Introduction
CxCa is the second most common form of malignant
tumor that results in female mortalities worldwide. Persistent
infection with high-risk human papilloma viruses (HPVs),
particularly HPV-16 and HPV-18, is a key factor in the development
of CxCa (1). The incidence of
high-risk HPV strains has been identified in up to 99.7% of
cervical squamous cell carcinomas, and 94–100% of cervical
adenocarcinoma and adenosquamous carcinomas (2,3). The
expression of viral E6 and E7 oncoproteins by host cells during HPV
infection is critical for the induction of CxCa. The E6 protein
abrogates p53 function primarily through the binding of
ubiquitin-like enzyme E6-associated protein, while the E7 protein
inactivates the retinoblastoma protein and p130. Together, these
changes result in malignant transformation (4,5). In
addition, E6 and E7 expression may be associated with host genome
instability (6).
Bicistronic or polycistronic transcription, with two
or more open reading frames (ORFs), is a feature of HPVs.
Regulatory elements (including E2-binding elements or those that
bind to activator protein-1 and octamer-binding protein-1
transcription factors), early promoters (including the p97 of
HPV-16, and p99 of HPV-31), early splice sites and early
polyadenylation signal sites (7,8), are
all involved in the regulation of HPV early gene expression. HPV
early gene transcripts are always concomitant with
post-transcriptional RNA splicing processes to eliminate non-coding
introns from transcripts (7).
Different RNA splicing patterns may produce different transcripts,
resulting in the production of diverse proteins (7,8).
Furthermore, the course of CxCa is always concomitant with HPV
integration. This integration into the host genome can considerably
change the expression pattern of HPV genes due to the effect of
transcriptional and post-transcriptional regulation. Moreover, HPV
genome integration may also affect the function of cellular genes
through the activation of oncogenes, or the inactivation of
tumor-suppressor genes.
While HPV-16 early gene transcription patterns have
been reported in recent years, HPV-16 E6 transcription patterns,
during the course of CxCa genesis have not been systematically
analyzed. Therefore, the present study used the amplification of
papillomavirus oncogene transcripts (APOT) to determine the
profiles of E6-associated early gene transcripts and
comprehensively investigate the variation of transcriptional
patterns in eight low-grade squamous intraepithelial lesions
(LSILs), 24 high-grade squamous intraepithelial lesions (HSILs) and
eight CxCa HPV-16-positive cervical biopsy samples.
Materials and methods
Patients and specimens
Samples from 63 hospitalized or outpatient subjects,
with HPV-16-positive cervical malignancies, were collected from the
Second Affiliated Hospital of Wenzhou Medical University (Wenzhou,
Zhejiang, China) between December 2010 and April 2012. These
samples included eight cases of LSILs, 38 cases of HSIL consisting
of 22 cervical intra-epithelial neoplasia (CIN) II cases and 16 CIN
III cases, and 17 CxCa cases. The patient age range was 25–59
years, with a median age of 43 years. HPV genotyping and
pathological confirmation was conducted for all specimens during
the collection. The patients did not receive any radiotherapy or
chemotherapy prior to the surgery. In addition, eight control
cervical tissue samples negative for HPV and with normal cytology
were obtained from patients who underwent a hysterectomy owing to
benign gynecological diseases. Subsequent to ex-vivo
procedures, the specimens were preserved in liquid nitrogen until
further experimentation. This study was approved by the Medical
Ethics Committee of the First Affiliated Hospital of Wenzhou
Medical University (Wenzhou, Zhejiang, China). All participating
patients provided informed consent.
RNA isolation
The cryopreserved cervical tissues were crushed to a
powder in liquid nitrogen, and the RNA was extracted with TRIzol
reagent according to the manufacturer’s instructions (Invitrogen,
Carlsbad, CA, USA). To remove the residual contaminating DNA, the
RNA preparation was treated with RNase-free DNase I (Takara
Biotechnology, Co., Ltd., Dalian, China). The purified RNA was
dissolved in RNase-free water (Toyobo Co., Ltd. Osaka, Japan) and
stored at −80°C. The concentration and purity of total RNA were
quantified by an ultraviolet spectrophotometer (DU640, Beckman
Coulter, Miami, FL, USA) at 260 and 280 nm, respectively, and the
integrity of the RNA was examined by 1% agarose gel
electrophoresis. Only RNA samples with an A260/A280 ratio of
1.8–2.0 and high integrity were used for further experiments.
Reverse transcription and amplification
of oncogene transcripts
The APOT assay was used to amplify HPV oncogene
transcripts. The total RNA (1 μg) was reverse transcribed using an
oligo (dT)17 RT primer, coupled to a linker sequence
(5′-GAC TCGAGTCGACATCGATTTTTTTTTTTTTTTTT’3) (9). Reverse transcription was conducted for
1 h at 42°C in a reaction system containing 2.5 μM RT primer, 200
units of Moloney murine leukemia virus reverse transcriptase
(Toyobo Co., Ltd.), 20 units of RNase Inhibitor (Toyobo Co., Ltd)
and 1X RT buffer, in a final volume of 20 μl. In order to open the
RNA stem-loop structures, the RNA samples were incubated at 65°C
for 10 min prior to mixing with reaction buffer and reverse
transcriptase. The cDNA obtained was subsequently amplified by
polymerase chain reaction (PCR) in a total volume of 50 μl, using 1
unit of KOD-plus DNA Polymerase (Toyobo Co., Ltd), 0.2 μM HPV-16
E6-specific forward primer P1 (5′-CGACCCAGAAAG TTACCAC-3′) and 0.2
μM P0 reverse primer (5′-GACTCGAGT CGACATCGA-3′). The reaction
mixture was subjected to an initial denaturation step for 90 sec,
followed by 35 cycles of denaturation at 94°C for 30 sec, annealing
at 59°C for 30 sec, elongation at 68°C for 2 min, and a final
elongation step at 68°C for 6 min to ensure the integrity of the
amplified fragments.
Southern hybridization
The final PCR products were electrophoresed in 2.5%
agarose gels, and the DNA was transferred to a nitrocellulose
membrane (Amersham Life Sciences, Buckinghamshire, England) and
fixed for 2 h at 80°C prior to mixing with hybridization solution.
Following pre-hybridization for 1 h, the membranes were incubated
overnight at 46°C in hybridization solution, with a
5′-biotin-labeled HPV-16 E6-specific probe (5′-CTGCGACGTGAGGTATAT
GACTTTG-3′) at a final concentration of 100 ng/ml (Thermo
Scientific, Co., Ltd., Waltham, MA, USA). Following hybridization,
the membranes were washed twice with pre-warmed 2X saline sodium
citrate (SSC) solution containing 0.1% sodium dodecyl sulfate (SDS)
at 50°C for 20 min, and twice with pre-warmed 0.2X SSC solution
containing 0.1% SDS at 53°C for 15 min. Detection of the probe
signal was performed using a chemiluminescence detection system
(Thermo Scientific, Co., Ltd.) according to the manufacturer’s
instructions, followed by film exposure.
Sequence analysis of HPV-16 E6-associated
transcripts
The APOT amplification products were visualized by
2.5% agarose gel electrophoresis. The PCR-amplified fragments of
interest were separated and purified from the gel using an agarose
gel DNA extraction kit, according to the manufacturer’s
instructions (TianGen, Beijing, China). The corresponding amplimers
were cloned into a pEASY™-blunt zero cloning vector
(TransGen, Beijing, China), and the sequencing of amplified
sequences was conducted using an ABI3730 XL Genetic Analyzer
(Applied Biosystems, Waltham, MA, USA), according to standard
procedures. The alignment analysis of the sequencing results was
conducted using the BLASTn service supplied by the National Center
for Biotechnology Information (Bethesda, MD, USA).
Results
Establishment of specific amplification
for HPV-16 E6-associated transcripts
The basis of an APOT assay is the 3′ rapid
amplification of cDNA ends, which enables amplification and cloning
of the region between a single short sequence in the cDNA molecule,
and its unknown 3′ end (10). In
order to analyze HPV-16 E6-associated transcripts, mRNA from tissue
samples was reverse transcribed to cDNA, using an RT primer
containing a conservative sequence in its 5′ end. PCR amplification
was conducted using HPV-16 E6-specific (P1) and -conserved (P0)
sequences, which resulted in amplification and cloning of the
region between a single short sequence in a cDNA molecule and its
unknown 3′ end (10). This primer
design amplified the E6-associated transcripts from episomal and
integrated viral genomes. To verify the specificity of the modified
APOT assay, cDNA from HPV-16-positive Caski cells, containing
integrated HPV-16 genome, and HPV-negative normal cervical tissues,
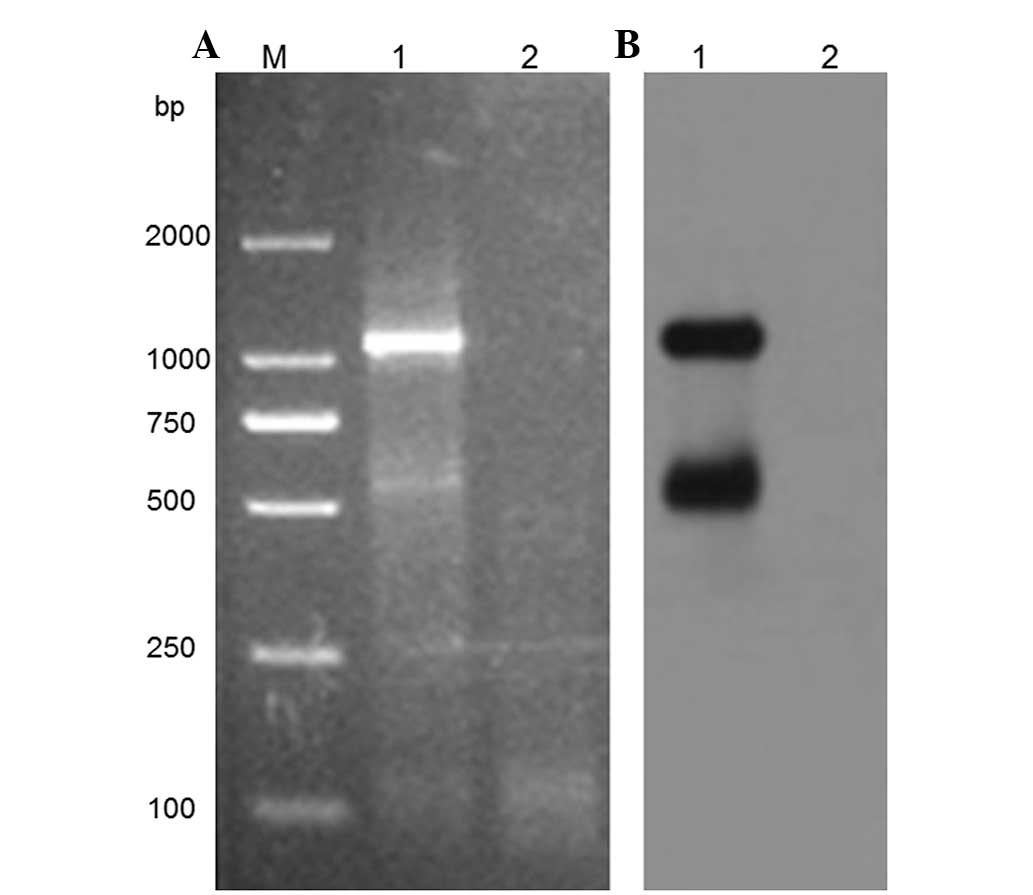
were used. Fig. 1A demonstrates
that the target amplimer could be detected in the HPV-16-positive
Caski cells, whereas it was absent in the normal cervical tissue
samples. The presence of HPV-16 E6-associated transcripts was
further confirmed by southern blotting using HPV-16 E6 probes
(Fig. 1B), which suggests that this
method may specifically amplify the HPV-16 E6-associated
transcripts.
Characteristics of the HPV-16
E6-associated transcriptional pattern in the tissues of CIN and
CxCa
To identify HPV-16 E6-associated transcripts, 63 RNA
samples of good quality were obtained from HPV-16-positive cervical
specimens (LSIL, n=8; HSIL, n=38; CxCa, n=17), and were amplified
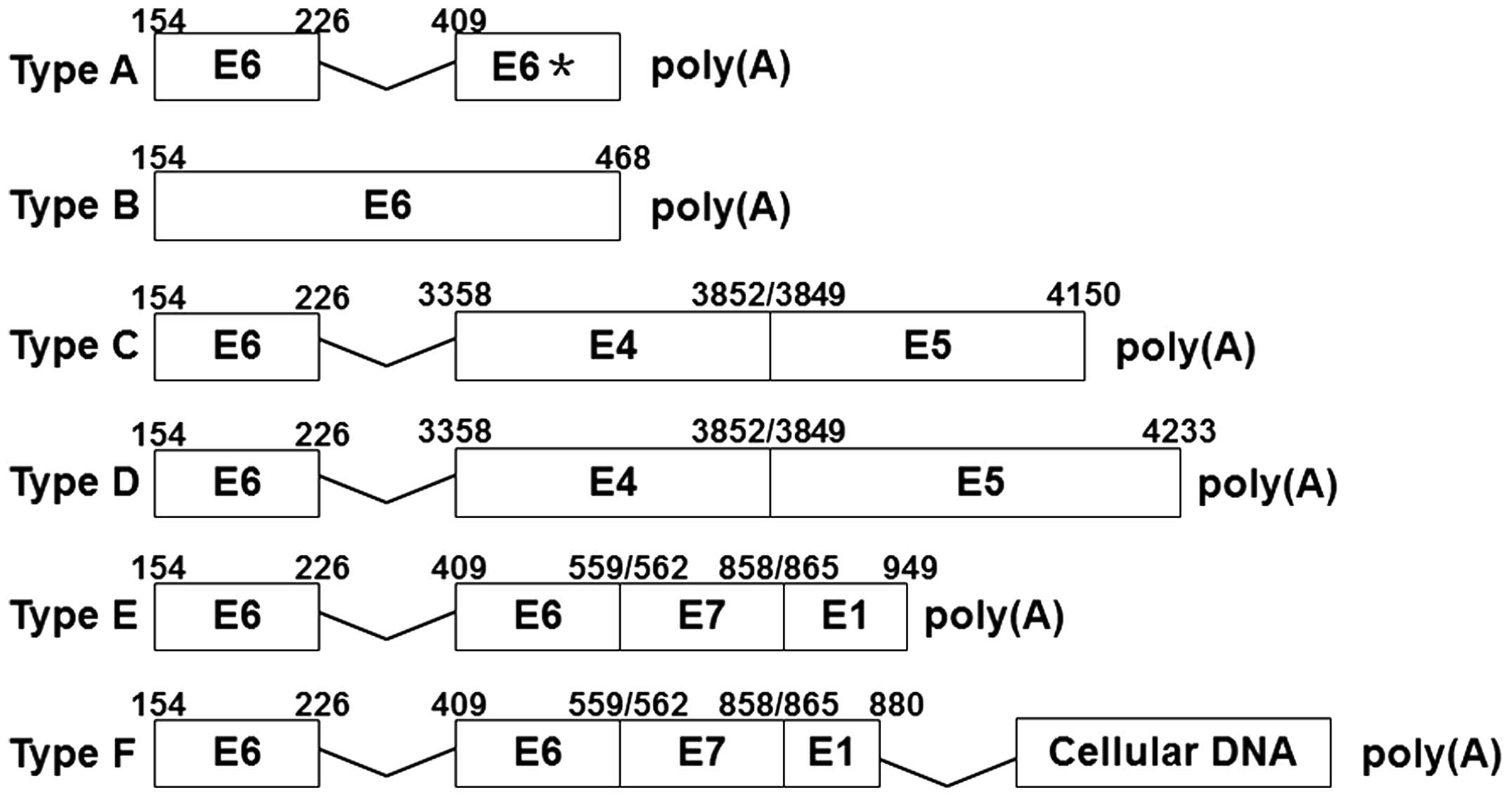
by PCR according to the aforementioned method. Six different types
of HPV-16 E6-associated transcription patterns were identified
(Fig. 2). Among these patterns,
types A, B, C, D and E were all directly connected with the
polyadenylation site and lacked host-cell genetic material.
Furthermore, transcription patterns C and D ended with an unbroken
E5 (the total sequence of E5), and were affected by an early
polyadenylation signal, which suggests that they are episomal
patterns. Meanwhile, the A, B and E transcripts contained
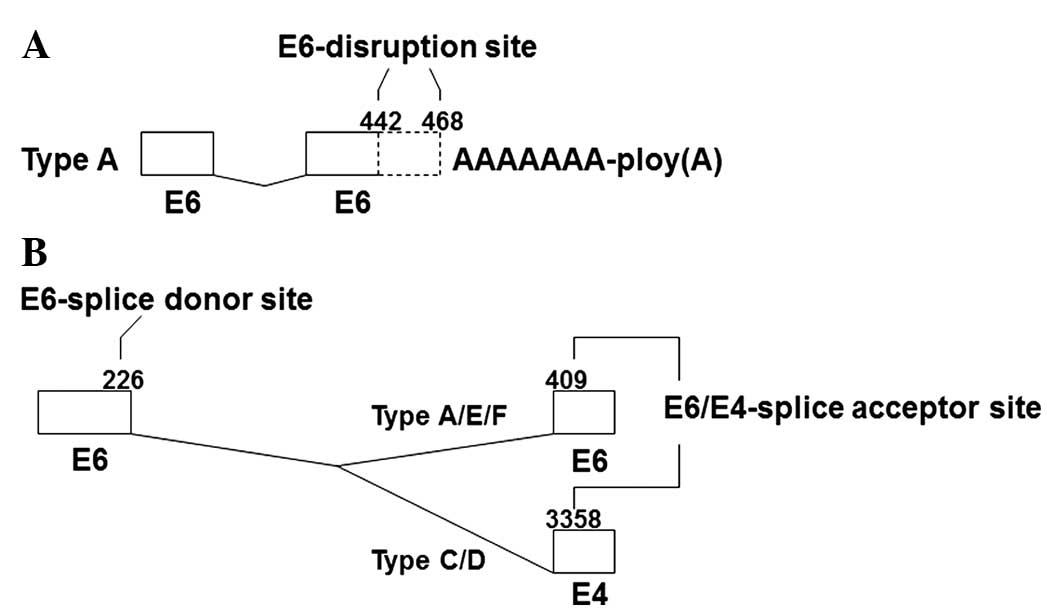
untraditional splice donor signals at nucleotides (nts) 442, 468
and 949, suggesting that they are potential integration transcripts
(Figs. 2 and 3A). The type F transcripts were connected
with host genome sequences, clearly identifying them as integration
transcripts. In pattern A, the HPV-16 E6 gene was spliced in the
sites of nt 226, a splice donor signal, and nt 409, a splice
acceptor signal (Figs. 2 and
3B). In addition, there were
different disruptions at the 3′ end of the E6 gene region, located
at nt 442 and 468 (Fig. 3A). In
pattern B transcripts, the site of disruption was located at nt 468
of the HPV-16 E6 gene, with no internal splicing observed. Patterns
C and D contained the same gene fragments of partial HPV-16 E6, E4
and E5 genes, with splice sites at nt 226 in E6, and nt 3,356 in E4
(Fig. 3B). However, the sequence of
E5 at their 3′ ends was different. Patterns E and F also contained
the same HPV-16 early gene fragments, with E6 internal splicing.
However, in pattern F, the HPV-16 E1 gene was disrupted at nt 880,
and was connected with host genome sequences. With the exception of
pattern F, all the HPV-16 E6-associated transcript patterns were
first reported by Wentzensen et al (11). Of these six HPV-16 E6-associated
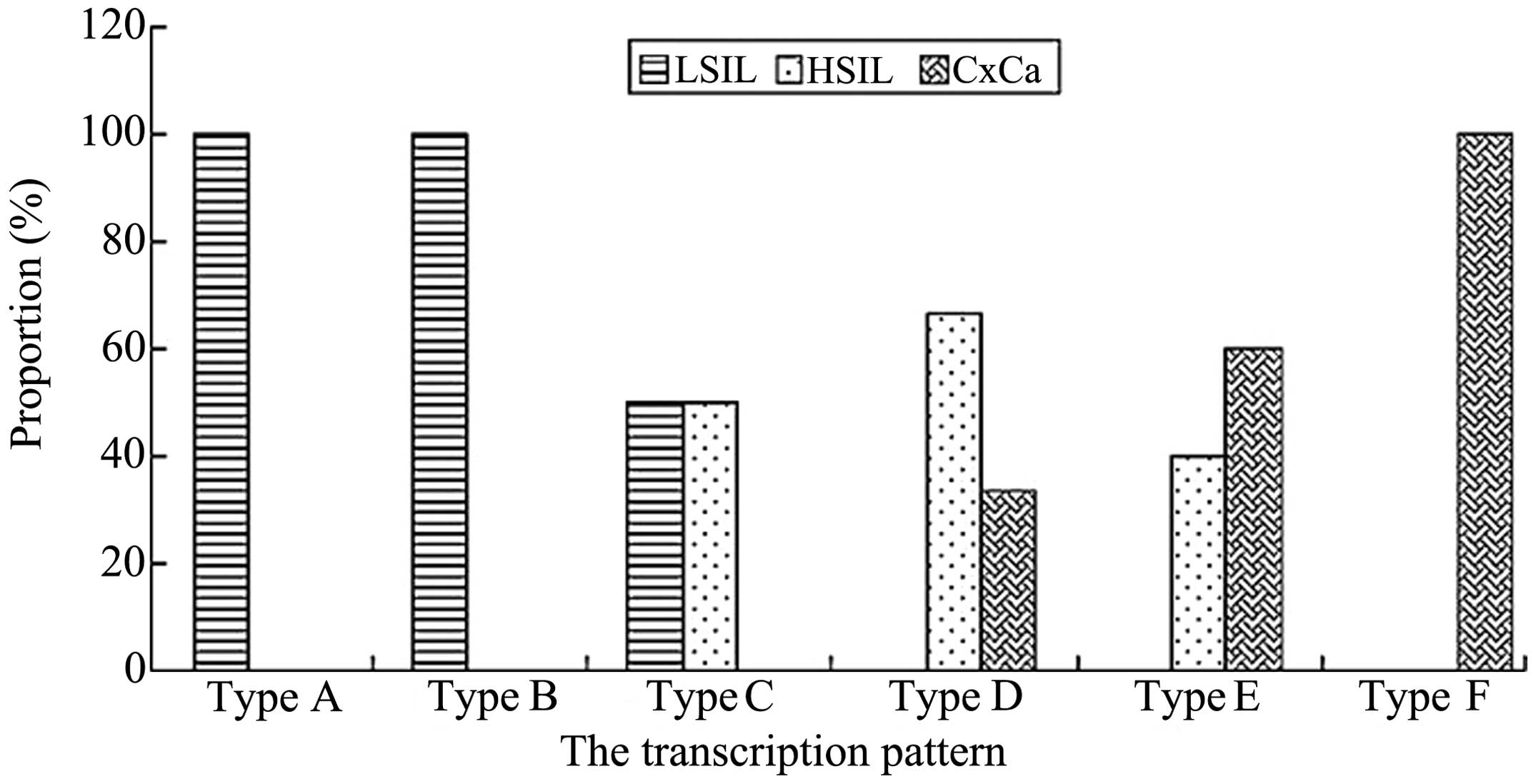
transcripts, patterns E and F accounted for 53.8%.
Changes in the HPV-16 E6 transcription
patterns during carcinogenesis and the development of CxCa
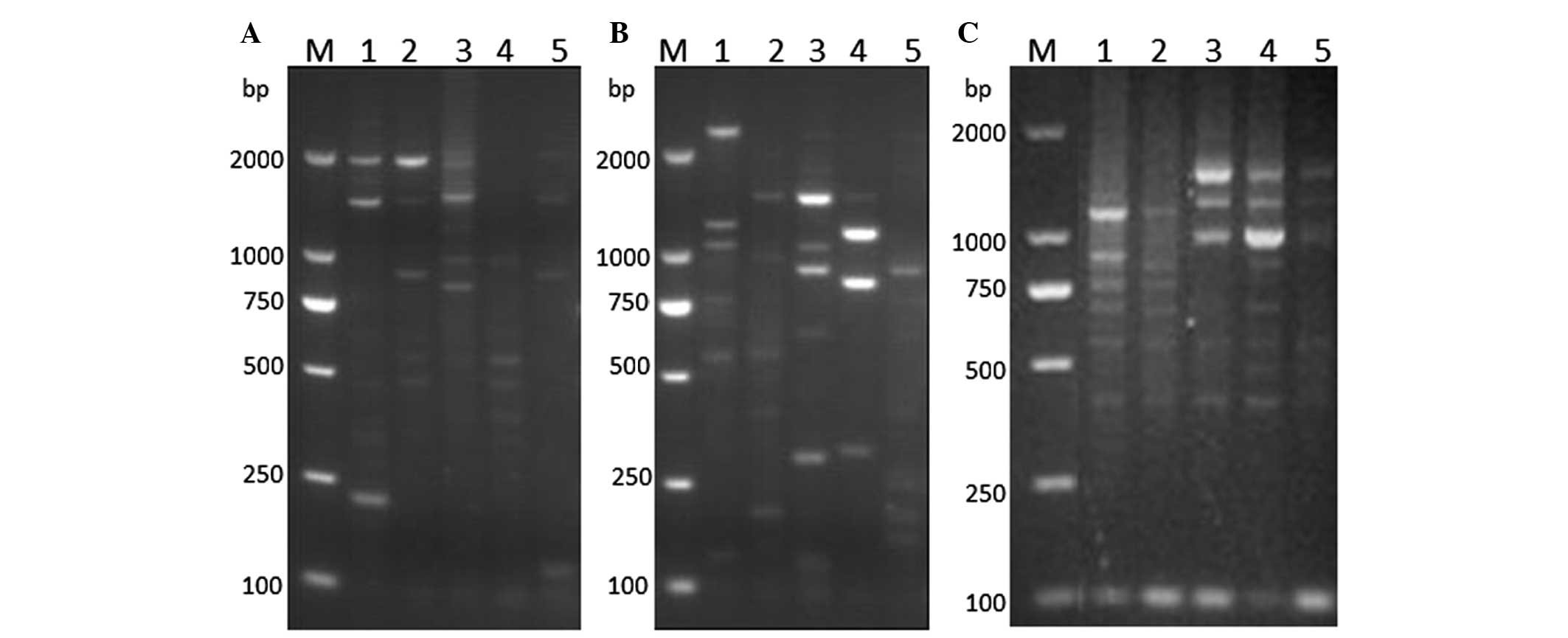
HPV-16 E6-associated transcriptional patterns were
systematically analyzed in HPV-16-positive tissues of LSIL, HSIL
and CxCa, and were found to significantly differ between the
different tissues. Over the course of LSIL progression to CxCa,
there was an observable difference in the expression of specific
HPV-16 E6-associated transcription patterns (Fig. 4 and 5). All samples positive for patterns A and
B were identified in cases of LSIL, while all samples positive for
pattern F were identified in cases of CxCa (Fig. 5). In total, pattern C accounted for
50% of the transcripts in LSIL and HSIL. Overall, 66.67% of cases
of HSIL were positive for pattern D transcripts. However, pattern D
represented only 33.33% of the transcripts in CxCa, and was absent
in LSIL. In total, 60% of pattern E transcripts were detected in
CxCa samples, with the rest identified in HSIL specimens.
Discussion
The double-stranded, circular DNA genome of HPV is
~7.9 kb in length and consists of the non-coding long control
region, the early gene region (encoding the early genes of E1, E2,
E4, E5, E6 and E7) and the late gene region (encoding the capsid
proteins L1 and L2) (7). Due to the
polycistronic characteristics of the HPV genome, different profiles
of HPV gene expression may result from different transcriptional
patterns and the splicing of these transcripts (7). The transcriptional pattern of episomal
HPV-16 infection has been previously elucidated (7), and it is recognized that the HPV
genome integrates into the host cell genome in cervical
carcinogenesis, which may alter the transcription pattern of HPV
genes (9). The majority of previous
studies have focused on E7-associated transcriptional patterns.
Several E7-associated transcriptional patterns, including the
episomal type E7-E1^E4 (where ‘^’ represents splicing), and the
integrated types E7-E1^-cellular and E7-E1^E4-cellular DNA, were
revealed in HPV-16-positive CxCa (9,11). In
addition to the HPV E6 full-length transcript, two E6-associated
transcripts, termed E6*I and E6*II (where ‘*’ represents the splice
site), have been previously reported (7). The E6*I and E6*II transcripts use the
site at nt 226 in the E6 ORF region as the donor splice site, and
the sites of nt 409 and nt 526 in the HPV-16 genome as the splicing
receptor sites. Previous studies have demonstrated that the E6*I
and E6*II patterns are associated with the promotion of E7
expression (12,13). In HPV-16-positive CxCa tissues,
additional E6-associated transcription patterns, including
integrate-derived transcripts of E6-E7-E1, E6-E7-E1^E4, E6-E7-E1
(integrated E1 gene), E6-E7-E1 (disintegrated E1 gene) and an
episomal transcript of E6-E7-E1^E4, have also been identified
(11,14). However, the presence of these
transcriptional patterns, and their correlation with carcinogenesis
and the development of CxCa, have not yet been investigated.
In order to analyze the HPV-16 E6-associated
transcription profile in the different stages of CxCa, an APOT
assay was used in the present study to determine E6-associated
transcript patterns in 63 HPV-16-positive cervical tumor tissues.
In total, six E6-associated transcription patterns were identified
in the HPV-16-positive cervical tumor tissues. In contrast with
previous studies (11,14,15),
the present study described five transcriptional patterns (A, B, C,
D and E) for the first time using the APOT method, but did not
identify a complete E6 gene. A potential reason for this is that
the APOT assay may not be sufficient in amplifying long
integrate-derived transcripts. In addition, it tends to amplify
those transcripts present at higher levels, and exclude those
expressed at lower levels (16).
Alternatively, post-transcriptional splicing may contribute to
these results (7,12,17).
It was also observed that the integrated E7 gene was preserved in
the E6-associated transcripts of patterns E and F. Therefore, E6
splicing may also contribute to HPV-16 E7 expression, as previously
reported for E6*I and E6*II transcripts (7).
The role of HPV-16 early gene transcription patterns
in the carcinogenesis and development of CxCa has not yet been
established by previous studies. In the present study, distinct
differences in HPV-16 early gene transcriptional patterns, at
different stages of CxCa progression, were observed. All samples
positive for patterns A and B originated from patients with LSIL,
while all samples positive for pattern F were identified in
patients with CxCa. In total, 50% of the samples from patients with
LSIL and HSIL were positive for pattern C transcripts, while the
majority of samples positive for patterns D and E were identified
in patients with HSIL and CxCa. Van Tine et al (18) proposed that the evolution of
transcription patterns may be a dynamic process in response to
environmental changes, to minimize gene expression associated with
cell proliferation (18). This may
also explain the existence of different HPV-16 E6-associated
transcriptional patterns with the progression of CxCa.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81172463), Zhejiang
Provincial Natural Science Foundation (no. Y14H190021), the
Department of Education of Zhejiang Province (nos. Y200907403 and
Y201327980) and Wenzhou Science and Technology Bureau (nos.
Y20120159, Y20090103 and H20100063). These sponsors provided the
funding for the experiments and the collection of specimens.
Abbreviations:
|
HPV
|
human papilloma virus
|
|
ORFs
|
open reading frames
|
|
APOT
|
amplification of papillomavirus
oncogene transcripts
|
|
HSILs
|
high-grade squamous intraepithelial
lesions
|
References
|
1
|
zur Hausen H: Human papillomaviruses in
the pathogenesis of anogenital cancer. Virology. 184:9–13. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frisch M, Fenger C, van den Brule AJ, et
al: Variants of squamous cell carcinoma of the anal canal and
perianal skin and their relation to human papillomaviruses. Cancer
Res. 59:753–757. 1999.PubMed/NCBI
|
|
3
|
Castellsagué X, Díaz M, de Sanjosé S, et
al; International Agency for Research on Cancer Multicenter
Cervical Cancer Study Group. Worldwide human papillomavirus
etiology of cervical adenocarcinoma and its cofactors: implications
for screening and prevention. J Natl Cancer Inst. 98:303–315. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scheffner M, Werness BA, Huibregtse JM,
Levine AJ and Howley PM: The E6 oncoprotein encoded by human
papillomavirus types 16 and 18 promotes the degradation of p53.
Cell. 63:1129–1136. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Sampath A, Raychaudhuri P and
Bagchi S: Both Rb and E7 are regulated by the ubiquitin proteasome
pathway in HPV-containing cervical tumor cells. Oncogene.
20:4740–4749. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Incassati A, Patel D and McCance DJ:
Induction of tetraploidy through loss of p53 and upregulation of
Plk1 by human papillomavirus type-16 E6. Oncogene. 25:2444–2451.
2006. View Article : Google Scholar
|
|
7
|
Zheng ZM and Baker CC: Papillomavirus
genome structure, expression, and post-transcriptional regulation.
Front Biosci. 11:2286–2302. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schwartz S: HPV-16 RNA processing. Front
Biosci. 13:5880–5891. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klaes R, Woerner SM, Ridder R, et al:
Detection of high-risk cervical intraepithelial neoplasia and
cervical cancer by amplification of transcripts derived from
integrated papillomavirus oncogenes. Cancer Res. 59:6132–6136.
1999.
|
|
10
|
Frohman MA, Dush MK and Martin GR: Rapid
production of full-length cDNAs from rare transcripts:
amplification using a single gene-specific oligonucleotide primer.
Proc Natl Acad Sci USA. 85:8998–9002. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wentzensen N, Ridder R, Klaes R,
Vinokurova S, Schaefer U and Doeberitz Mv: Characterization of
viral-cellular fusion transcripts in a large series of HPV16 and 18
positive anogenital lesions. Oncogene. 21:419–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smotkin D, Prokoph H and Wettstein FO:
Oncogenic and nononcogenic human genital papillomaviruses generate
the E7 mRNA by different mechanisms. J Virol. 63:1441–1447.
1989.PubMed/NCBI
|
|
13
|
Sedman SA, Barbosa MS, Vass WC, et al: The
full-length E6 protein of human papillomavirus type 16 has
transforming and trans-activating activities and cooperates with E7
to immortalize keratinocytes in culture. J Virol. 65:4860–4866.
1991.PubMed/NCBI
|
|
14
|
Kraus I, Driesch C, Vinokurova S, et al:
The majority of viral-cellular fusion transcripts in cervical
carcinomas cotranscribe cellular sequences of known or predicted
genes. Cancer Res. 68:2514–2522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmitt M, Dalstein V, Waterboer T, Clavel
C, Gissmann L and Pawlita M: Diagnosing cervical cancer and
high-grade precursors by HPV16 transcription patterns. Cancer Res.
70:249–256. 2010. View Article : Google Scholar
|
|
16
|
Schmitz M, Driesch C, Jansen L, Runnebaum
IB and Dürst M: Non-random integration of the HPV genome in
cervical cancer. PLoS One. 7:e396322012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cornelissen MT, Smits HL, Briët MA, et al:
Uniformity of the splicing pattern of the E6/E7 transcripts in
human papillomavirus type 16-transformed human fibroblasts, human
cervical premalignant lesions and carcinomas. J Gen Virol.
71:1243–1246. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Van Tine BA, Kappes JC, Banerjee NS, et
al: Clonal selection for transcriptionally active viral oncogenes
during progression to cancer. J Virol. 78:11172–11186. 2004.
View Article : Google Scholar : PubMed/NCBI
|