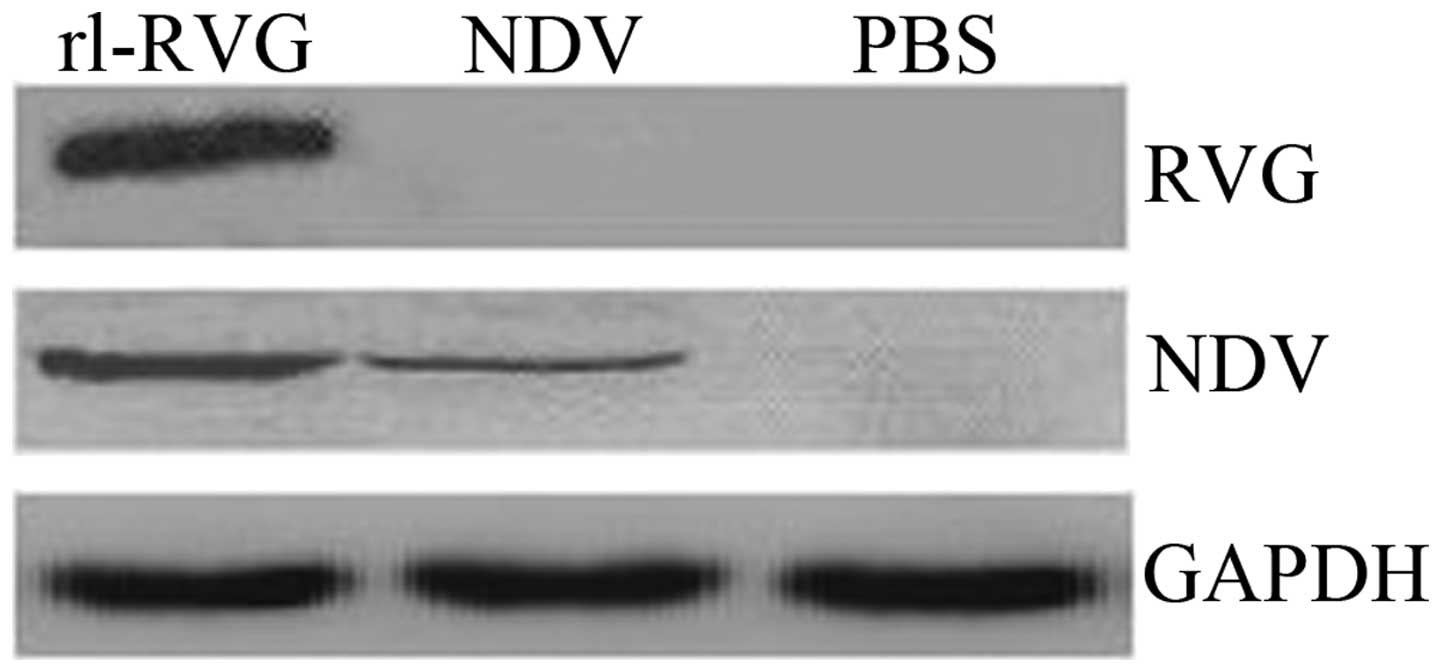
Western blot analysis was used to determine RVG and
NDV protein expression. The results revealed that RVG was expressed
following transfection with rl-RVG, whereas in the PBS and NDV
groups, RVG was not expressed. NDV was expressed in the rl-RVG and
NDV groups but was not expressed in the PBS group (Fig. 4).
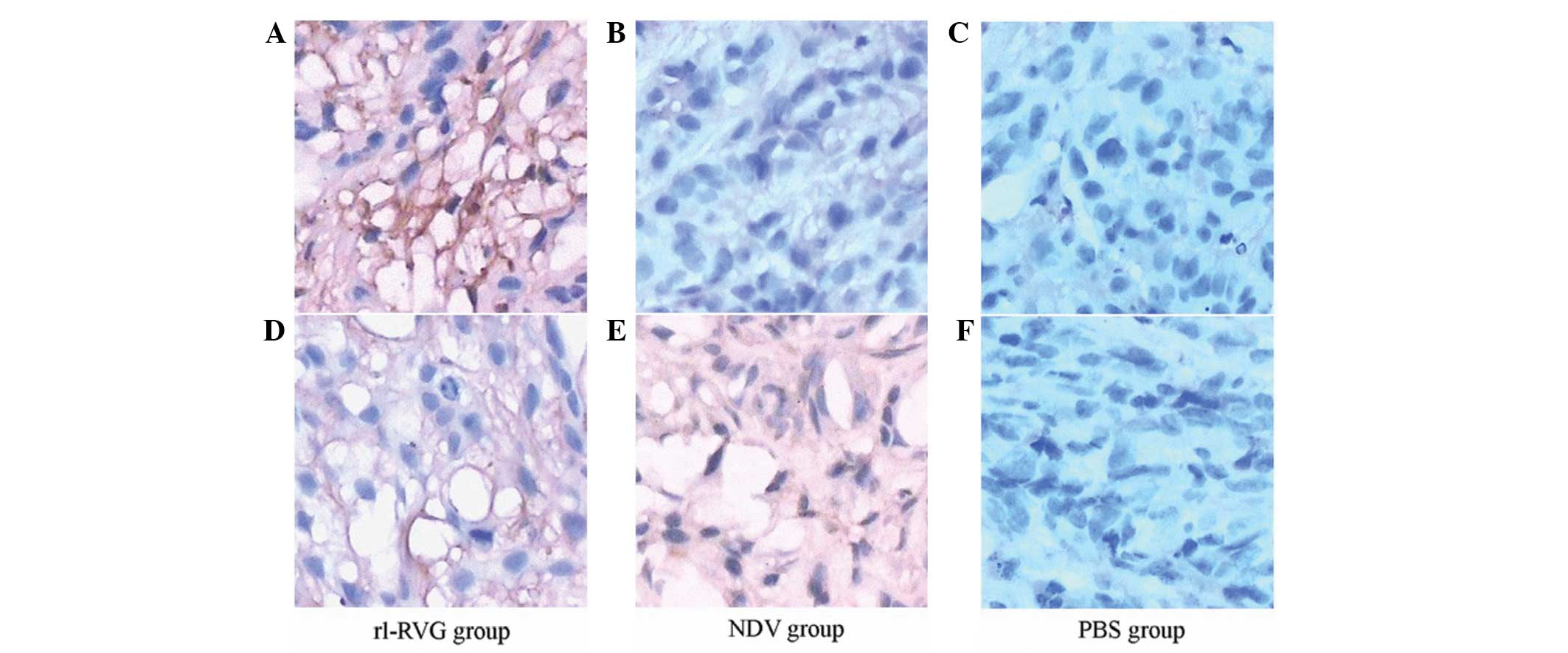
Immunohistochemistry
Immunohistochemistry with primary antibodies for NDV
and RVG was used to confirm the expression of the NDV and RVG
proteins in the tumor tissues. The results revealed that, in the
rl-RVG group, RVG was expressed in the tumor cell cytoplasm, but
there was no RVG expression in the NDV and PBS groups (Fig. 5A1–A3). The
cytoplasm of the cells from the rl-RVG and NDV groups was positive
for RVG expression, and the rl-RVG group exhibited stronger
expression compared with the NDV group, while the PBS group was
negative for RVG expression (Fig.
5B1–B3). These results indicated that the
NDV proteins were expressed in the cytoplasm of the tumor cells in
the rl-RVG and NDV groups and were expressed to a greater extent in
the rl-RVG group. Additionally, there were notably more mucous
lakes in the RVG group (Fig.
5).
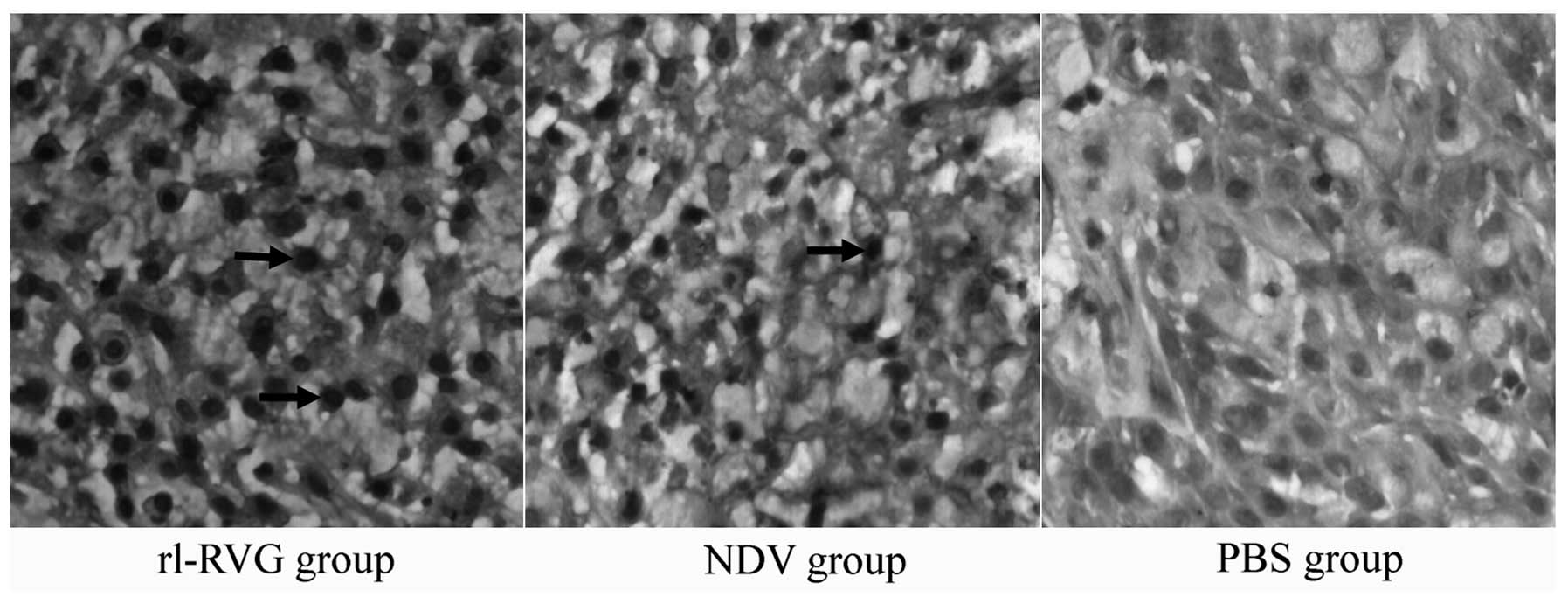
Apoptosis of tumor cells
A TUNEL assay was performed to detect apoptosis in
the tumor cells. The results revealed that the number of apoptotic
cells and the apoptotic index were markedly higher in the rl-RVG
and NDV groups compared with the PBS group (P<0.01), with the
rl-RVG group exhibiting a higher number and index compared with the
NDV group (P<0.05) (Fig. 6;
Table II).
 | Table IIAI of tumors in mice transfected with
rl-RVG. |
Table II
AI of tumors in mice transfected with
rl-RVG.
| Group | AI (n=10) | F-value | P-value |
|---|
| rl-RVG | 0.300±0.0408a | | |
| NDV | 0.225±0.0470b | 120.249 | <0.001 |
| PBS | 0.044±0.0212 | | |
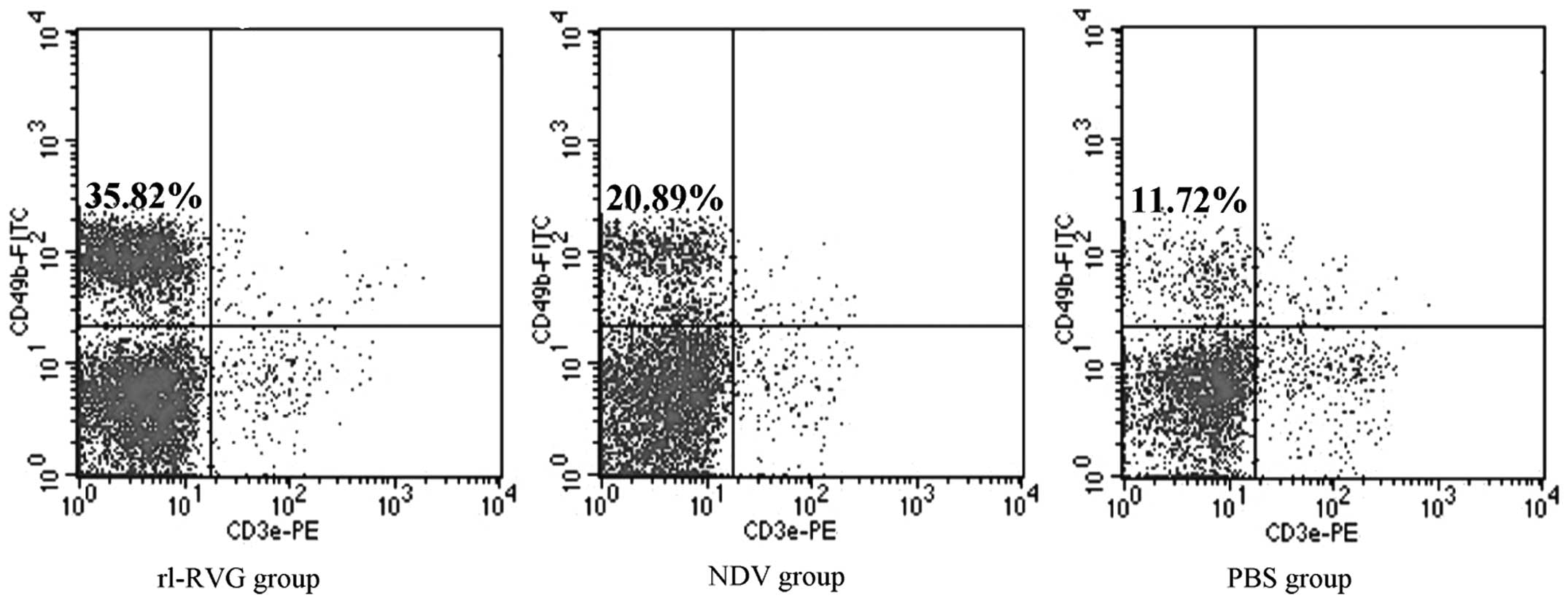
Number of NK cells in the spleen
Flow cytometry was used to determine the number of
NK cells. The results proved that the number of NK cells was
markedly higher in the rl-RVG and NDV groups compared with the PBS
group (P<0.001), with the rl-RVG group exhibiting the highest
levels (Fig. 7; Table III).
 | Table IIIRatio of natural killer cells
transfected with rl-RVG. |
Table III
Ratio of natural killer cells
transfected with rl-RVG.
| T cell | rl-RVG | NDV | PBS | F-value | P-value |
|---|
| CD49+ | 43.2±5.26a | 28.8±2.38b | 18.8±5.90 | 31.58 | <0.001 |
Discussion
Lung cancer is one of the most common malignant
tumors and its incidence is increasing. The majority of lung cancer
patients have already lost the opportunity to undergo surgery prior
to obtaining a clear diagnosis. Also, radiation and chemotherapy
can produce evident side-effects and treatment resistance.
Oncolytic therapy is one of the most widely researched novel
biological treatments. Oncolytic viral therapy is a method that
harnesses the natural ability of a virus to infect, duplicate
within and lyse a host cell as part of its natural life cycle
(1).
A variety of viruses have been revealed to possess
oncolytic, antitumoral activity, including herpes simplex virus
type 1, vaccinia virus and adenovirus (9).
NDV is a type of fowl cholera virus that mainly
infects poultry. NDV is a non-segmented, negative-sense,
single-stranded RNA virus of the Paramyxoviridae family, with a
natural avian host range. The NDV genome codes for the following
six genes, listed in order from the 3′-end: Nucleocapsid protein,
phosphoprotein, matrix protein, fusion protein, HN and large
protein (5,8).
NDV has been reported to selectively duplicate in
and destroy tumor cells, while sparing normal cells, and therefore
its application as a oncolytic agent in cancer treatment has been
explored (10–14). Immunotherapy using NDV has also been
used for the treatment of neuroblastoma (15), melanoma (16,17)
and other malignancies. However, to the best of our knowledge,
there have been no studies on the anti-lung carcinoma effect of RVG
expressing the recombinant avirulent NDV LaSota strain.
RV, a highly neurotropic virus, leads to deadly
encephalomyelitis in almost all mammals, including humans. The
genome of RV encodes five structural proteins, namely,
nucleoprotein, phosphoprotein, matrix protein, G protein and large
protein. RVG, a type I transmembrane protein, consists of
cytoplasmic, transmembrane and external domains that are exposed as
trimers on the surface of the mature virus particle (18). The external domain alone has been
revealed to induce the production of neutralizing antibodies and
thereby provide complete protection against RV (19). Rabies viruses can spread to
contiguous or non-contiguous cells, which are encompassed by the
interstitial space. Despite being merged into the surface of NDV
virions, RVG does not alter the trypsin-dependent infectivity of
NDV in mammalian cell cultures. RVG expression does not affect the
initiation of the innate immune response to NDV in mammalian cells.
RVG gene expression does not augment the toxicity of the NDV vector
in poultry or mice (20). In the
present study, rL-NDV was used, which was generated using the RVG
gene from a non-pathogenic RV, the ERA strain. Numerous animal
studies have revealed that rL-RVG is safe in mice, poultry, dogs
and cats (20–23).
It has been demonstrated that the majority of
oncolytic NDV strains induce apoptosis through the extrinsic and
intrinsic caspase-dependent cell death pathways (24). In the present study, the TUNEL assay
demonstrated that the number of apoptotic cells and the apoptotic
index were markedly higher in the rl-RVG and NDV groups than in the
PBS group, and that the levels in the rl-RVG group were higher than
in the NDV group. NDV can duplicate more rapidly in human tumor
cells compared with normal cells and then exert oncolytic effects
(25). This selective effect is
likely due to the restrained production of V proteins and
virus-induced cytokines in the host (26). NDV can infect the majority of tumor
cells, and the viral duplication in the cells can be tested by
detecting the augmentation of viral antigens on the cell surface
(27). In the present study, the
analysis revealed that the tumor volume was clearly decreased
following rl-RVG and NDV transfection.
Despite leading to direct oncolytic effects on tumor
cells, NDV can modulate the human immune system. It has been
reported that NDV stimulates host immunity to generate cytokines,
including interferon (IFN)-β, IFN-α, interleukin (IL)-1 and TNF-α,
which in turn, leads to the activation of macrophages, sensitized T
cells and NK cells (28). NDV
augments antitumor cytotoxic activity through activation of human
NK cells. HN is a potent inducer of IFN production by human
peripheral blood mononuclear cells and is able to upregulate
TNF-related apoptosis inducing ligands (TRAIL) (29). The direct interaction between the HN
viral glycoprotein and sialic acid residues on the cell surface
could activate NK cells. Thus, those secreted cytokines, including
IL-2, IFN-γ and TNF-α, could be stimulated by activated NK cells,
further activating and affecting other immune cell functions. It
can therefore be speculated that activated NK cells have increased
cytolytic antitumor effects (20).
The analysis in the present study revealed that tumor necrosis,
spleen size, generation of multinucleated giant cells in the spleen
and the number of NK cells were markedly increased in the rl-RVG
and NDV groups compared with the control group. This phenomenon was
more pronounced in the rl-RVG group.
The present study aimed to assess the inhibitory
effect of rl-RVG on lung adenocarcinoma A549 cells in tumor-bearing
mice and its likely mechanism. The analysis revealed that following
rl-RVG or NDV transfection, the tumor volumes were markedly
decreased. Immunohistochemistry indicated that in the rl-RVG group,
RVG was expressed in the tumor cell cytoplasm, while there was no
RVG expression in the NDV or PBS groups; in the rl-RVG and NDV
groups, NDV was expressed in the tumor cell cytoplasm, and was
expressed to a greater extent in the rl-RVG group compared with the
NDV group. There was no NDV expression in the PBS group. RT-PCR and
western blotting suggested that the rl-RVG vector was successfully
transfected into the adenocarcinoma A549 cells in the tumor-bearing
mice, as an increase in the RVG gene and protein expression in the
rl-RVG group was observed, and expression of the NDV gene and
protein was observed in the rl-RVG and NDV groups. The level of
tumor necrosis increased and the spleen became enlarged, with
multinucleated giant cell formations. Flow cytometry indicated that
the number of NK cells was markedly higher in the rl-RVG and NDV
groups than in the control group. This phenomenon was more
pronounced in the rl-RVG group. TUNEL assay demonstrated that the
apoptotic cell number and the apoptotic index were markedly higher
in the rl-RVG and NDV groups compared with the PBS group, with the
rl-RVG group exhibiting a higher apoptotic index compared with the
NDV group.
In conclusion, the results of the present study
indicated that rl-RVG inhibits the growth of lung cancer cells and
accelerates apoptosis to a certain extent. rl-RVG can modulate the
immune system and strengthen the cell immune response, leading to
an anti-tumor effect. The present study is expected to provide an
experimental basis for further clinical application of rl-RVG in
lung cancer therapy.
Acknowledgements
This study was supported by the Social Development
Technological Support Projects of Zhenjiang, Jiangsu, China (grant
no. SH2013041).
References
|
1
|
Foucault C, Mordant P, Grand B, et al:
Unexpected extensions of non-small-cell lung cancer diagnosed
during surgery: revisiting exploratory thoracotomies and incomplete
resections. Interact Cardiovasc Thorac Surg. 16:667–672. 2013.
|
|
2
|
Wheelock EF and Dingle JH: Observations on
the repeated administration of viruses to a patient with acute
leukamia. A preliminary report. N Engl J Med. 271:645–651.
1964.
|
|
3
|
Cassel WA and Garrett RE: Newcastle
disease virus as an antineoplastic agent. Cancer. 18:863–868.
1965.
|
|
4
|
Reichard KW, Lorence RM and Cascino CJ:
Selective replication of Newcastle disease virus (NDV) in cancer
cells is associated with virus-induced cell fusion. Proc Am Assoc
Cancer Res. 33:5211992.
|
|
5
|
Bukreyev A, Huang Z, Yang L, et al:
Recombinant Newcastle disease virus expressing a foreign viral
antigen is attenuated and highly immunogenic in primates. J Virol.
79:13275–13284. 2005.
|
|
6
|
DiNapoli JM, Kotelkin A, Yang L, et al:
Newcastle disease virus, a host range-restricted virus, as a
vaccine vector for intranasal immunization against emerging
pathogens. Proc Natl Acad Sci USA. 104:9788–9793. 2007.
|
|
7
|
DiNapoli JM, Nayak B, Yang L, et al:
Newcastle disease virus-vectored vaccines expressing the
hemagglutinin or neuraminidase protein of H5N1 highly pathogenic
avian influenza virus protect against virus challenge in monkeys. J
Virol. 84:1489–1503. 2010.
|
|
8
|
Martinez-Sobrido L, Gitiban N,
Fernandez-Sesma A, et al: Protection against respiratory syncytial
virus by a recombinant Newcastle disease virus vector. J Virol.
80:1130–1139. 2006.
|
|
9
|
Donahue JM, Mullen JT and Tanabe KK: Viral
oncolysis. Surg Oncol Clin N Am. 11:661–680. 2002.
|
|
10
|
Reichard KW, Lorence RM, Cascino CJ, et
al: Newcastle disease virus selectively kills human tumor cells. J
Surg Res. 52:448–453. 1992.
|
|
11
|
Elankumaran S, Rockemann D and Samal SK:
Newcastle disease virus exerts oncolysis by both intrinsic and
extrinsic caspase-dependent pathways of cell death. J Virol.
80:7522–7534. 2006.
|
|
12
|
Hrabák A, Csuka I, Bajor T and Csatáry LK:
The cytotoxic anti-tumor effect of MTH-68/H, a live attenuated
Newcastle disease virus is mediated by the induction of nitric
oxide synthesis in rat peritoneal macrophages in vitro. Cancer
Lett. 231:279–289. 2006.
|
|
13
|
Bian H, Fournier P, Peeters B and
Schirrmacher V: Tumor-targeted gene transfer in vivo via
recombinant Newcastle disease virus modified by a bispecific fusion
protein. Int J Oncol. 27:377–384. 2005.
|
|
14
|
Lorence RM, Rood PA and Kelley KW:
Newcastle disease virus as an antineoplastic agent: induction of
tumornecrosis factor-alpha and augmentation of its cytotoxicity. J
Natl Cancer Inst. 80:1305–1312. 1988.
|
|
15
|
Lorence RM, Reichard KW, Katubig BB, et
al: Complete regression of human neuroblastoma xenografts in
athymic mice after local Newcastle disease virus therapy. J Natl
Cancer Inst. 86:1228–1233. 1994.
|
|
16
|
Cassel WA and Murray DR: Treatment of
stage II malignant melanoma patients with a Newcastle disease virus
oncolysate. Nat Immun Cell Growth Regul. 7:351–352. 1988.
|
|
17
|
Wallack MK, Sivanandham M, Balch CM, et
al: Surgical adjuvant active specific immunotherapy for patients
with stage III melanoma: the final analysis of data from a phase
III, randomized, double-blind, multicenter vaccinia melanoma
oncolysate trial. J Am Coll Surg. 187:69–79. 1998.
|
|
18
|
Ashraf S, Singh PK, Yadav DK, et al: High
level expression of surface glycoprotein of rabies virus in tobacco
leaves and its immunoprotective activity in mice. J Biotechnol.
119:1–14. 2005.
|
|
19
|
Barth R, Diderrich G and Weinmann E: NIH
test, a problematic method for testing potency of inactivated
rabies vaccine. Vaccine. 6:369–377. 1988.
|
|
20
|
Ge J, Wang X, Tao L, et al: Newcastle
disease virus-vectored rabies vaccine is safe, highly immunogenic,
and provides long-lasting protection in dogs and cats. J Virol.
85:8241–8252. 2011.
|
|
21
|
Zamarin D and Palese P: Oncolytic
Newcastle disease virus for cancer therapy: old challenges and new
directions. Future Microbiol. 7:3347–3367. 2012.
|
|
22
|
Bazzoni F and Beutler B: The tumor
necrosis factor ligand and receptor families. N Engl J Med.
334:1717–1725. 1996.
|
|
23
|
Zou H, Li Y, Liu X and Wang X: An
APAF-1.cytochrome c multimeric complex is a functional apoptosome
that activates procaspase-9. J Biol Chem. 274:11549–11556.
1999.
|
|
24
|
Ravindra PV, Tiwari AK, Sharma B and
Chauhan RS: Newcastle disease virus as an oncolytic agent. Indian J
Med Res. 130:507–513. 2009.
|
|
25
|
Nelson NJ: Scientific interest in
Newcastle disease virus is reviving. J Nat Cancer Inst.
91:1708–1710. 1999.
|
|
26
|
Schirrmacher V, Haas C, Bonifer R, et al:
Human tumor cell modification by virus infection: an efficient and
safe way to pro duce cancer vaccine with pleiotropic immune
stimulatory properties when using Newcastle disease virus. Gene
Ther. 6:63–73. 1999.
|
|
27
|
Fiola C, Peeters B, Fournier P and Unsal
A: Tumor selective replication of Newcastle disease virus:
association with defects of tumor cells in antiviral defence. Int J
Cancer. 119:328–338. 2006.
|
|
28
|
Avki S, Turutoglu H, Simsek A and Unsal A:
Clinical and immunological effects of Newcastle disease virus
vaccine on bovine papillomatosis. Vet Immunol Immunopathol.
98:9–16. 2004.
|
|
29
|
Zeng J, Fournier P and Schirrmacher V:
Induction of interferon-alpha and tumor necrosis factor-related
apoptosis-inducing ligand in human blood mononuclear cells by
hemagglutinin-neuraminidase but not F protein of Newcastle disease
virus. Virology. 297:19–30. 2002.
|