Spandidos Publications style
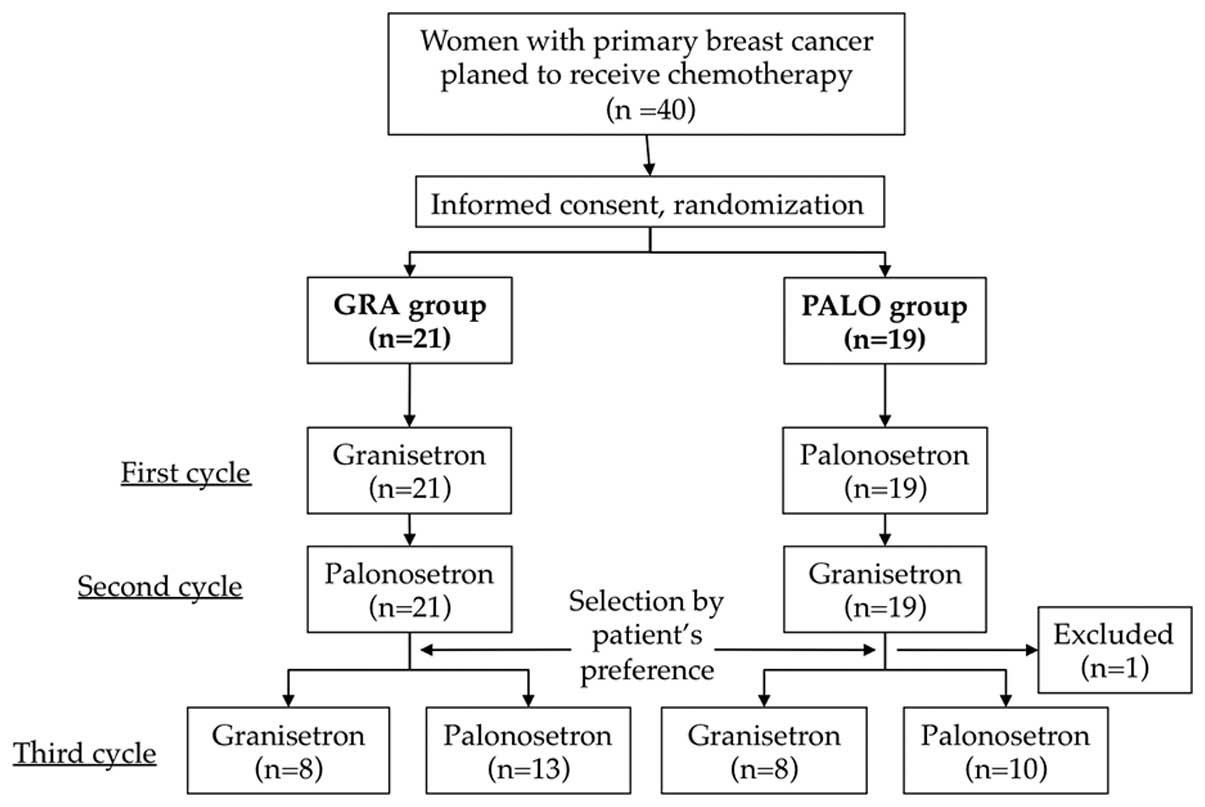
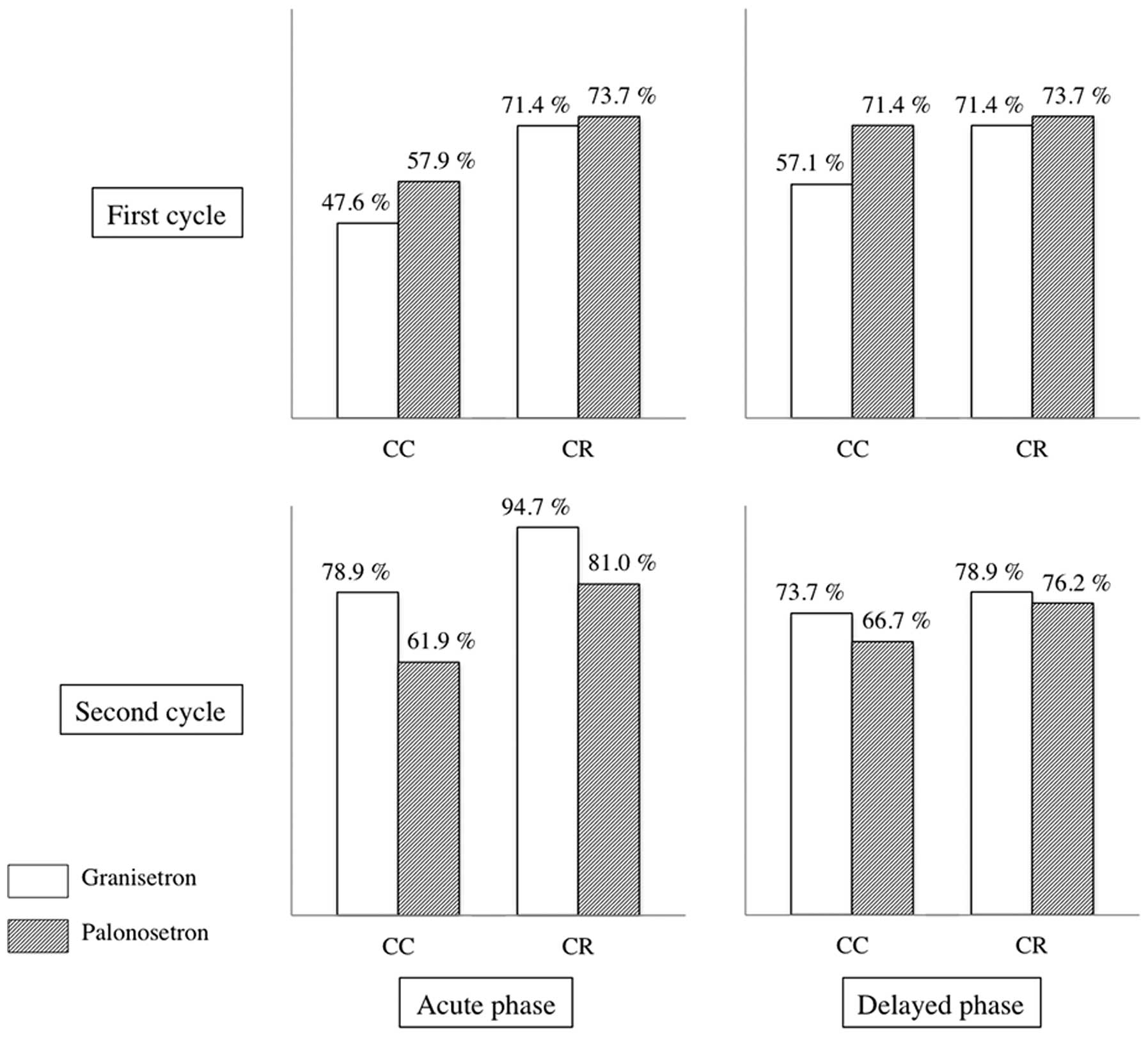
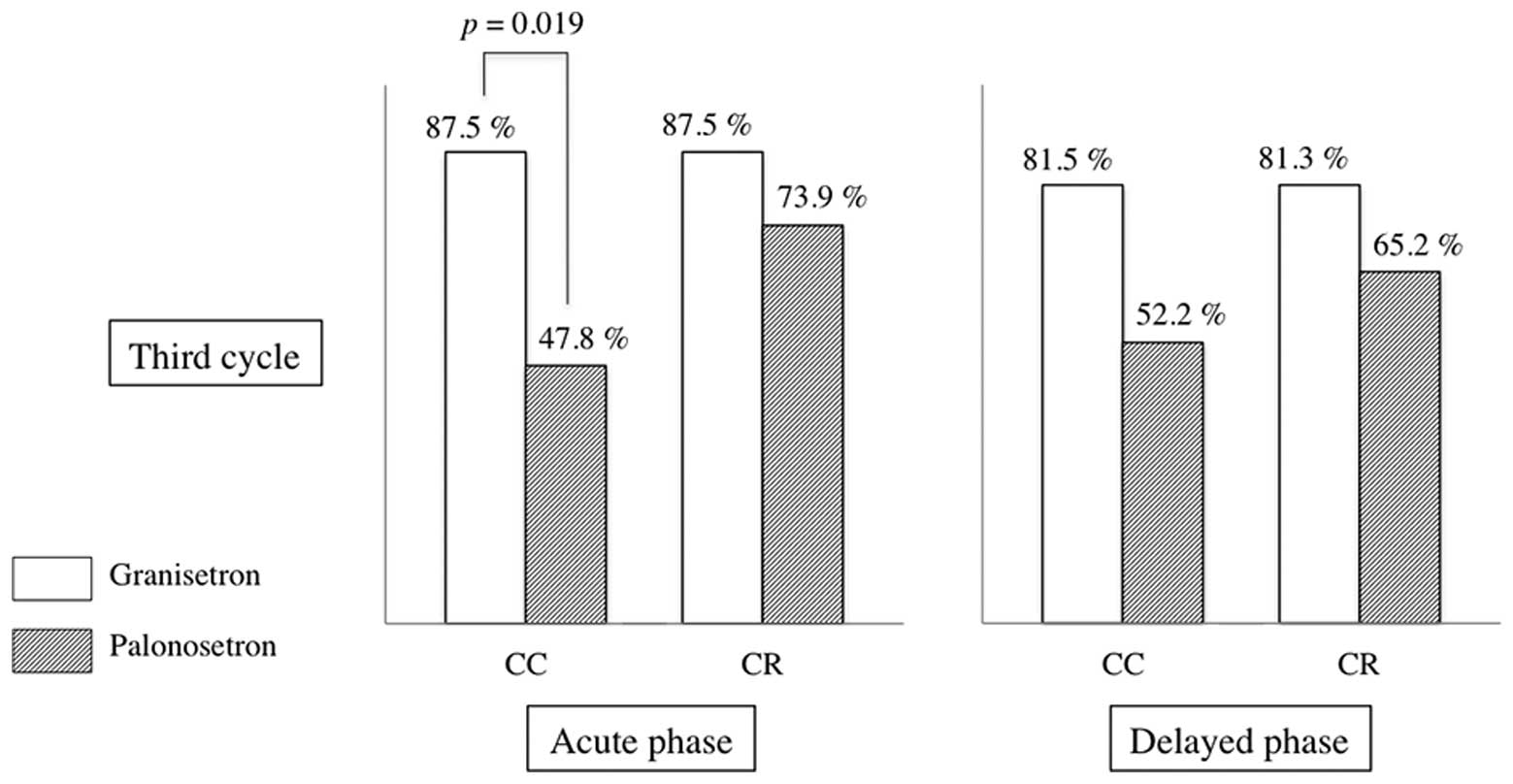
Ohzawa H, Miki A, Hozumi Y, Miyazaki C, Sagara Y, Tanaka Y, Shiba S, Joutoku H, Sakuragi M, Takehara M, Takehara M, et al: Comparison between the antiemetic effects of palonosetron and granisetron in breast cancer patients treated with anthracycline‑based regimens. Oncol Lett 9: 119-124, 2015.
APA
Ohzawa, H., Miki, A., Hozumi, Y., Miyazaki, C., Sagara, Y., Tanaka, Y. ... Yasuda, Y. (2015). Comparison between the antiemetic effects of palonosetron and granisetron in breast cancer patients treated with anthracycline‑based regimens. Oncology Letters, 9, 119-124. https://doi.org/10.3892/ol.2014.2640
MLA
Ohzawa, H., Miki, A., Hozumi, Y., Miyazaki, C., Sagara, Y., Tanaka, Y., Shiba, S., Joutoku, H., Sakuragi, M., Takehara, M., Sakuma, Y., Nishimura, W., Fujii, H., Yasuda, Y."Comparison between the antiemetic effects of palonosetron and granisetron in breast cancer patients treated with anthracycline‑based regimens". Oncology Letters 9.1 (2015): 119-124.
Chicago
Ohzawa, H., Miki, A., Hozumi, Y., Miyazaki, C., Sagara, Y., Tanaka, Y., Shiba, S., Joutoku, H., Sakuragi, M., Takehara, M., Sakuma, Y., Nishimura, W., Fujii, H., Yasuda, Y."Comparison between the antiemetic effects of palonosetron and granisetron in breast cancer patients treated with anthracycline‑based regimens". Oncology Letters 9, no. 1 (2015): 119-124. https://doi.org/10.3892/ol.2014.2640