Introduction
In recent decades, cancer has become a main cause of
mortality in humans. Worldwide, ~12.73 million cases of cancer
occur annually, resulting in 7.6 million mortalities. Colorectal
cancer is one of the most common types of gastrointestinal
malignancies. Of all cancers, it has the third highest global
incidence, the second highest mortality rate in developed countries
(1) and the fifth highest mortality
rate in China (2).
The pathogenesis of malignancy is complex and
depends on the genetic, environmental, lifestyle and other factors
of the host. Furthermore, infectious factors exhibit a significant
influence on the development and progression of malignancy
(3–6). It is clear that there is a close
association between certain cancers and infection by pathogenic
microorganisms, including hepatitis B, hepatitis C, Helicobacter
pylori, Clonorchis sinensis and Schistosoma
mansoni. Human cytomegalovirus (HCMV) is also considered to be
closely associated with cancer (7).
As the HCMV gene and protein expression can be detected in
colorectal cancer tissue (8,9), it
was hypothesized that HCMV infection may be associated with
colorectal cancer. However, certain studies have opposed this
hypothesis; the expression of HCMV in frozen tissues of colorectal
carcinoma was investigated by immunohistochemistry and nested
polymerase chain reaction (PCR) and negative results were
demonstrated and, thus, the authors did not identify an association
between HCMV and colorectal cancer (10–12).
Therefore, further study is required to determine whether the
occurrence and development of colorectal cancer is closely
associated with HCMV and, if they are associated, the mechanism of
this association also remains to be elucidated.
The present study aimed to examine the mechanism of
the occurrence and development of colorectal cancer, which is
associated with HCMV infection and the signal transduction pathways
of TLRs. Furthermore, the expression levels of inflammatory
cytokines were detected in colorectal carcinoma.
Materials and methods
Collection of specimens and clinical
data
Samples were collected from the Department of
General Surgery, the Second Affiliated Hospital of Qingdao
University Medical College (Qingdao, China) between March and
September 2012, which included 56 cases of colorectal cancer and 36
cases of colon adenoma. Samples of the normal mucosa adjacent to
the cancerous tissue were used as controls. The cancer cases
included 30 males and 26 females with a median age of 67 years
(range, 46–78 years). A total of 12 cases of highly differentiated
adenocarcinoma, 26 cases of moderately differentiated
adenocarcinoma and 18 cases of poorly differentiated adenocarcinoma
were identified. None of the patients had received chemotherapy or
radiotherapy. The specimens were embedded in paraffin for
immunohistochemical staining. This study was approved by the ethics
committee of Qingdao University (Qingdao, China) and written
informed consent was obtained from all patients.
Immunohistochemistry
Mouse anti-human monoclonal HCMV IE1–72 antibody was
purchased from Abcam (Cambridge, UK) and used at a 1:40 dilution.
Rabbit anti-human polyclonal antibodies against TLR2, TLR4, NF-κB,
interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α were
purchased from Wuhan Boster Biological Engineering Co., Ltd.
(Wuhan, China) and used at dilutions of 1:100. The
immunohistochemistry kits were also purchased from Wuhan Boster
Biological Engineering Co. Ltd.
For streptavidin-biotin complex (SABC)
immunohistochemistry, the biopsy specimens were paraffin-embedded,
cut into 4-μm sections, dewaxed and antigen-repaired in the
microwave for 10 min. The sections were then treated with the
primary antibody at 37°C for 65 min and the secondary antibody at
37°C for 20 min in a water bath. The SABC was added at 37°C for 20
min and washed with phosphate-buffered saline (PBS) four times,
subsequent coloration with 3,3-diaminobenzidine for 15 min and
washing with distilled water, the samples were stained with
hematoxylin followed by dehydration, clearing and mounting with
neutral gums and observed under a microscope (C5050, Olympus
Corporation, Tokyo, Japan) PBS was used instead of primary antibody
as a negative control. The tissues The positive TLR2, TLR4, NF-κB
and TNF-α (Wuhan Boster Biological Engineering Co., Ltd.) were used
as positive controls for TLR2, TLR4, NF-κB and TNF-α expression.
IE1–72, NF-κB and TNFα localized in the cytoplasm and nucleus; TLR2
and TLR4 localized in the cytoplasm and membrane. The result was
determined by the phase-weighting method based on the number of
positive cells and staining intensity (14). Samples in which the number of
positive cells was <5% received a score of 0, 5–24% received a
score of 1, 25–49% received a score of 2, 50–74% received a score
of 3, ≥75% received a score of 4. Staining intensity was scored as
follows: Yellow staining, 1; brown staining, 2; and tan staining,
3. The total staining score was calculated as the sum of the
percentage positivity and staining intensity scores; a total score
of 1–2 was considered negative staining (−), while a total score of
≥3 was considered positive staining (+).
Infection of SW480 cells in vitro
SW480 cells, a colon cancer cell line from the
Department of Microbiology (Qingdao University), were used and the
AD169 strain of HCMV was donated by the Pasteur Institute (Paris,
France). Virus multiplicity of infection (MOI) was calculated as
the virus plaque forming units per ml divided by the cell count. In
the preliminary experiment, the majority of cells were infected
when the MOI was 5. Thus, an MOI of 5 was used throughout the rest
of the study.
For infection, a single cell suspension of SW40
cells was cultured in a 0.01% polylysine-coated 60-mm dish. A total
of five dishes were used for the experimental group (infection
group) and a control group. The infection group was treated with
HCMV (MOI=5) and the control group was treated with an equivalent
volume of D/F12 (200μl; 1:1) medium prior to culturing the cells
for 3 days at 37°C. Morphological changes were observed with an
inverted microscope (XD-101, Jiangnan Photoelectricity Co., Ltd.,
China) every day. Cells were then collected to extract the mRNA for
reverse transcription (RT)-PCR in the pre-infection stage (0 h) and
at 6, 12, 24, 48 and 72 h following infection.
RT-PCR
Total RNA was extracted from cells using TRIzol
reagent (Wuhan Boster Biological Engineering Co., Ltd.) and a UV
spectrophotometer (Beijing Purkinje General Instrument Co., Ltd.,
Beijing, China) was used to determine RNA concentration and purity.
cDNA was reverse transcribed using a reverse transcription kit
[Promega (Beijing) Biotech Co., Ltd, Beijing, China] in accordance
with the manufacturer’s instructions. The reaction product was
preserved at −20°C. The primers were designed according to the HCMV
AD l69-specific gene sequence of IE1–72 (GenBank accession no.
X17403.1). The primers were synthesized by Shanghai Sangon
Biological Engineering Technology & Services Co., Ltd., and the
sequences are shown in Table I.
RT-PCR was performed. Briefly, the target gene was amplified by
PCR, using the following reaction conditions: initial denaturation
at 94°C for 5 min, denaturation at 94°C for 30 sec, primer
annealing at 55°C for 30 sec, elongation at 72°C for 30 sec, all
for 30 cycles, final elongation at 72°C for 10 min.
 | Table IPrimers used in this study. |
Table I
Primers used in this study.
| Genetic element | PCR product size | Sequence |
|---|
| IE1–72 (F) | 251 bp |
5′-GAGTCCTCTGCCAAGAGAAA-3′ |
| IE1–72 (R) | |
5′-GAGTTCTGCCAGGACATCTTT-3′ |
| TLR2 (F) | 405 bp |
5′-TCGGAATGTCACAGGACAGC-3′ |
| TLR2 (R) | |
5′-CAGTTCATACTTGCACCACTCAC-3′ |
| TLR4 (F) | 170 bp |
5′-TGAGCAGTCGTCCTGGTATC-3′ |
| TLR4 (R) | |
5′-CAGGGCTTTTCTGAGTCGTC-3′ |
| NF-κB (F) | 398 bp |
5′-GAAGAAGCGAGACCTGGAG-3′ |
| NF-κB (R) | |
5′-TCCGGAACACAATGGCCAC-3′ |
| TNF-α (F) | 171 bp |
5′-TCGGAATGCACAGGACAGC-3′ |
| TNF-α (R) | |
5′-CAGTTCATACTTGCACCACTCAC-3′ |
| β-actin (F) | 154 bp |
5′-TGGAACGGTGAAGGTGACAG-3′ |
| β-actin (R) | |
5′-GGCTTTTAGGATGGCAAGGG-3′ |
The PCR products were analyzed by 1.5% agarose gel
electrophoresis, and the results were observed under ultraviolet
illumination. When a band clearly appeared in the appropriate size
range, it was considered to be positively amplified. Images of the
resulting gels were captured using the UVP gel imaging system (UVP
Co. Ltd., Upland, CA, USA). Quantitative and qualitative analyses
of the results were performed based on gray-scale values by the
Quantity One imaging system (Bio-Rad Laboratories, Hercules, CA,
USA). The gray-scale value ratio of the target gene and the
internal reference, β-actin, was considered as the relative content
of the mRNA of each gene. The experiment was performed in
triplicate for each sample.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA) to perform
the χ2 test, Spearman’s correlation analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of IE1–72, TLR2, TLR4, NF-κB
and TNF-α in colorectal carcinoma and adenoma tissue is
significantly higher than in normal mucosal tissues adjacent to
cancer
Immunohistochemical analysis revealed that the
expression of IE1–72, TLR2, TLR4, NF-κB and TNF-α protein in
colorectal carcinoma was significantly higher than that in the
normal tissues. The differences were statistically significant
(P<0.05), and the expression levels of IE1–72, TLR4 and TNF-α in
the colon adenoma were evidently higher than those in normal tissue
(P<0.05) (Table II; Fig. 1). The results indicated that HCMV
infection may be associated with colorectal cancer and adenoma and
inflammatory responses may be mediated by the TLR2 or TLR4
signaling pathways. Therefore, further experiments at the cellular
level were conducted to confirm this.
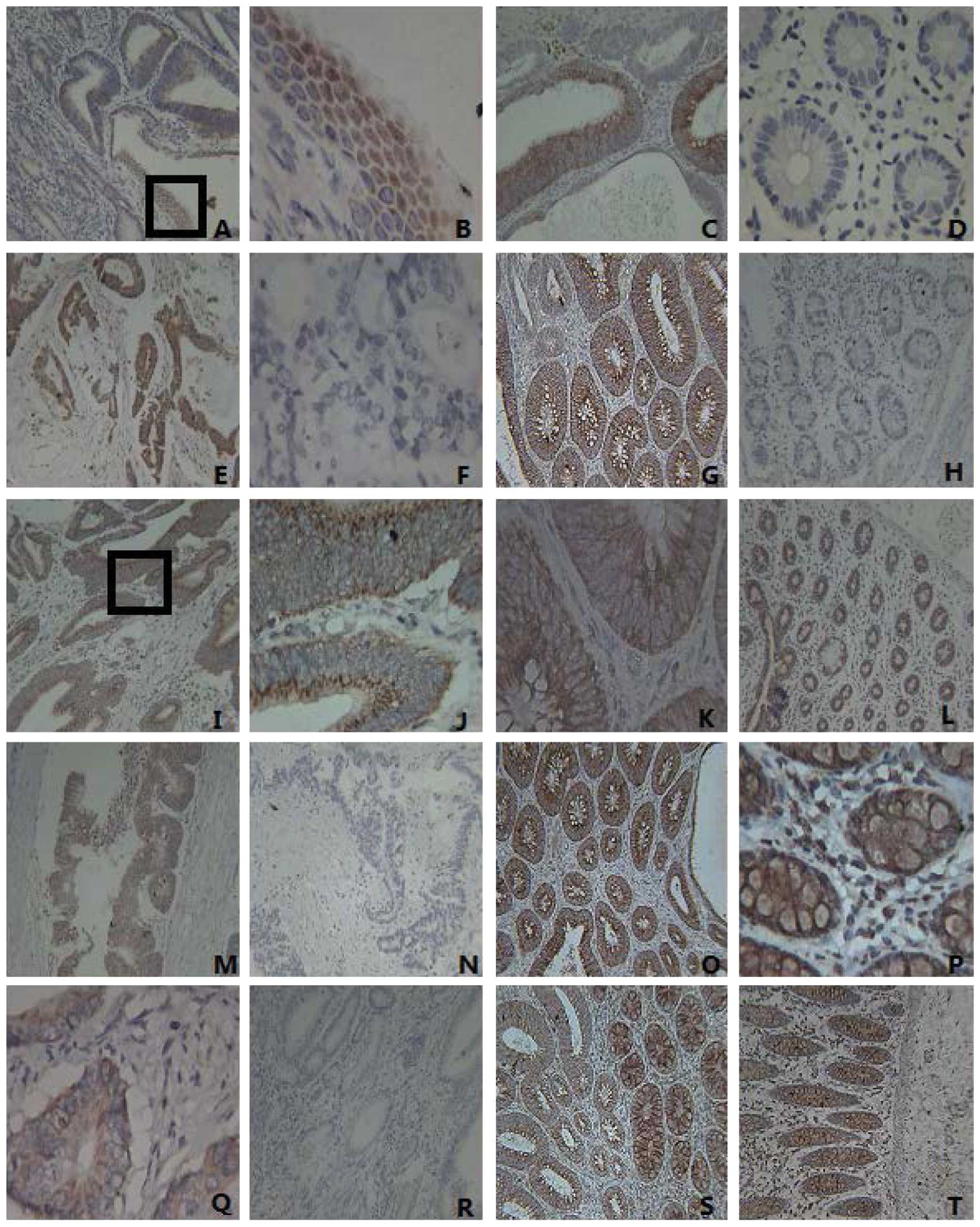 | Figure 1Expression of IE1–72, TLR2, TLR4,
NF-κB and TNF-α in colorectal cancer tissues, adenoma and normal
tissues. (A) The expression of IE1–72 in colorectal carcinomas was
positive (magnification, ×100). The IE1–72 protein is visible as
brown granules located in the nucleus. (B) IE1–72 expression from
part A (enclosed by a black box) is shown at a higher magnification
(x400). (C) The positive expression of IE1–72 was identified in
adenoma, visualized as brown granules located in the cytoplasm
(magnification, ×100). (D) The negative expression of IE1–72 in
normal tissues (magnification, ×400). (E) The positive expression
of TLR2 in colorectal carcinomas was indicated by brown particles
located in the cytoplasm (magnification, ×100). (F) The negative
expression of TLR2 in colorectal cancer (magnification, ×400). (G)
The positive expression of TLR2 in adenoma (magnification, ×100).
(H) The negative expression of TLR2 in normal tissues
(magnification, ×100). (I) The positive expression of TLR4 in
colorectal carcinomas (x100) was localized by brown particles in
the cell membrane and cytoplasm. (J) TLR4 expression from part I
(enclosed by a black box) is shown at a higher magnification
(x400). (K) The positive expression of TLR4 in adenoma corresponds
to the colored portions of cytoplasm and membrane (magnification,
×400). (L) The positive expression of TLR4 in normal tissues
(magnification, ×100). (M) The positive expression of NF-κB in
colorectal cancer tissues (magnification, ×100×). (N) The negative
expression of NF-κB in colorectal cancer tissues (magnification,
×100). (O) The positive expression of NF-κB in adenoma
(magnification, ×100). (P) The positive expression of NF-κB in
adjacent normal tissue, where it appears as clear dark brown
particles in the cytoplasm (magnification, ×400). (Q) Positive
TNF-α protein expression observed mainly in the cytoplasm (brown
particles) in colorectal cancer tissues (magnification ×400). (R)
The negative expression of TNF-α in colorectal cancer
(magnification, ×100). (S) The positive expression of TNF-α in
colorectal adenoma (magnification, ×100). (T) The positive
expression of TNF-α in normal tissue (magnification, ×100). TLR,
Toll-like receptor; NF, nuclear factor; TNF, tumor necrosis
factor. |
 | Table IIExpression of IE1–72, TLR2, TLR4,
NF-κB and TNF-α in colorectal cancer, adenoma and normal tissues,
which were adjacent to the cancer tissues. |
Table II
Expression of IE1–72, TLR2, TLR4,
NF-κB and TNF-α in colorectal cancer, adenoma and normal tissues,
which were adjacent to the cancer tissues.
| Positive expression,
% (n) |
|---|
|
|
|---|
| Tissue type | IE1–72 | TLR2 | TLR4 | NF-κB | TNF-α |
|---|
| Adenocarcinoma
(n=56) | 44.6 (25) | 69.6 (39) | 73.2 (41) | 80.4 (45) | 80.4 (45) |
| Normal mucosa
(n=56) | 12.5 (7) | 42.8 (24) | 35.7 (20) | 39.2 (22) | 33.9 (19) |
| Adenoma (n=36) | 41.7 (15) | 55.5 (20) | 83.3 (30) | 83.3 (20) | 66.6 (24) |
Correlation between the protein
expression of IE1–72, TLR2 and TLR4 in colorectal carcinoma
In 25 positive cases of IE1–72, there were 24
positive cases of TLR2 and 24 positive cases of TLR4 in the
colorectal cancer samples. Statistical analysis of the results
revealed that the correlation coefficients between IE1–72 and TLR2
and TLR4 were 0.515 and 0.462, respectively. HCMV infection was
found to correlate with the expression of TLR2 and TLR4 in
colorectal cancer.
mRNA levels of TLR2 IE1–72, TLR4 and
NF-κB in SW480 cells following infection
Prior to infection (0 h), no expression of IE1–72
was identified, the expression levels of TLR2 and TNF-α were low,
and the expression levels of TLR4 and NF-κB were high. The
expression of IE1–72 increased after 6 h, reached a peak after 48 h
and decreased after 72 h. The expression of TLR2 and TNF-α
increased gradually with IE1–72 and reached a peak at 48 h. No
significant differences in TLR4 and NF-κB expression were
identified (Figs. 2 and 3). HCMV infection may upregulate the
expression of TLR2 and TNF-α.
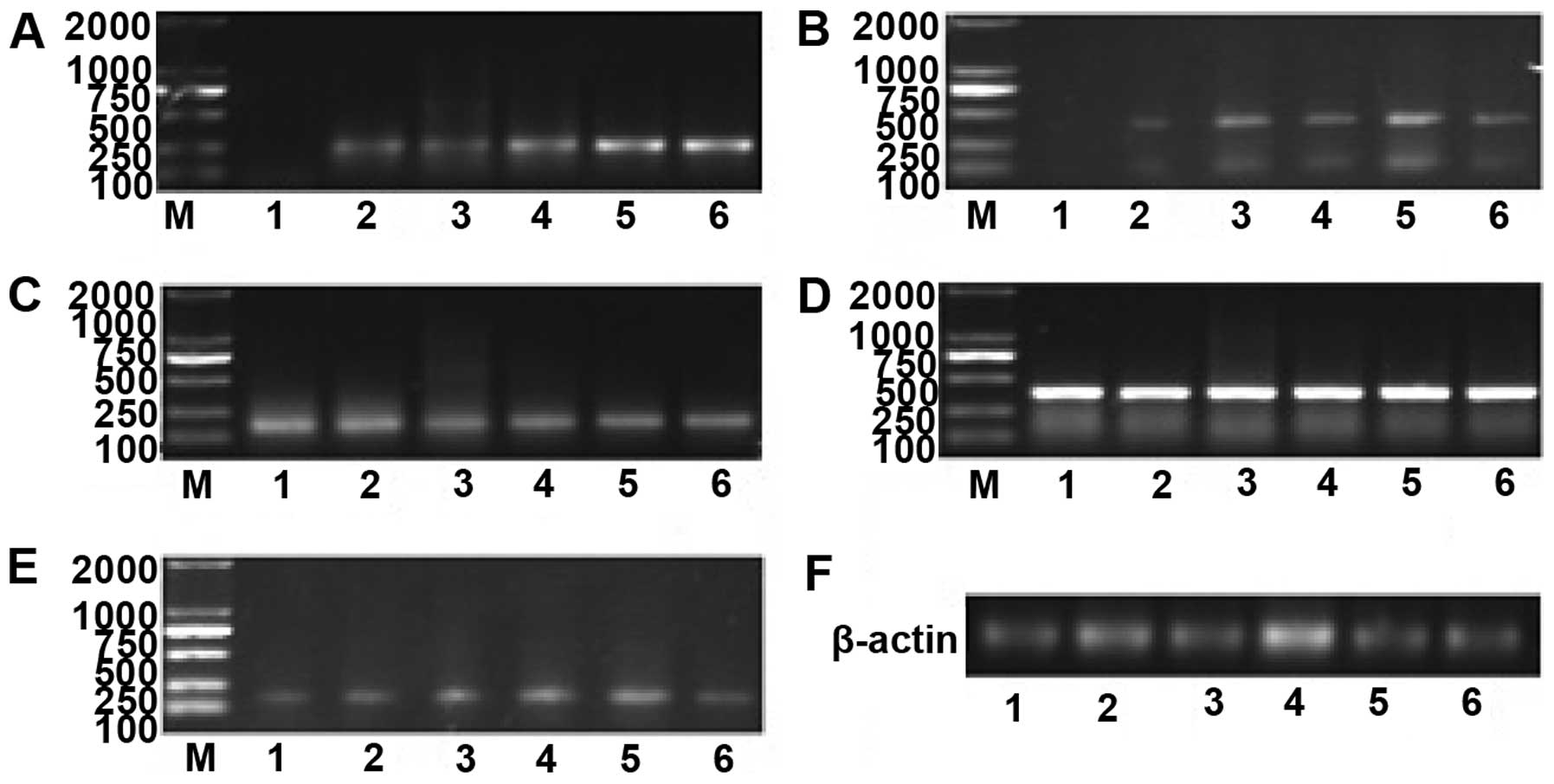 | Figure 2mRNA expression of IE1–72, TLR2, TLR4,
NF-κB and TNF-α at various time points after SW480 cells were
infected. (A) mRNA expression of IE1–72 increased in a
time-dependent manner. (B) TLR2 mRNA levels were upregulated at 6
h, exhibited a prominent increase at 12 h, reached their peak at 48
h and decreased gradually after 48 h. (C)No significant differences
in TLR4 mRNA were identified. (D) NF-κB mRNA was expressed at high
levels, and no significant difference in expression was identified
over time. (E) TNF-α mRNA began to increase at 6 h, peaked at 48 h,
and decreased gradually after 48 h. (F) The internal reference
gene, β-actin. 1, prior to HCMV infection (0 h); 2, 6 h after
infection; 3, 12 h after infection; 4,24 h after infection; 5, 48 h
after infection; and 6, 72 h after infection; M: marker. TLR,
Toll-like receptor; NF, nuclear factor; TNF, tumor necrosis factor;
HMCV, human cytomegalovirus. |
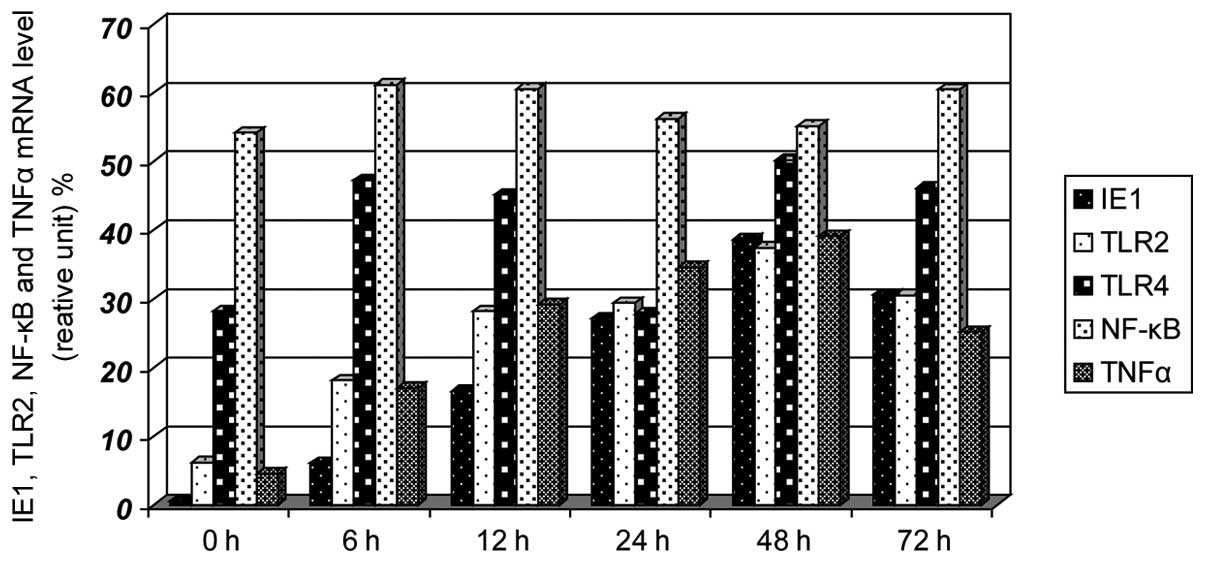 | Figure 3Effects on the mRNA expression levels
of IE1–72, TLR2, TLR4, NF-κB and TNF-α following SW480 cell HCMV
infection. After SW480 cells were infected with HCMV, the mRNA
levels of TLR2 and TNF-α increased at 6 h and had increased
significantly by 12 h, reaching peak levels at 48 h, the levels
then decreased. TLR4 and NF-κB showed no clear changes. TLR,
Toll-like receptor; NF, nuclear factor; TNF, tumor necrosis factor;
HMCV, human cytomegalovirus. |
Discussion
HCMV belongs to the β-herpes virus family and is the
largest DNA virus among the human herpes viruses. The human
population is generally susceptible to HCMV. In healthy
individuals, HCMV exhibits the characteristics of
latency-reactivation, which is an important immune escape mechanism
that HCMV uses to establish a long-term coexistence process with
the host. The complex interactions between HCMV and the immune
system are considered to be closely associated with numerous
complex diseases. Although the mechanism of occurrence and
development of cancer is not clear, a number of studies have
detected the expression of HCMV in different tumor tissues. The
expression of the cytomegalovirus gene and protein has been
detected in colorectal cancer, malignant glioma and prostate cancer
and, in these cases, the adjacent normal tissues exhibited a low
expression (15–17). Therefore, the authors hypothesized
that HCMV may cause chronic inflammation and lead to cancer,
similar to other viruses, and that HCMV is important in colon
tumorigenesis development. In vitro studies of HCMV have
also demonstrated that HCMV could induce cell malignancy and
disrupt cell regulatory pathways. In addition, HCMV was found to
exhibit characteristics of tumorigenicity, such as causing gene
mutations and abnormal regulation of the cell cycle,
inhibiting/promoting apoptosis, inducing angiogenesis, inducing
cell immune escape, and regulating the synthesis and secretion of
cytokines in host cells, in addition to other behaviors (18–21).
Although numerous studies have hypothesized that
HCMV infection is involved in the occurrence and development of
cancer, no direct evidence of the underlying mechanism has been
provided. Based on the characteristics of the complex
latent-reactivation of HCMV, studies have used the concepts of ‘hit
and run’ and ‘tumor control’ to describe the mechanism of action of
HCMV in tumorigenesis. The researchers emphasized that the genetic
environment provided by tumor cells is different from that of
normal cells (including transcription factors, signal transduction
pathways and the dysfunction of anti-oncogenic proteins), and that
this environment is advantageous for persistent infection by HCMV
and exhibits a regulatory role in cancer (12,22).
The results of the present study demonstrated that
the positive expression rate of IE1–72 protein in colorectal cancer
and colon adenoma was 44.6 and 41.7%, respectively, which was
significantly higher than the expression in normal tissues
(P<0.05). These results are consistent with the results of the
study by Cobbs et al (15), which
demonstrated a close association between HCMV infection and the
occurrence of colorectal cancer and adenomas. The results of the
present study also show colon adenoma may be the early stage of
precancerous lesions of the colon.
The mechanism by which HCMV enters the colonic
mucosal cells remains unclear. The process by which HCMV causes
chronic inflammation, leading to malignant transformation is also
unclear. It has been reported that glycoproteins on cell membranes
mediated and guided the process of HCMV entry into the host cell
(23). Compton et al
(13) identified a TLR, TLR2, that
recognizes the capsid protein of HCMV, which further activates
NF-κB and, thus, activates the innate immune response and causes
the secretion of inflammatory cytokines. TLRs were identified as
pathogen recognition receptors of the innate immune system; the
activated signal transduction pathway of TLRs is involved in the
classical MyD88 signal transduction pathway. After ligand binding,
TLRs initiate activation of the NF-κB pathway and mitogen activated
protein kinases pathway, resulting in the transcription and release
of cytokines and inflammatory chemotactic factors. TLRs are
important for the body’s resistance to the invasion of pathogenic
organisms (24). It has been found
that the signal transduction pathways mediated by TLRs are
important in the process of tumorigenesis (25). TLR2 may rapidly recognize the
envelope protein of HCMV and trigger immune responses immediately,
prior to the virus entering the cells, which may provide a timing
advantage in the recognition reaction resulting in cells infected
by HCMV (26). TLR2 is one of the
main ways for HCMV to activate innate immune responses (27). As a result of this activation,
certain intracellular components of the infected cells will be
altered or cell disruption occurs and cell lysates are released.
Components of these lysates may be recognized by TLR2 as endogenous
ligands, thus potentiating the immune response via dendritic cells
and macrophages to initiate antiviral effects.
TNF-α is a critical pro-inflammatory cytokine, which
is involved in cancer progression. High expression of TNF-α in the
tumor microenvironment is a common characteristic of numerous
malignant tumors, which are usually associated with poor prognoses.
A number of studies have proposed that TNF-α is essential for
carcinogenesis that is associated with inflammation (10,28,29).
In the present study, the mRNA of TLR2 and its
downstream inflammatory factor, TNF-α, were upregulated with the
increased expression of IE1–72 in SW480 cells following HCMV
infection. This indicated that the release of chronic inflammation
factors caused by infection with HCMV may be achieved via the TLR2
signaling pathway in the tumor microenvironment, thereby further
inducing cell malignancy. To confirm this hypothesis, future
studies are required to evaluate whether the expression of IE1–72
and TNF-α and other inflammatory factors is downregulated following
the blockade of the TLR2 signaling pathway. As tumorigenesis is a
long process, it is difficult to confirm a close association
between viral infection and cancer. Therefore, the examination of
other signaling pathways is required to investigate the effects of
HCMV on malignant transformation in normal colorectal mucosa.
This study also demonstrated that the expression of
TLR4 and NF-κB was increased in colorectal cancer tissue specimens;
however, no significant differences in the mRNA expression of TLR4
and NF-κB were identified. NF-κB is not usually in the active state
in the cytoplasm. Following activation, NF-κB translocates to the
nucleus, binds its target and promotes transcription of the gene
(30,31). Assessment of the activity and
translocation of NF-κB is required in order to understand the
underlying mechanism.
A broad and in-depth examination of the mechanism,
existence and significance of HCMV in colorectal cancer may reveal
novel insights with regard to the etiology of cancer. These
insights may present novel strategies for the prevention of cancer
and identify novel antiviral treatments for patients with HCMV,
leading to the development of highly efficient vaccines and novel
therapeutic approaches.
Acknowledgements
This study was supported by the National Natural
Science Fund (grant no’s. 81070501 and 81471958) and the Planning
Project of Science and Technology in Colleges and Universities in
Shandong Province (grant no. J12LL11). The authors would like to
thank Dr Xu Jing and Dr Qi Yao (Department of Pathology, Second
Affiliated Hospital, Medical College, Qingdao University), and Mr.
Wang Zhihao and Mr. Wang Yanlei (Department of Microbiology,
Medical College, Qingdao University), for their technical
assistance during the study.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
JH, PZ and WQC: 2012, China cancer
registration report. Military medical press; pp. 15–16. 2013
|
|
3
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hussain SP and Harris CC: Inflammation and
cancer: an ancient link with novel potentials. Int J Cancer.
121:2373–2380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuper H, Adami HO and Trichopoulos D:
Infections as a major preventablecause of human cancer. J Intern
Med. 248:171–183. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fidler IJ: Modulation of the organ
microenvironment for treatment of cancer metastasis. J Natl Cancer
Inst. 87:1588–1592. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soroceanu L and Cobbs CS: Is HCMV a tumor
promoter? Virus Res. 157:193–203. 2011. View Article : Google Scholar :
|
|
8
|
Harkins L, Volk AL, Samanta M, et al:
Specific localization of human cytomegalovirus nucleic acids and
proteins in human colorectal tumor. Lancet. 360:1557–1563. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen HP, Jiang JK, Chen CY, et al: Human
cytomegalovirus preferentially infects the neoplastic epithelium of
colorectal cancer: a quantitative and histological analysis. J Clin
Virol. 54:240–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akintola-Ogunremi O, Luo Q, He TC and Wang
HL: Is cytomegalovirus associated with human colorectal
tumorigenesis? Am J Clin Pathol. 123:244–249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knösel T, Schewe C, Dietel M and Petersen
I: Cytomegalovirus is not associated with progression and
metastasis of colorectal cancer. Cancer Lett. 211:243–247. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bender C, Zipeto D, Bidoia C, et al:
Analysis of colorectal cancers for human cytomegalovirus presence.
Infect Agent Cancer. 4:62009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Compton T, Kurt-Jones EA, Boehme KW, et
al: Human cytomegalovirus activates inflammatory cytokine responses
via CD14 and Toll-like receptor 2. J Virol. 77:4588–4596. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawasaki H, Altieri DC, Lu CD, et al:
Inhibition of apoptosis by survivin predicts shorter survival rates
in colorectal cancer. Cancer Res. 58:5071–5074. 1998.PubMed/NCBI
|
|
15
|
Harkins L, Volk AL, Samanta M, et al:
Specific localization of human cytomegalovirus nucleic acids and
proteins in human colorectal tumor. Lancet. 360:31557–1563. 2002.
View Article : Google Scholar
|
|
16
|
Cobbs CS, Harkins L, Samanta G, et al:
Human cytomegalovirus infection and expression in human malignant
glioma1. Cancer Res. 62:3347–3350. 2002.PubMed/NCBI
|
|
17
|
Samanta M, HarIim L, Klemm K, et al: High
prevalence of humancytomegalovims in prostatic intraepithelial
neoplasm and prostatic carcinoma. J Urol. 170:998–1002. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barami K: Oncomodulatory mechanisms of
human cytomegalovirus in gliomas. J Clin Neurosci. 17:819–823.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maussang D, Verzijl D, van Walsum M, et
al: Human cytomegalovirus-encoded chemokine receptor US28 promotes
tumorigenesis. Proc Natl Acad Sci USA. 103:13068–13073. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cobbs CS, Sorocean UL, Denham S, et al:
Modulation of oncogenic phenotype in human glioma cells by
cytomegalovirus IE1mediated mitogenicity. Cancer Res. 68:724–730.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cobbs CS, Soroceanu L, Denham S, et al:
Human cytomegalovirus induces cellular tyrosine kinase signaling
and promotes glioma cell invasiveness. J Neurooncol. 85:271–280.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cinatl J Jr, Scholz M and Doerr HW: Role
of tumor cell immune escapemechanis ms in cytomegalovirus-mediated
oncomodulation. Med Res Rev. 25:167–185. 2005. View Article : Google Scholar
|
|
23
|
Lopper M and Compton T: Coiled-coil
domains in glycoproteins B and H are involved in human
cytomegalovirus membrane fusion. J Virol. 78:8333–8341. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thompson JM and Iwasaki A: Toll-like
receptors regulation of viral infection and disease. Adv Drug Deliv
Rev. 60:786–794. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsan MF: Toll-like receptors, inflammation
and cancer. Semin Cancer Biol. 16:32–37. 2006. View Article : Google Scholar
|
|
26
|
Boehme KW, Guerrero M and Compton T: Human
cytomegalovirus envelope glycoproteins B and H are necessary for
TLR2 activation in permissive cells. J Immunol. 177:7094–7102.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Juckem LK, Boehme KW, Feire AL and Compton
T: Differential initiation of innate immune responses induced by
human cytomegalovirus entry into fibroblast cells. J Immunol.
180:4965–4977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rosen EM, Goldberg ID, Liu D, et al: Tumor
necrosis factor stimulates epithelial tumor cell motility. Cancer
Res. 51:5315–5321. 1991.PubMed/NCBI
|
|
29
|
Arnott CH, Scott KA, Moore RJ, et al:
Expression of both TNFalpha receptor subtypes is essential for
optimal skin tumour development. Oncogene. 23:1902–1910. 2004.
View Article : Google Scholar
|
|
30
|
Karin M: Nuclear factor-κB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghosh S and Karin M: Missing pieces in the
NF-κB puzzle. Cell Suppl. 109:S81–S96. 2002.
|

















