Introduction
Gastrointestinal stromal tumors (GISTs) are the most
prevalent mesenchymal neoplasms of the GI tract. The annual
incidence of GISTs is reported to be 7–19 cases per million
individuals (1). Typically, GISTs
arise from the muscularis propria in the wall of the GI tract, and
are believed to originate from the interstitial cells of Cajal, the
majority of which are positive for KIT [cluster of differentiation
(CD)117] and tend to be positive for CD34. The current histological
classification for GISTs includes spindle, epithelioid and mixed
cell subtypes (2). The stomach is
the most common location for GISTs to occur (60–70%), followed by
the small intestine (20–30%), colorectum (10%) and esophagus
(<5%) (3). However, a small
number of mesenchymal tumors with similar histopathological and
immunohistochemical characteristics to GISTs have been increasingly
described in the omentum, mesentery and retroperitoneum (<7%).
These are known as extragastrointestinal stromal tumors (EGISTs)
(4–6).
The computed tomography (CT) and magnetic resonance
imaging (MRI) features of GISTs have been reported previously.
Numerous studies have identified malignant imaging signs for GISTs,
such as a large size, irregular surface, ill-defined margins,
tissue invasion, distant metastasis, peritoneal dissemination and
satellite nodules (7–10). However, few studies describe the
radiological findings of EGISTs (3). The purpose of the present study was to
review the CT and MRI images of EGISTs and analyze the correlations
between the radiological findings and pathological features.
Patients and methods
Subjects
The clinical, pathological and radiological findings
of 24 patients with primary EGISTs who were treated at East
Hospital, Tongji University School of Medicine (Shanghai, China)
and Shanghai Tenth People’s Hospital, Tongji University School of
Medicine (Shanghai, China) between May 2004 and August 2013 were
reviewed. All EGISTs were histologically proven by surgery. The
criteria for diagnosing the EGISTs were as follows: i) The mass had
no definite connection with the GI tract wall by intraoperative or
pathological observations; ii) the mass had typical GIST
morphology, as observed by light microscopy; and iii) the mass
expressed KIT and/or CD34. Written informed consent was obtained
from all patients.
CT and MRI technique
In total, 23 of the 24 patients underwent CT scans
of the abdomen and/or pelvis at the time of presentation. CT
examinations were performed with a 16-slice spiral CT scanner
(Sensation; Siemens Medical Solutions, Erlangen, Germany; n=12) or
a 64-sclice spiral CT scanner (Philips Brilliance; Philips Medical
Systems, Best, the Netherlands; n=11). The main parameters of the
CT scans were as follows: Tube voltage, 120 kVp; tube current, 250
mAs; slice thickness, 3–8 mm; field of view, 350 mm; matrix,
512×512; gantry speed, 0.75 sec/rotation; and pitch, 1.0–1.2. Oral
diatrizoate meglumine (concentration, 3%; dose, 800–1,000 ml;
Gastrografin; Bayer Schering, Berlin, Germany) was administered to
ten patients prior to the scans. Dual-phase dynamic contrast
enhancement was performed to obtain images of the arterial phase
(30–35 sec) and venous phase (65–70 sec) subsequent to the
intravenous administration of contrast agent (Omnipaque 300;
Nycomed Amersham, Princeton, NJ, USA; dose, 1.5 ml/kg body weight;
injection rate, 2.5–3.5 ml/sec). Multiplanar reformation and
maximum intensity projection images were achieved at an affiliated
workstation.
Six out of 24 patients underwent abdominal and/or
pelvic examinations with a 3.0-Tesla MRI scanner (Philips Achieva;
Philips Medical Systems) using a body coil. The main parameters of
the MRI examination were as follows: Field of view, 375 mm; matrix
size, 252×192; and slice thickness, 3–6 mm. T1WI [spin echo
sequence; repetition time (TR)/echo time (TE), 500/7.9 msec; number
of signal averages (NSA), 2], T2WI (fast spin echo sequence; TR/TE,
3,000/65 msec; NSA, 2) and DWI (EPI sequence; TR/TE, 1,147/70 msec;
NSA, 2; b value, 800 sec/mm2) were obtained in the axial
plane, and T2-weighted short time inversion recovery images (TR/TE,
1,822/60 msec; NSA, 2) were obtained in the axial, coronal and
sagittal planes. Following the intravenous administration of
gadopentetate dimeglumine (Magnevist®; Bayer Schering,
Berlin, Germany; dose, 0.1 mmol/kg body weight; injection rate, 1.5
ml/sec), dual-phase dynamic contrast enhancement was performed to
obtain fat-saturated T1WI (fast field echo sequence; flip angle,
10°; TR/TE, 4.1/2.0 msec; NSA, 2) of the arterial phase (30 sec)
and venous phase (60 sec) in the axial, coronal and sagittal
planes. In five out of 24 patients, both CT and MRI images were
available.
Imaging and pathological analyses
On the CT images, the attenuation of each tumor was
recorded as a hypo-, iso- or hyperdensity compared with the
adjacent muscle. On MRI images, the signal intensity of each tumor
was recorded as a hypo-, iso- or hyperintensity compared with the
adjacent muscle. The cystic-necrotic component was defined as the
center of the tumor having a density of <20 Hounsfield units
(HU) on contrast-enhanced images or water-like signal without
enhancement on MRI images. The radiological images were used to
measure the largest dimension of each tumor. The degree of tumor
enhancement was classified as mild (<30 HU) or marked (≥30 HU).
The enhancement patterns were recorded as homogeneous or
heterogeneous. Tumor vessels were defined as engorged vascular
structures within the mass. Lymphadenopathy was determined as
present if a nodular soft-tissue lesion existed that was >10 mm
in the short-axis diameter.
Two experienced radiologists who were blinded to the
pathological results of the EGISTs retrospectively reviewed the
radiological images, and the findings were reported as a consensus
of opinion. Tumor characteristics, including localization, size,
contours, borders, cystic-necrotic components, calcification,
hemorrhage and tumor vessels, were recorded. The attenuation and
intensity, as well as the degree and pattern of enhancement of the
EGISTs were evaluated. Radiological findings were also evaluated
for ascites, tumor invasion, lymphadenopathy and distant
metastasis.
The pathological findings in the surgical specimens
were retrospectively reviewed by one experienced pathologist, with
a particular emphasis on the presence of morphology, mitotic
activity and the immunoreactivity of KIT and CD34. On light
microscopy, ≤5 mitoses/50 high-power fields (HPFs) is generally
considered to indicate a low-grade EGIST, whereas >5 mitoses per
50 HPFs is generally considered to indicate a high-grade EGIST
(1,7). This was also the grading system used
for the present study.
Statistical analysis
Quantitative variables are expressed as the mean ±
standard deviation (SD) and categorical variables are expressed as
frequencies or percentages. Statistical analyses to compare the
radiological characteristics of EGISTs of differing grades were
performed with χ2 or Fisher’s exact tests (SPSS, version
13.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical and pathological features
Based on the diagnostic criteria, 24 surgically
resected EGISTs were identified. A slight male predominance (13
males and 11 females) existed within the study group. The mean age
at the time of presentation was 53 years (SD, 13 years; range,
34–81 years). The mean tumor size was 12.8 cm (SD, 5.3 cm; range,
4.5–25.1 cm). The clinical symptoms were an abdominal or pelvic
mass (n=14); abdominal pain (n=8) and abdominal distension (n=7).
The primary EGISTs occurred in the omentum (n=4; 16.7%), mesentery
(n=19; 79.2%) and retroperitoneum (n=1; 4.2%). The pathological
subtype of the 24 EGISTs was classified as spindle cell (n=21;
87.5%), epithelioid cell (n=1; 4.2%) and mixed cell (n=2; 8.3%).
Immunohistochemistry showed that 91.7% (22/24) and 70.8% (17/24) of
the tumors were positive for KIT and CD34, respectively. Two
KIT-negative EGISTs were both of mesenteric origin and epithelioid
or mixed cell subtype. The clinical data, pathological subtypes and
immunohistochemical results are shown in Tables I and II. According to the mitotic counts, seven
(29.2%) EGISTs were of low grade and 17 (70.8%) were of high
grade.
 | Table IGeneral information of 24 patients
with extragastrointestinal stromal tumors. |
Table I
General information of 24 patients
with extragastrointestinal stromal tumors.
| Variable | Omentum (n=4) | Mesentery (n=19) | Retroperitoneum
(n=1) |
|---|
| Gender |
| Male | 1 | 11 | 1 |
| Female | 3 | 8 | - |
| Age, years |
| ≤40 | 1 | 2 | - |
| 41–50 | 2 | 7 | 1 |
| 51–60 | 1 | 4 | - |
| 61–70 | - | 3 | - |
| 71–80 | - | 1 | - |
| ≥81 | - | 2 | - |
| Pathological
subtype |
| Spindle cell | 4 | 16 | 1 |
| Epithelioid
cell | - | 1 | - |
| Mixed cell | - | 2 | - |
| Size, cm |
| ≤5 | - | 2 | - |
| 5–10 | - | 4 | 1 |
| >10 | 4 | 13 | - |
 | Table IIImmunohistochemical results of 24
patients with extragastrointestinal stromal tumors. |
Table II
Immunohistochemical results of 24
patients with extragastrointestinal stromal tumors.
| Result | n (%) |
|---|
| KIT(+) | 22 (91.7) |
| CD34(+) | 17 (70.8) |
| KIT(+) and
CD34(+) | 15 (62.5) |
| KIT(+) and
CD34(−) | 7 (29.2) |
| KIT(−) and
CD34(+) | 2 (8.3) |
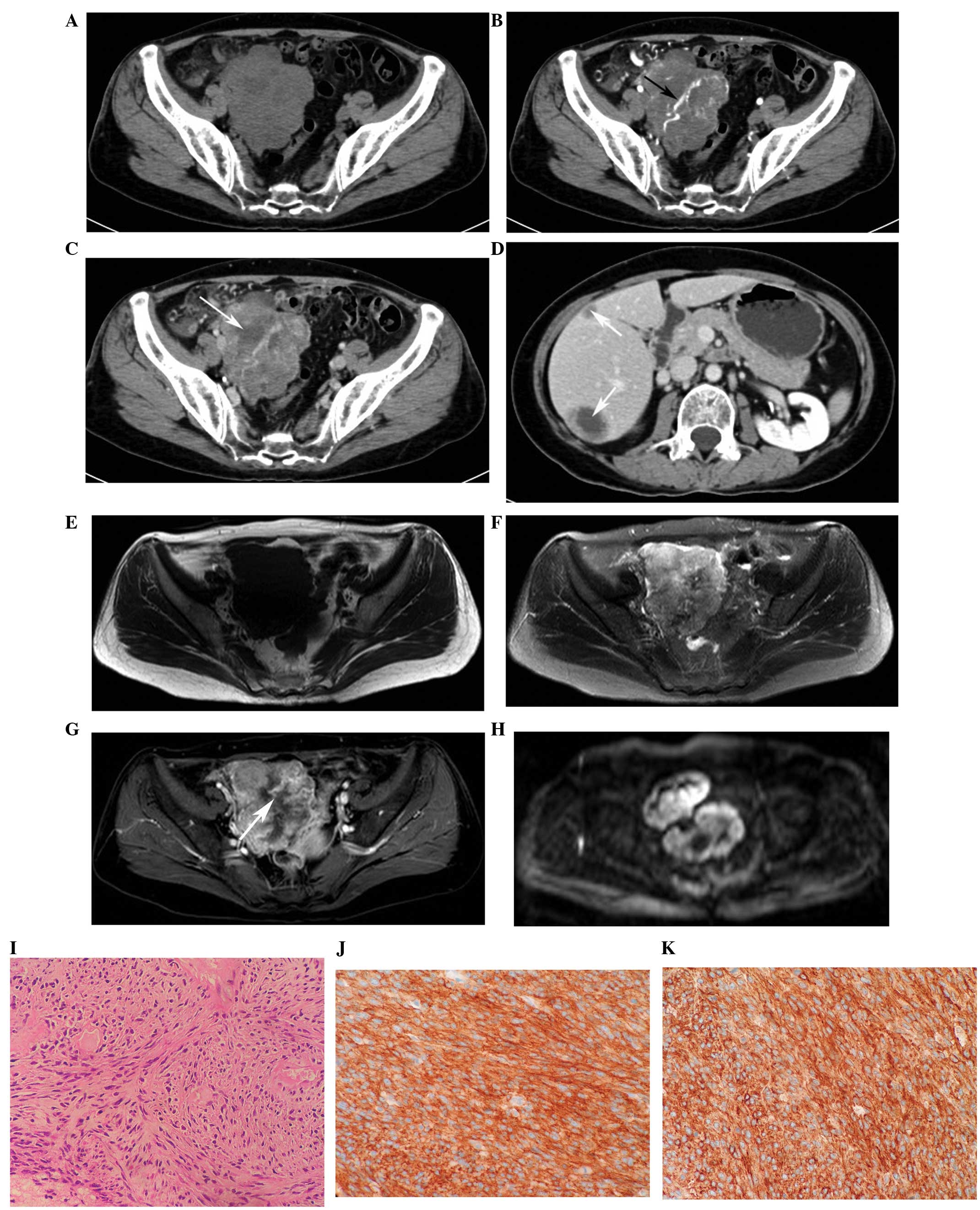
CT and MRI findings
On the CT (n=23; seven low-grade EGISTs and 16
high-grade EGISTs) and MRI (n=6; three low-grade EGISTs and three
high-grade EGISTs) images, 16 tumors (66.7%) exhibited round or
oval contours and eight (33.3%) showed an irregular appearance. The
masses were regarded as ill-defined in 16 patients (66.7%). The
tumors appeared as a hypodensity (n=7), slight hypodensity (n=9) or
isodensity (n=7) on precontrast CT images, as a slight
hypointensity (n=3), isointensity (n=2) or slight hyperintensity
(n=1) on T1WI, and as a hyperintensity (n=6) on T2WI and DWI
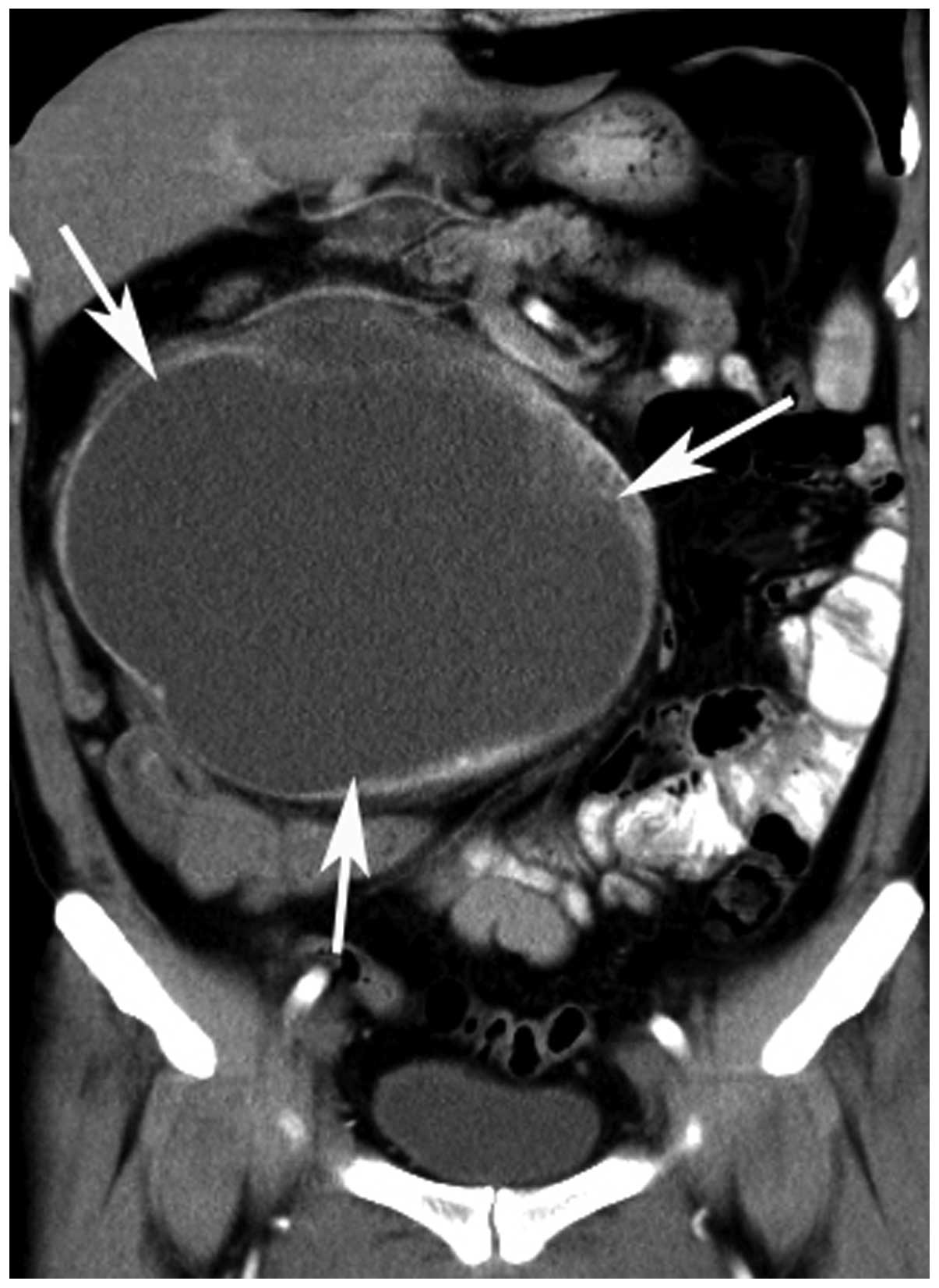
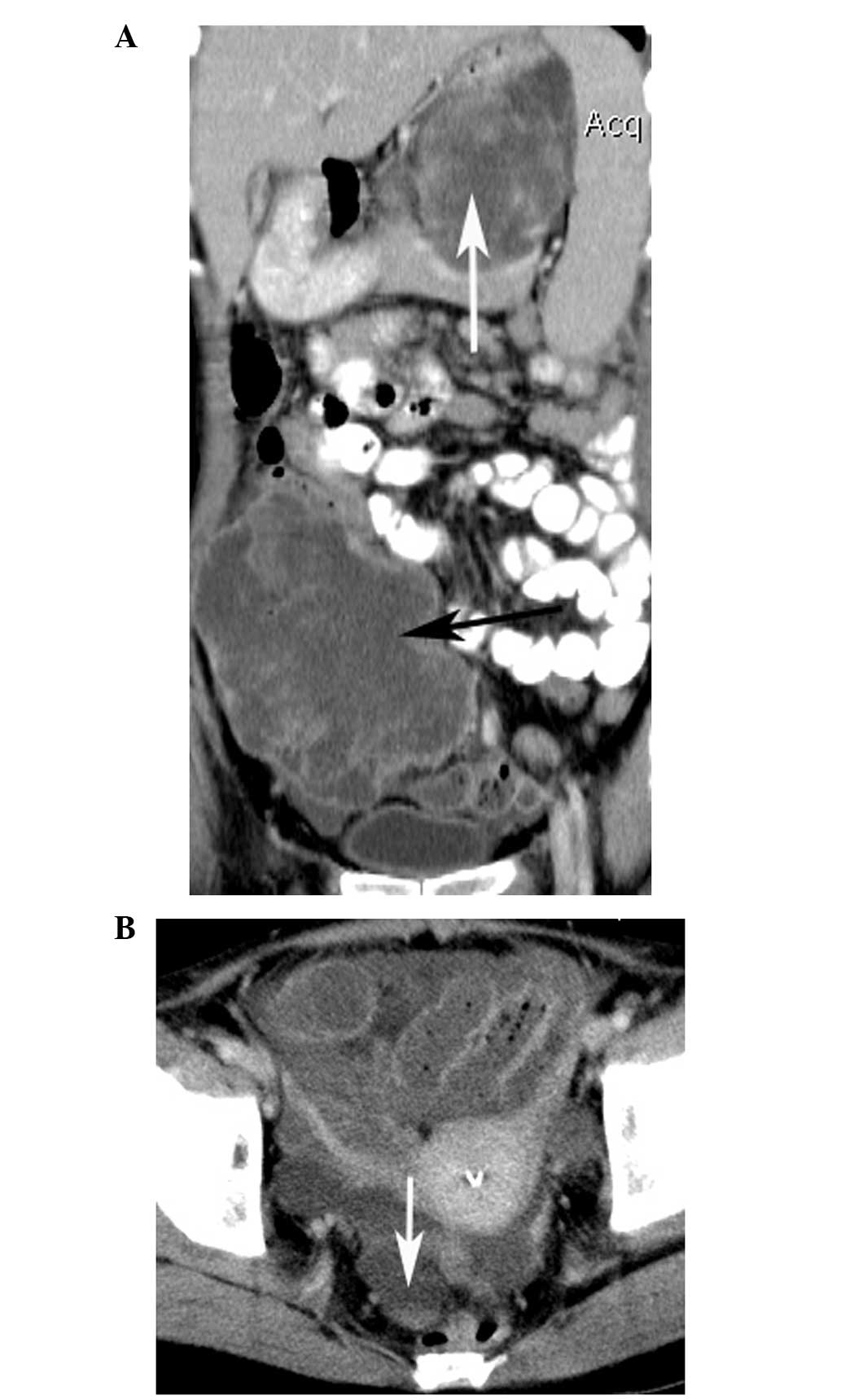
(Figs. 1 and 2). A total of 21 tumors (87.5%) showed a
cystic-necrotic component (Figs.
1–5). Only one tumor showed
mild enhancement, while the others (n=23; 95.8%) demonstrated
marked enhancement (Figs. 1 and
3–6). Overall, 21 tumors (87.5%) showed
heterogeneous enhancement in the arterial and venous phases
(Figs. 1 and 4–6).
Calcification was found in one tumor and hemorrhage in two tumors
(Fig. 7). Engorged tumor vessels
were apparent in 13 masses (54.2%; Figs. 1 and 4). Ascites was observed in three patients
(12.5%; Fig. 6B). Enlarged
mesenteric lymph nodes without necrosis were observed in one tumor
and were proved to be metastases during surgery (Fig. 5). Distant metastases were present in
10 patients (41.7%). The locations of metastases were the adrenal
gland alone (n=1; Fig. 6A), the
liver alone (n=8; Fig. 1D), and the
liver and peritoneum (n=1). All metastases were heterogeneously
enhanced and the hepatic metastases were peripherally enhanced with
necrotic centers (Figs. 1D and
6A).
Correlations between tumor grade and
radiological findings
Statistical analyses showed that tumor size
(P=0.041), tumor borders (P=0.021), tumor vessels (P=0.023) and
distant metastasis (P=0.019) correlated with high-grade EGISTs.
However, tumor localization, tumor contours, cystic-necrotic
components, calcification, hemorrhage, degree and pattern of
enhancement, ascites and lymphadenopathy did not exhibit
significant differences (P>0.05) between the low- and high-grade
EGISTs. The radiological findings of the EGISTs of differing grades
are summarized in Table III.
 | Table IIIComputed tomography and magnetic
resonance imaging findings of 24 patients with
extragastrointestinal stromal tumors. |
Table III
Computed tomography and magnetic
resonance imaging findings of 24 patients with
extragastrointestinal stromal tumors.
| Criteria | Low-grade
(n=7) | High-grade
(n=17) | P-value |
|---|
| Localization |
| Omentum | 1 | 3 | 0.678 |
| Mesentery | 6 | 13 | |
|
Retroperitoneum | - | 1 | |
| Size, cm |
| ≤5 | 2 | 0 | 0.041 |
| 5–10 | 2 | 3 | |
| >10 | 3 | 14 | |
| Contours |
| Round or oval | 5 | 11 | 1.000 |
| Irregular | 2 | 6 | |
| Borders |
| Ill-defined | 2 | 14 | 0.021 |
| Well-defined | 5 | 3 | |
| Cystic-necrotic
component |
| Present | 6 | 15 | 1.000 |
| Absent | 1 | 2 | |
| Hemorrhage |
| Present | - | 2 | 1.000 |
| Absent | 7 | 15 | |
| Calcification |
| Present | 1 | - | 0.292 |
| Absent | 6 | 17 | |
| Degree of
enhancement |
| Mild | 1 | - | 0.292 |
| Marked | 6 | 17 | |
| Pattern of
enhancement |
| Homogeneous | 1 | 2 | 1.000 |
| Heterogeneous | 6 | 15 | |
| Tumor vessels |
| Present | 1 | 12 | 0.023 |
| Absent | 6 | 5 | |
| Ascites |
| Present | 1 | 2 | 1.000 |
| Absent | 6 | 15 | |
|
Lymphadenopathy |
| Present | - | 1 | 1.000 |
| Absent | 7 | 16 | |
| Distant
metastasis |
| Present | - | 10 | 0.019 |
| Absent | 7 | 7 | |
Discussion
The interstitial cells of Cajal, pace-maker cells
that control GI track peristalsis and express the KIT antigen, are
believed to be the origin of GISTs. The occurrence of GISTs as
primary tumors in extragastrointestinal intra-abdominal tissues,
such as the mesentery, omentum, retroperitoneum, abdominal wall,
gallbladder, pancreas and rectovaginal septum, occurs rarely
(3,4,11–13).
Due to the similar histological appearance and immunophenotype
compared with GISTs, EGISTs are believed to be representations of
either GISTs that have separated from the GI tract wall or
independent mesenchymal cell growth of the mesentery, omentum and
retroperitoneum (5,14). The incidence of EGISTs is uncertain
with regard to gender (3,4,13,15–17).
The present study exhibits a slight male predominance (54.2%;
13/24). However, EGISTs occur predominantly in adults, with a mean
age of between 50 and 60 years (4,13,16).
In the present study, all patients were of an advanced age (mean
age, 53 years old), with none being children or adolescents.
Previous studies have shown that the majority of EGISTs are large
when first diagnosed, with a mean size ranging between 10 and 18 cm
(4,13,16).
Small EGISTs rarely produce symptoms due to their atypical site.
EGISTs are often diagnosed incidentally during investigations for
other symptoms. In the present patient group, 70.8% (17/24) of the
EGISTs were >10 cm and the most common clinical symptoms,
including an abdominal or pelvic mass, abdominal pain and abdominal
distension, were non-specific. EGISTs have a predilection for the
areas of the mesentery (22.2–42.9%), omentum (25–28.6%) and
retroperitoneum (10.7–33.3%) (4,13,16).
In the present study, EGISTs in the mesentery, omentum and
retroperitoneum were involved in 79.2, 16.7 and 4.2% of cases
respectively.
The histopathological appearance of EGISTs is
variable, but, in general, three subtypes, including spindle,
epithelioid and mixed cell types, are noted. In the present study,
the EGISTs predominantly displayed the spindle cell subtype (87.5%;
21/24), which is consistent with previous studies (13,16,17).
KIT is overexpressed at a high frequency (96.4–100%) when detected
by an immunohistochemical method and has been shown to be a good
immunomarker for diagnosing EGISTs (16,18).
Thus, KIT-negative EGISTs are rare and their clinicopathological
features have not been well documented (2,16,19).
Yamamoto et al (2) reported
that a preference for an omental origin and an epithelioid cell
subtype characterized KIT-negative EGISTs. The present study showed
that 91.7% (22/24) of the tumors were positive for KIT, with only
two KIT-negative EGISTs, both of mesenteric origin and of
epithelioid or mixed cell subtype. CD34 staining was positive in
70.8% of the tumors, which is similar to the values previously
reported in EGISTs (4,15). The accurate risk stratification of
EGISTs has become increasingly important owing to emerging adjuvant
imatinib therapy. Based on GIST size and mitotic count, the
National Institutes of Health consensus classification system is
commonly used to assess prognosis subsequent to surgery (20). However, Yamamoto et al
(18) found that in KIT-positive
EGISTs, the mitotic count, but not the tumor size, was correlated
with a worse prognosis. Consequently, the present study adopted
their findings to define a grading method on the basis of mitotic
count.
Numerous studies have reported the CT and MRI
features of primary GISTs, including heterogeneous enhancement,
exophytic growth, a size of >5 cm, a necrotic or cystic center,
mucosal ulceration, tumor vessels and aneurysmal dilatation
(7–10). Metastases are found most commonly in
the liver (15.9–34.6%) followed by the mesentery (26%) and
peritoneum (11.5–13.0%) (7,9,10). The
water-like attenuation or signal intensity in the center of
metastases indicates necrosis or cystic degeneration, and the
peripheral hypervascular portion represents solid tumor.
Lymphadenopathy is not a feature of GISTs (8,9).
Calcification, hemorrhage and ascites are rare characteristics in
GISTs (7–10). Tateishi et al (10) reported that CT findings of a large
tumor size ≥11.1 cm, unclear boundaries, an irregular surface,
heterogeneous enhancement, the presence of invasion, hepatic
metastasis and peritoneal dissemination were favorable for a
diagnosis of high-grade GIST and affected the five-year survival
rate. Similarly, Ulusan et al (7) found that heterogeneous enhancement,
size (>10 cm), localization, cystic-necrotic components and
metastases were correlated with malignant GIST. To the best of our
knowledge, few studies have reported the CT and MRI findings of
EGISTs. Due to the rarity of EGISTs, much of the available
radiological information is derived from small case series, which
identify EGISTs as large masses with solid and cystic components
and without an air-fluid level (3–6,11–13,15–19).
In the present study, the majority of the EGISTs appeared as round
or oval (66.7%; 16/24), cystic-solid (87.5%; 21/24) and ill-defined
(66.7%; 16/24) soft-tissue masses. The EGISTs were hypodense
(69.6%; 16/23) or isodense (30.4%; 7/23) on CT images, hypointense
(50%; 3/6), isointense (33.3%; 2/6) or hyperintense (16.7%; 1/6) on
T1WI, and hyperintense on T2WI (100%; 6/6) and DWI (100%; 6/6).
These results show that the attenuation and intensity of EGISTs are
non-specific and that DWI is unable to differentiate low- and
high-grade EGISTs. In the study, 54.2% (13/24) of EGISTs displayed
tumor vessels, 95.8% (23/24) of the masses showed marked
enhancement and 87.5% (21/24) demonstrated heterogeneous
enhancement. Calcification, hemorrhage, ascites and lymphadenopathy
were rare signs, and metastases were most common in the liver
(37.5%; 9/24). Analyses revealed that tumor size, borders and
vessels, and distant metastasis correlate with high-grade EGISTs.
The imaging findings of EGISTs in the present study exhibit certain
differences compared with those found in GISTs and EGISTs (3,4,7–10).
The discrepancy may be caused by the small number of EGISTs or the
differing pathological behavior between GISTs and EGISTs.
Differentiation between EGISTs and other
intra-abdominal tumors, including benign cystic masses,
leiomyosarcoma, malignant fibrous histiocytoma, fibrosarcoma,
liposarcoma and solitary fibrous tumors, by radiology is difficult
without surgical pathology (2,3). These
non-EGISTs may share the majority of imaging characteristics with
EGISTs. The present results showed that EGISTs tend to be
characterized by certain features, such as advanced patient age,
large tumor size, cystic-necrotic components, rare lymphadenopathy,
a pattern of heterogeneous enhancement and hepatic metastasis.
Also, several radiological characteristics of EGISTs, including the
size, borders, tumor vessels and distant metastasis, can provide
useful information in the differentiation between low- and
high-grade EGISTs.
The present study has several limitations. Firstly,
the study is retrospective. Secondly, the number of patients is
small. Owing to the rarity of EGISTs, a large multi-institutional
study on the radiological diagnosis of EGISTs is therefore
required.
In conclusion, EGISTs are rare and aggressive tumors
with a predilection for the mesentery, omentum and retroperitoneum.
CT and MRI can accurately reveal the location and extent of EGISTs,
and certain features, such as advanced patient age, large tumor
size, cystic-necrotic components, rare lymphadenopathy, a pattern
of heterogeneous enhancement and hepatic metastasis may aid in the
diagnosis of EGISTs. Also, radiological characteristics, such as a
large tumor size (>10 cm), ill-defined borders, tumor vessels
and distant metastasis, can provide useful information in
identifying the malignant behavior of EGISTs.
Acknowledgements
The current study was supported by the Foundation of
Shanghai Science and Technology Committee (no. 41902502) and the
Shanghai Health Bureau (no. 2012198).
References
|
1
|
Joensuu H: Risk stratification of patients
diagnosed with gastrointestinal stromal tumor. Hum Pathol.
39:1411–1419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamamoto H, Kojima A, Nagata S, Tomita Y,
Takahashi S and Oda Y: KIT-negative gastrointestinal stromal tumor
of the abdominal soft tissue: a clinicopathologic and genetic study
of 10 cases. Am J Surg Pathol. 35:1287–1295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim HC, Lee JM, Kim SH, et al: Primary
gastrointestinal stromal tumors in the omentum and mesentery: CT
findings and pathologic correlations. AJR Am J Roentgenol.
182:1463–1467. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barros A, Linhares E, Valadão M, Gonçalves
R, Vilhena B, Gil C and Ramos C: Extragastrointestinal stromal
tumors (EGIST): a series of case reports. Hepatogastroenterology.
58:865–868. 2011.PubMed/NCBI
|
|
5
|
Fagkrezos D, Touloumis Z, Giannila M,
Penlidis C, Papaparaskeva K and Triantopoulou C:
Extra-gastrointestinal stromal tumor of the omentum: a rare case
report and review of the literature. Rare Tumors. 4:e442012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goukassian ID, Kussman SR, Toribio Y and
Rosen JE: Secondary recurrent multiple EGIST of the mesentery: A
case report and review of the literature. Int J Surg Case Rep.
3:463–466. 2012. View Article : Google Scholar
|
|
7
|
Ulusan S, Koc Z and Kayaselcuk F:
Gastrointestinal stromal tumours: CT findings. Br J Radiol.
81:618–623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim HC, Lee JM, Choi SH, et al: Imaging of
gastrointestinal stromal tumors. J Comput Assist Tomogr.
28:596–604. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sandrasegaran K, Rajesh A, Rushing DA,
Rydberg J, Akisik FM and Henley JD: Gastrointestinal stromal
tumors: CT and MRI findings. Eur Radiol. 15:1407–1414. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tateishi U, Hasegawa T, Satake M and
Moriyama N: Gastrointestinal stromal tumor. Correlation of computed
tomography findings with tumor grade and mortality. J Comput Assist
Tomogr. 27:792–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Peng Z and Xu L:
Extragastrointestinal stromal tumor arising in the rectovaginal
septum: report of an unusual case with literature review. Gynecol
Oncol. 113:399–401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alkhatib L, Albtoush O, Bataineh N,
Gharaibeh K, Matalka I and Tokuda Y: Extragastrointestinal stromal
tumor (EGIST) in the abdominal wall: Case report and literature
review. Int J Surg Case Rep. 2:253–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agaimy A and Wünsch PH: Gastrointestinal
stromal tumours: a regular origin in the muscularis propria, but an
extremely diverse gross presentation. A review of 200 cases to
critically re-evaluate the concept of so-called
extra-gastrointestinal stromal tumours. Langenbecks Arch Surg.
391:322–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goh BK, Chow PK, Kesavan SM, Yap WM, Chung
YF and Wong WK: A single-institution experience with eight
CD117-positive primary extragastrointestinal stromal tumors:
critical appraisal and a comparison with their gastrointestinal
counterparts. J Gastrointest Surg. 13:1094–1098. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reith JD, Goldblum JR, Lyles RH and Weiss
SW: Extragastrointestinal (soft tissue) stromal tumors: an analysis
of 48 cases with emphasis on histologic predictors of outcome. Mod
Pathol. 13:577–585. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim KH, Nelson SD, Kim DH, et al:
Diagnostic relevance of overexpressions of PKC-θ and DOG-1 and
KIT/PDGFRA gene mutations in extragastrointestinal stromal tumors:
a Korean six-centers study of 28 cases. Anticancer Res. 32:923–937.
2012.PubMed/NCBI
|
|
17
|
Patnayak R, Jena A, Parthasarathy S, et
al: Primary extragastrointestinal stromal tumors: a
clinicopathological and immunohistochemical study - a tertiary care
center experience. Indian J Cancer. 50:41–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto H, Oda Y, Kawaguchi K, et al:
c-kit and PDGFRA mutations in extragastrointestinal stromal tumor
(gastrointestinal stromal tumor of the soft tissue). Am J Surg
Pathol. 28:479–488. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ogawa H, Gotoh K, Yamada T, et al: A case
of KIT-negative extra-gastrointestinal stromal tumor of the lesser
omentum. Case Rep Gastroenterol. 6:375–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fletcher CD, Berman JJ, Corless C, et al:
Diagnosis of gastrointestinal stromal tumors: A consensus approach.
Hum Pathol. 33:459–465. 2002. View Article : Google Scholar : PubMed/NCBI
|