Introduction
The human P2X7 receptor is a trimeric ligand-gated
cation channel encoded by the P2RX7 gene, which is located on
chromosome 12q24 (1). P2X7 is
expressed in a wide variety of normal and disease-associated cell
types. Activation of this receptor by extracellular adenosine
5′-triphosphate results in numerous downstream events, including
the release of proinflammatory mediators, cell proliferation or
death, and killing of intracellular pathogens. As a result, P2X7 is
significant and exhibits several functions in neoplasia. P2X7 and
its regulation is of considerable interest with regard to human
health and disease, including in the development and publication of
a number of patents (2). At
present, little is known with regard to how epithelial cells
regulate expression of the P2X7 receptor. However, a preliminary
study revealed that P2X7 expression is also regulated
post-transcriptionally (3). In
addition, putative microRNA (miRNA) binding sites within the P2RX7
3′-untranslated region (UTR) have also been identified (4,5).
miRNAs are small, noncoding 18–25-nucleotide RNAs that regulate
mRNA targets and control critical functions in a variety of
biological processes (6). Animal
miRNAs form imperfectly base-paired duplexes with miRNA response
elements in target mRNAs. This interaction may inhibit translation
and lead to degradation of mRNA (7,8). For
effective repression, base-pairing between the miRNA-response
elements and the first 2–7 nucleotides of the miRNA, referred to as
the ‘seed’ region, is important (9–11).
miR-21 is overexpressed in numerous types of tumors, including
non-small cell lung cancer (NSCLC), indicating its significance in
cancer development. However, the underlying mechanism of
miR-21-mediated tumorigenesis remains unclear, largely due to
limited knowledge with regard to miR-21 targets. Various
computer-aided algorithms have predicted a number of putative
miR-21 targets, however, these targets have not been validated
experimentally. The aim of this study was to investigate whether
miRNAs (miR-21, let-7 g, and miR-205) regulate P2X7 mRNA stability
and to evaluate the prognostic impact of P2RX7 expression in
patients with NSCLC.
Materials and methods
Patients
In total, 96 NSCLC patients were retrospectively
selected from patients who had undergone resection at the Unit of
Thoracic Surgery of Pisa, Azienda Ospedaliero-Universitaria Pisana
(Pisa, Italy) between 1993 and 2012. Histological diagnoses were
formulated according to the World Health Organization
classification (12,13). Clinicopathological characteristics
were collected in 82 cases, while survival data were obtained for
53 patients. All patients provided written informed consent prior
to the molecular analyses. This study was approved by the ethics
committee of the University of Pisa (Pisa, Italy).
RNA and DNA isolation
RNA and DNA were isolated from 5–10 μm sections of
82 formalin-fixed paraffin-embedded (FFPE) tissues and 14
cytological specimens. The cytological samples were obtained from
bronchial brushing, aspirative transbronchial needle aspiration and
transthoracic needle aspiration. The cyctolgical preparations were
conducted according to a standard specimen processing procedure in
our laboratory. All samples were fixed in Cytofix (BD Biosciences,
San Jose, CA, USA) and stained with Papanicolaou for 20 min in an
automated stainer (Leica Stainer; ST5020, Leica, Mannheim,
Germany). For aspirative cytology (transbronchial needle aspiration
and transthoracic needle aspiration), the specimens were fixed in
Cytofix and stained with Papanicolaou staining (Kaltek s.r.l.,
Padua, Italy). Bronchial brushing samples and cellular material
were centrifuged (Sorvall T6000D, Thermo Scientific, Rockford, IL,
USA) at 1366 x g for 10 min. Next, 10% neutral buffered formalin
(Diapath S.p.A., Bergamo, Italy) was added to the pellet and the
pellet placed into a tissue processing and embedding cassette (Bio
Optica S.p.A., Milan, Italy) and embedded with paraffin.
P2X7, miRNA expression and mutational
analysis
Quantification of mRNA expression was performed in
triplicate using quantitative reverse transcription polymerase
chain reaction (PCR) with the following primers: Forward,
5′-CTCCCATCTCAACTCCCTGA-3′ and reverse, 5′ACCAGCTTCCTGAACAGCTC-3′,
for P2X7. Specific TaqMan® miRNA Assays (Applied
Biosystems Life Technologies, Foster City, CA, USA) were used for
Let-7 g, miR-21, miR-205 and RNU6B according to the manufacturer’s
instructions. The threshold cycle (Ct) and baselines were
determined using manual settings, where Ct was set in the linear
phase of the amplification and baseline in the initial cycles of
PCR. Expression was calculated by relative quantification and fold
expression changes were determined by the
2−ΔΔCT method using the
DataAssist software (Applied Biosystems Life Technologies).
Mutational analysis was also performed for all
samples. The mutational status of the codons 12–13 of the K-Ras
gene was analyzed by pyrosequencing using the anti-epidermal growth
factor receptor (EGFR) MoAb response® kit (K-Ras status)
(Diatech Pharmacogenetics, Jesi, Italy) according to the
manufacturer’s instructions.
PCR-single stranded conformation polymorphism and
sequencing analysis were used for EGFR genotyping in exons 18–21,
as previously described (14).
Statistical analysis
One-way analysis of variance and χ2 tests
were used to determine the association between miRNA expression,
P2X7 mRNA expression and the different parameters. The survival
analysis was performed using the Kaplan-Meier method. Statistical
analyses were performed using the JMP10 software (SAS, Cary, NC,
USA) and a two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
This study was conducted in 96 patients with NSCLC,
which included 63 adenocarcinomas, 31 squamous cell carcinomas and
two large cell carcinomas. The median age at diagnosis was 66.5
years (range, 46–83 years). Using the TNM staging system, the
distribution of the patients was as follows: 18 cases, stage I; 44
cases, stage II; 14 cases, stage III; and six cases, stage IV; 34
cases, negative lymph node status; 20 cases, N1 status; 23 cases,
N2 status; and five cases, Nx status (13).
Survival data were obtained for 53 patients (median
follow-up duration, 36 months; range, 7–98 months). Disease
progression and mortality from lung cancer were observed in 32
(60.3%) and 18 (34%) of the 53 NSCLC patients, respectively. The
median progression-free survival (PFS) and overall survival (OS)
were 18 months (95% CI, 2–89 months) and 25 months (95% CI, 2–90
months), respectively.
P2X7 and miRNA expression and
clinicopathological characteristics
P2X7 mRNA expression was quantified and normalized
to the glyceraldehyde 3-phosphate-dehydrogenase housekeeping gene.
The expression of mature Let-7 g, miR-21, and miR-205 was
quantified and normalized to the RNU6B endogenous control in 96
NSCLC tissues using quantitative PCR. The samples were divided into
high and low expression groups based on the median fold change
values (0.56, P2X7; 1.02, Let-7 g; 5.81, miR-21; and 0.01,
miR-205). No statistically significant associations were identified
between P2X7 mRNA levels and the main clinicopathological
characteristics of the NSCLC patients (Table I). With regard to the association
between P2X7 expression levels and the miRNA profile, it was found
that samples with low P2X7 expression exhibited a higher miR-21
fold change (11.42±2.28) when compared with samples expressing high
levels of P2X7 (7.41±2.28). In addition, the mutational analysis
revealed K-Ras and EGFR mutations in 19.7 and 23.9% of the NSCLC
patients, respectively. K-Ras and EGFR mutations were mutually
exclusive (P=0.0006), observed only in the NSCLC patients with
adenocarcinoma (P<0.0001). The mutations were associated with
female gender (P<0.0001) and patients that did not smoke
(P<0.0001). Notably, significantly higher miR-21 expression was
observed in the NSCLC tumors that expressed mutant K-Ras when
compared to that of tumors that expressed wild-type K-Ras
(P=0.003). In addition, the majority of the tumors that expressed
mutant K-Ras exhibited low P2X7 expression.
 | Table ICorrelations between P2X7 mRNA
expression and the main clinicopathological characteristics of the
non-small cell lung cancer patients. |
Table I
Correlations between P2X7 mRNA
expression and the main clinicopathological characteristics of the
non-small cell lung cancer patients.
| P2X7 mRNA expression,
n (%) | |
|---|
|
| |
|---|
| Parameter | Low | High | P-value |
|---|
| Age, years |
| ≤68 | 28 (56) | 22 (44) | 0.21 |
| >68 | 20 (43.5) | 26 (56.5) | |
| Gender, n |
| Male | 33 (45.8) | 39 (54.2) | 0.15 |
| Female | 15 (62.5) | 9 (37.5) | |
| Histology, n |
| ADC | 32 (50.8) | 31 (49.2) | 0.21 |
| SCC | 14 (45.2) | 17 (54.8) | |
| LCC | 2 (100) | 0 (0) | |
| Tumor stagea, n |
| T1 (T1a–T1b) | 6 (46.1) | 7 (53.9) | 0.70 |
| T2 (T2a–T2b) | 25 (51) | 24 (49) | |
| T3–T4 | 8 (40) | 12 (60) | |
| Lymph-node status,
n |
| Negative | 21 (61.8) | 13 (38.2) | 0.09 |
| Positive | 16 (37.2) | 27 (62.8) | |
| Nx | 2 (40) | 3 (60) | |
Survival analysis
To evaluate the association between P2X7 expression
and the prognosis of NSCLC patients, a survival analysis using the
Kaplan-Meier method was conducted. In this analysis, disease
recurrence and overall postoperative survival were used as
endpoints.
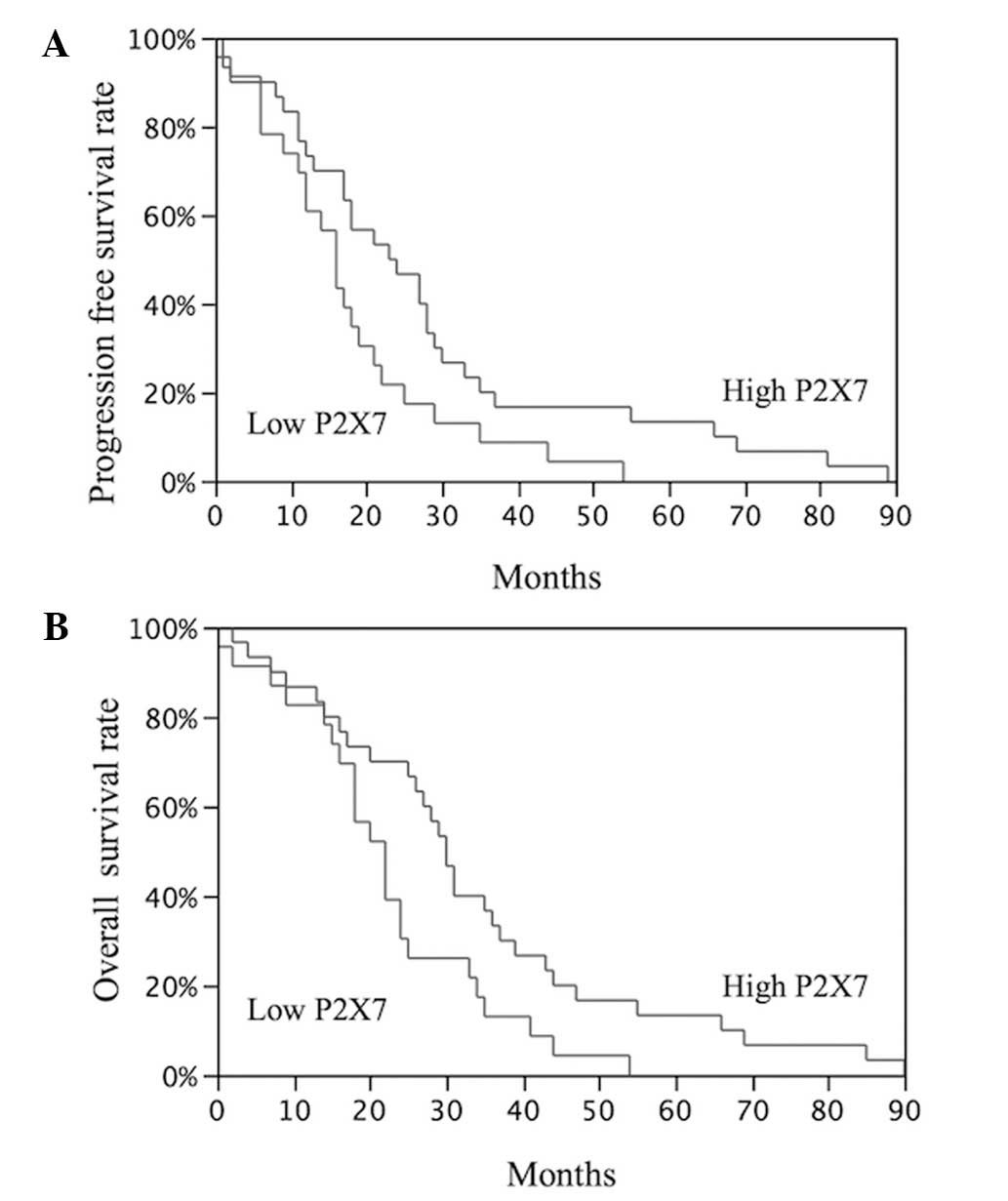
A significant difference was identified between the
PFS (P=0.03) and OS (P=0.02) of the NSCLC patients with high P2X7
expression and the patients with low P2X7 expression (Fig. 1).
Discussion
P2X7 is characterized by high plasticity, as its
expression is modulated in a cell type- or
differentiation-dependent manner (15). Transcriptional mechanisms may
underlie the increased P2X7 expression observed in lung cancer, and
the expression of individual isoforms of the same protein may or
may not correlate with the mRNA, indicating that separate
post-translational mechanisms may account for the regulation of
P2X7 expression (2). In the current
study, P2X7 mRNA levels were evaluated and quantified by
quantitative PCR in NSCLC cases. One of the aims of this study was
to explore whether particular miRNAs (miR-21, let-7 g and miR-205)
are important for P2X7 mRNA regulation. Samples with low P2X7
expression were found to exhibit a higher miR-21 fold change when
compared with samples exhibiting high levels of P2X7 expression. In
animals, miRNAs are hypothesized to bind via a partially homologous
sequence in the target gene at the 3′-UTR, causing translational
repression (6). With regard to
oncogenic miRNAs, a limited number of target genes have been
characterized experimentally, however, increasing evidence
indicates putative targets, predicted by different algorithm
programs. For example, the Sanger miRNA database target search
(www.mirbase.org.) reveals >900 targets for miR-21,
which is not consistent with the prediction of ~100 target genes
per single miRNA (16). Therefore,
it is likely that only a small fraction of predicted targets may be
true targets and thus, it would be difficult to validate them. In
the present study, an exact putative target site was not identified
for miR-21 within the 3′-UTR (1272 nucleotides) of the human P2X7
gene (NM_002562). However, at the 499 nucleotide position, a
partial target site (3′-GAAT-5′) was identified for the miR-21 seed
sequence (5′-AGCUUA-3′). Further studies are required to
investigate whether P2X7 is a target of miR-21, considering that in
animals, the miRNA does not bind to its target as efficiently as in
plants. Furthermore, polymorphisms in the miRNA ‘seed region’
(5) or in their binding sites in
target genes have been identified and appear to be involved in
cancer (17).
Furthermore, significantly higher miR-21 expression
was observed in NSCLC patients with K-Ras-mutated tumors, when
compared with K-Ras wild-type specimens, according to our previous
study (18). High levels of miR-21
expression in NSCLC patients harboring K-Ras mutations may indicate
a synergistic assocation between miR-21 and K-Ras oncogenes,
including P2X7 downregulation. Taken together, it is likely that
these processes promote tumor progression. The second aim of the
study was to evaluate the putative prognostic role for P2X7
expression, as this role remains controversial. Based on previous
studies, it appears that low levels of P2X7 activation facilitates
the growth or survival of certain tumor cell types (19). However, at high levels, the
extracellular receptor promotes cell death (20). Previous reports have linked
increased P2X7 expression with a poor prognosis in several types of
leukemia (21,22) and solid tumors (23–25).
By contrast, Souza et al (26) revealed that extracellular ATP
effectively inhibited proliferation and induced apoptosis or
necrosis of tumor cells (26).
These studies also demonstrated that the brief exposure of tumor
cells to ATP was able to efficiently induce cell death (reduction
of cell growth and induction of autophagy), which was largely
mediated via P2X7, indicating the anti-tumor potential of
purine-based drugs (27). The
results of the current study are consistent with those found by
Souza et al (26), showing
that defective P2X7 expression, as a result of miR-21 activation by
a K-Ras mutation, may lead to reduced tumor-killing activity,
resulting in a poorer prognosis. The identification of putative
associations of P2X7 with biological behavior in NSCLC would be of
considerable interest, and further studies will aid in the
understanding of P2X7 gene regulation and its role in lung cancer.
The significant differences in clinical outcome of NSCLC patients
with high P2X7 expression identified in this study indicate that
expression of the P2X7 receptor may be a useful prognostic marker,
as well as a novel target for therapy. Further studies, including
the investigation of P2X7 regulation by various micro-RNA or other
epigenetic mechanisms, may provide more insight with regard to the
results of this study.
Acknowledgements
This study was supported by a grant from the Italian
Ministry for University and Scientific Research (grant no. PRIN
2009LMEEEH_004).
References
|
1
|
Buell GN, Talabot F, Gos A, Lorenz J, Lai
E, Morris MA and Antonarakis SE: Gene structure and chromosomal
localization of the human P2X7 receptor. Receptors Channels.
5:347–354. 1998.PubMed/NCBI
|
|
2
|
Sluyter R and Stokes L: Significance of
P2X7 receptor variants to human health and disease. Recent Pat DNA
Gene Seq. 5:41–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li X, Qi X, Zhou L, Catera D, Rote NS,
Potashkin J, Abdul-Karim FW and Gorodeski GI: Decreased expression
of P2X7 in endometrial epithelial pre-cancerous and cancer cells.
Gynecol Oncol. 106:233–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou L, Qi X, Potashkin JA, Abdul-Karim FW
and Gorodeski GI: MicroRNAs miR-186 and miR-150 down-regulate
expression of the pro-apoptotic purinergic P2X7 receptor by
activation of instability sites at the 3′-untranslated region of
the gene that decrease steadystate levels of the transcript. J Biol
Chem. 283:28274–28286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rahman OA, Sasvari-Szekely M, Szekely A,
Faludi G, Guttman A and Nemoda Z: Analysis of a polymorphic
microRNA target site in the purinergic receptor P2RX7 gene.
Electrophoresis. 31:1790–1795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bagga S, Bracht J, Hunter S, Massirer K,
Holtz J, Eachus R and Pasquinelli AE: Regulation by let-7 and lin-4
miRNAs results in target mRNA degradation. Cell. 122:553–563. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lim LP, Lau NC, Garrett-Engele P, et al:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lai EC: Predicting and validating microRNA
targets. Genome Biol. 5:1152004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie X, Lu J, Kulbokas EJ, et al:
Systematic discovery of regulatory motifs in human promoters and 3′
UTRs by comparison of several mammals. Nature. 434:338–345. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Travis WD, Brambilla E, Muller-Hemerlink
HK and Harris CC: World Health Organization Classification of
Tumors. Pathology and Genetics of Tumors of the Lung, Pleura,
Thymus and Heart Lyon, France: IARC Press; 2004
|
|
13
|
Travis WD, Brambilla E, Noguchi M, et al:
International association for the study of lung cancer/American
thoracic society/European respiratory society international
multidisciplinary classification of lung adenocarcinoma. J Thorac
Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Capodanno A, Boldrini L, Alì G,
Pelliccioni S, Mussi A and Fontanini G:
Phosphatidylinositol-3-kinase α catalytic subunit gene somatic
mutations in bronchopulmonary neuroendocrine tumors. Oncol Rep.
28:1559–1566. 2012.PubMed/NCBI
|
|
15
|
Fuller SJ, Stokes L, SkarRatt KK, Gu BJ
and Wiley JS: Genetics of the P2X7 receptor and human disease.
Purinergic Signal. 5:257–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brennecke J, Stark A, Russell RB and Cohen
SM: Principles of microRNA-target recognition. PLoS Biol.
3:e852005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mulrane L, McGee SF, Gallagher WM and
O’Connor DP: miRNA dysregulation in breast cancer. Cancer Res.
73:6554–6562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Capodanno A, Boldrini L, Proietti A, et
al: Let-7g and miR-21 expression in non-small cell lung cancer:
correlation with clinicopathological and molecular features. Int J
Oncol. 43:765–774. 2013.PubMed/NCBI
|
|
19
|
Adinolfi E, Raffaghello L, Giuliani AL, et
al: Expression of P2X7 receptor increases in vivo tumor growth.
Cancer Res. 72:2957–2969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
White N and Burnstock G: P2 receptors and
cancer. Trends Pharmacol Sci. 27:211–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adinolfi E, Melchiorri L, Falzoni S, et
al: P2X7 receptor expression in evolutive and indolent forms of
chronic B lymphocytic leukemia. Blood. 99:706–708. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chong JH, Zheng GG, Zhu XF, et al:
Abnormal expression of P2X family receptors in Chinese pediatric
acute leukemias. Biochem Biophys Res Commun. 391:498–504. 2010.
View Article : Google Scholar
|
|
23
|
Raffaghello L, Chiozzi P, Falzoni S, Di
Virgilio F and Pistoia V: The P2X7 receptor sustains the growth of
human neuroblastoma cells through a substance P-dependent
mechanism. Cancer Res. 66:907–914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Solini A, Cuccato S, Ferrari D, et al:
Increased P2X7 receptor expression and function in thyroid
papillary cancer: a new potential marker of the disease?
Endocrinology. 149:389–396. 2008. View Article : Google Scholar
|
|
25
|
Slater M, Danieletto S, Pooley M, Cheng
TL, Gidley-Baird A and Barden JA: Differentiation between cancerous
and normal hyperplastic lobules in breast lesions. Breast Cancer
Res Treat. 83:1–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Souza CO, Santoro GF, Figliuolo VR, et al:
Extracellular ATP induces cell death in human intestinal epithelial
cells. Biochim Biophys Acta. 1820:1867–1878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bian S, Sun X, Bai A, et al: P2X7
integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to
mediate tumor cell death. PLoS One. 8:e601842013. View Article : Google Scholar : PubMed/NCBI
|