Introduction
Shikonin, a naphthoquinone extracted from the roots
of Lithospermum erythrorhizon, is a potent antioxidant with
anti-inflammatory, antiviral and cancer-preventing properties
(1). Shikonin exhibits significant
cytotoxic activity against multiple cancer cell types in
vitro and in vivo (2,3), and a
number of studies have previously established a potential role for
shikonin as a candidate therapeutic agent in the treatment of
cancer (4,5). However, the mechanism by which
shikonin achieves this effect has yet to be fully elucidated
(6).
Ovarian carcinoma is the most lethal type of
gynecological malignancy. The response to traditional
platinum-based chemotherapy is poor in numerous patients,
therefore, current research is focused on the development of novel
therapeutic strategies. Protein tyrosine kinases (PTKs) are
important in cellular signal transduction pathways and regulate
numerous cellular activities, including cell growth, migration,
differentiation and apoptosis (7,8).
Furthermore, the abnormal activation of PTKs is closely associated
with ovarian carcinoma (8),
therefore, PTKs are attractive targets for anticancer agents.
The expression and activity of the proto-oncogene
tyrosine kinase Src (Src) is associated with a poor prognosis and
advanced malignancy in a range of types of human cancer, including
ovarian carcinoma (9,10). Focal adhesion kinase (FAK), an
intracellular PTK recruited to focal adhesion sites, acts via cell
surface receptors as a major mediator of signal transduction
(11). FAK has been demonstrated to
be key factor in the regulation of cell survival (12), proliferation, differentiation,
migration, invasion (13) and
angiogenesis (14), all of which
are vital processes in the development of cancer. Furthermore, FAK
is overexpressed in ovarian cancer (15). Therefore, FAK may be involved in
promoting tumorigenesis and metastasis in cancer.
In the present study, it was hypothesized that
shikonin may have a role as an inhibitor of ovarian cancer cells
growth and migration, and therefore, could potentially serve as a
therapeutic agent for the management of human ovarian cancers.
Materials and methods
Preparation of shikonin
Shikonin was purchased from ChromaDex, Inc., (cat.
no., ASB-00019210-005; Irvine, CA, USA), dissolved in dimethyl
sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) and stored at
−20°C. For all experiments in the present study, the final
concentrations of the compounds analyzed were prepared by diluting
the stock solution with culture medium, while the control cultures
were diluted with the carrier solvent (0.1% DMSO).
Cell culture
SKOV-3 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA) and maintained in a
monolayer culture at 37°C and 5% CO2 in McCoy’s 5A
medium (Gibco Life Technologies, Carlsbad, CA, USA) with 10% fetal
bovine serum (Gibco Life Technologies).
Cytotoxicity assay
The cytotoxic effect of shikonin on the SKOV-3 cells
was measured by performing a Cell Counting kit (CCK)-8 assay
(Dojindo Laboratories, Kumamoto, Japan). Briefly, the cells were
dispensed into a 96-well flat-bottomed microtiter plate (Thermo
Scientific Nunc, Roskilde, Denmark) at a density of
1×104 cells/well, followed by treatment with various
concentrations of shikonin (1, 2, 4, 8, 16, 32, 64, 128 or 256 μM)
for 48 h. Cell growth was measured using an enzyme-linked
immunosorbent assay reader (Tecan Spectra, Wetzlar, Germany) to
analyze the CCK-8 assay.
Flow cytometric analysis
The rate of apoptosis was measured using an Annexin
V-fluorescein isothiocyanate/propidium iodide (FITC/PI) apoptosis
detection kit (Invitrogen Life Technologies, Carlsbad, CA, USA),
according to the manufacturer’s instructions. The cells were
exposed to various concentrations of shikonin (0, 4, 8 and 16
mmol/l), incubated for 48 h, collected and washed twice with
phosphate-buffered saline (PBS). Next, the cells were gently
resuspended in Annexin V binding buffer, incubated with Annexin
V-FITC/PI in the dark for 15 min and analyzed using flow
cytometry.
Caspase activity assay
The SKOV-3 cells (1×106) were incubated
without or with shikonin (16 μM). The cells were harvested at 0,
12, 24, 48 and 72 h, washed with PBS and pelleted. The supernatant
was aspirated, cell lysis buffer was added at 0.5
ml/1×106 cells and then the cells in the lysis buffer
were incubated on ice for 10 min. Reaction buffer containing 5 μl
dithiothreitol, 5 μl DEVD-AFC amino acid substrate and 380 μl
H2O was added to each aliquot of cell lysate and the
mixtures were incubated at 37°C for 1 h. The fluorescence emitted
by the cleaved substrates was determined using a spectrofluorometer
at an absorbance of 400 nm for excitation and 505 nm for emission.
One unit of enzyme activity corresponds to the activity required to
cleave 1 mg of substrate in 1 min at 37°C.
Migration assay
The SKOV-3 cells were plated onto the upper membrane
of a Transwell unit (8-μm pore size; Merck Millipore, Darmstadt,
Germany) at a density of 4×105 cells/well. The cells
were exposed to various concentrations of shikonin (0, 4, 8 and 16
μmol/l) and incubated for 24 h. Any non-migrated cells on the upper
membrane were removed using a cotton swab, while the migrated cells
(located on the lower surface of the Transwell filters) were fixed
for 5 min in methanol, stained with 0.1% crystal violet, eluted
with 33% ethylic acid and measured at an absorbance of 480 nm to
obtain the optical density values.
Western blot analysis
The SKOV-3 cells were harvested and lysed for 1 h on
ice in lysis buffer [50 mM Tris-Cl (pH 7.4), 1% NP-40, 0.25% sodium
deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM
Na3VO4, 1 mM NaF, mammalian protease
inhibitor cocktail and phosphatase inhibitor cocktail; Roche
Diagnostics, Indianapolis, IN, USA]. The caspase protein
concentrations were determined by performing a bicinchoninic acid
assay. The lysate was centrifuged at 16,000 × g and 4°C for 10 min,
and then equal quantities of total protein were mixed with loading
buffer, boiled for 5 min and subjected to electrophoresis on a 10%
SDS-polyacrylamide gel. Subsequently, the proteins were
electrotransferred onto polyvinylidene difluoride membranes.
Subsequent to blocking with Tris-buffered saline (TBS) containing
5% skimmed milk at room temperature, the membranes were incubated
for 2 h at room temperature with polyclonal rabbit anti-human
caspase-3,-8 and -9 (1:1,000; Cell Signaling Technology, In.,
Danvers, MA, USA) primary antibodies in TBS. Following three 10-min
washes in TBS, the membranes were incubated with a diluted
horseradish peroxidase-labeled secondary antibody for 1 h and after
an additional three washes, the protein expression levels were
detected using an enhanced chemiluminescence kit, according to the
manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA,
USA).
Statistical analysis
Data are presented as the mean ± standard deviation
of the results from three independent experiments. Differences were
assessed by the two-tailed Student’s t-test. Statistical analyses
were performed using SPSS version 16.0 software (SPSS, Inc.,
Chicago, IL, USA). All graphs were obtained using Microsoft Office
Excel 2010 software (Microsoft Research, Redmond, WA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
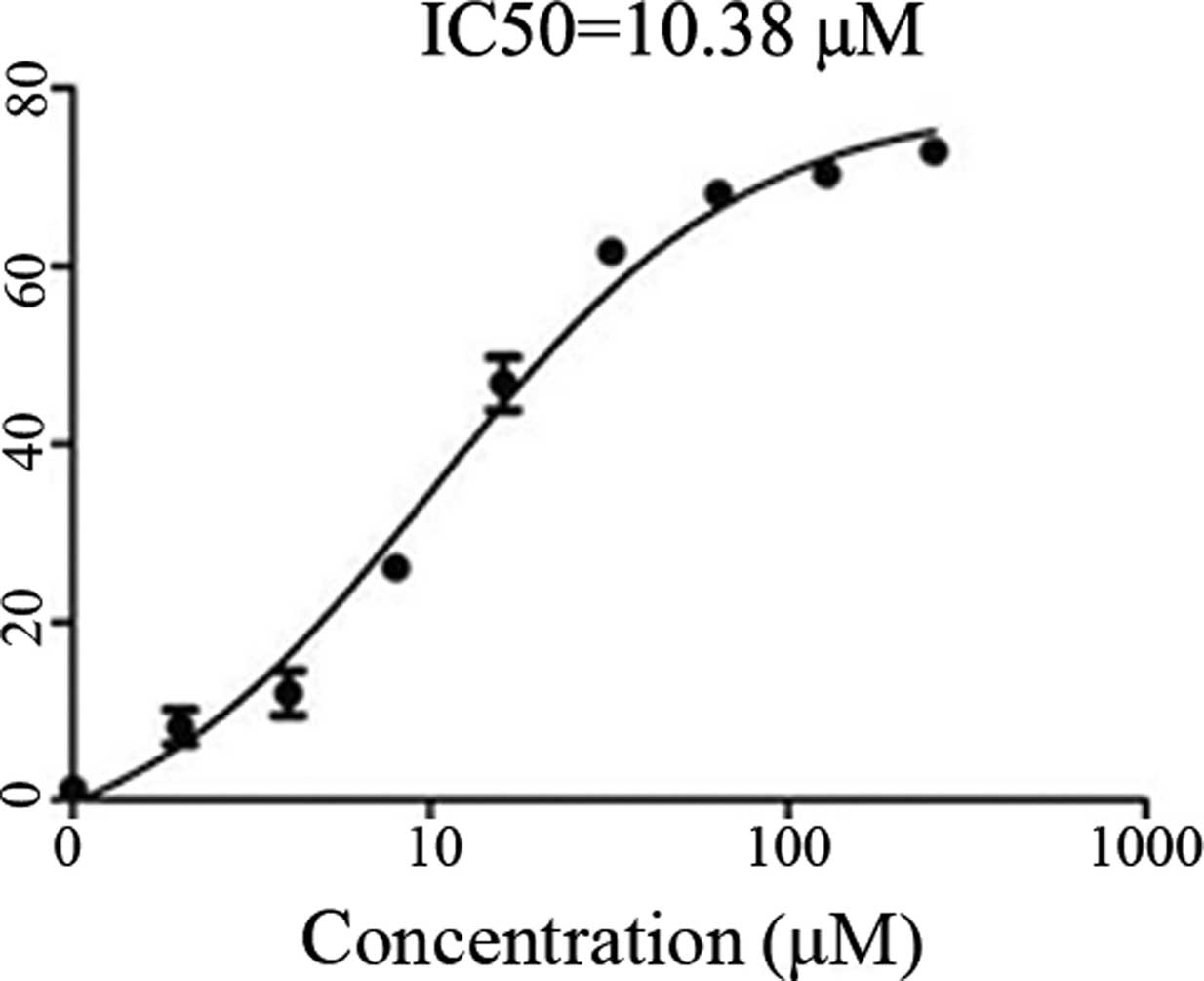
Inhibition of cell growth by
shikonin
To determine the inhibitory effects of shikonin on
the growth of cultured SKOV-3 cells and to ascertain the viability
of SKOV-3 cells in the presence of shikonin, a CCK-8 assay was
performed. Treating the SKOV-3 cells with increasing concentrations
of shikonin identified 10.38 μmol as the half maximal inhibitory
concentration of shikonin (Fig.
1).
Shikonin induces the apoptosis of SKOV-3
cells
The SKOV-3 cells were treated with various
concentrations of shikonin for 48 h (Fig. 2A) and the percentage of apoptotic
cells present following treatment is indicated in Fig. 2B. Increasing concentrations of
shikonin were associated with increased levels of SKOV-3 cell
apoptosis.
 | Figure 2Shikonin induces cell death in SKOV-3
cells. (A) SKOV-3 cells were treated with dimethyl sulfoxide (DMSO;
control) or 0, 4, 8 or 16 μM shikonin for 48 h, followed by use of
an Annexin V-FITC binding assay. Cell death was measured by
Annexin-V and PI staining, and flow cytometry; data are presented
as cytograms. Viable cells were negative for Annexin-V and PI
staining (lower left quadrant), early-stage apoptotic cells were
positive for annexin-V staining, but negative for PI staining
(lower right quadrant), and late-stage apoptotic cells were
positive for Annexin-V and PI staining (upper right quadrant). (B)
The percentage of Annexin V-stained cells following treatment with
with DMSO (control) or 0, 4, 8 or 16 μM shikonin. Data are
presented as the mean ± standard deviation (n=3).
*P<0.05 vs. control group. FITC, fluorescein
isothiocyanate; PI, propodium iodide. |
Effect of shikonin on caspase
activation
The changes in caspase-3, -8 and -9 protein
expression levels in response to shikonin administration were
determined by performing western blot analysis (Fig. 3A) and it was found that the levels
of cleaved caspase-3, -8 and -9 were increased following treatment
with shikonin for 48 h. The activity of caspase-3 protease
increased during shikonin-induced apoptosis (Fig. 3B)
Effect of shikonin on the motility and
invasion of SKOV-3 cells
Treatment of the SKOV-3 cells with increasing
concentrations of shikonin for 12 h resulted in a dose-dependent
decrease in cell migration (Fig. 4A and
B). Furthermore, the SKOV-3 cells that underwent longer
treatment periods with shikonin (24 h) also exerted a
dose-dependent inhibitory effect on cell invasion (Fig. 4C and D).
Effect of shikonin on the activity and
protein expression levels of Src and FAK in SKOV-3 cells
The activity of Src and FAK in the SKOV-3 cells was
measured by performing western blot analyses of
Tyr-397-phosphoryated FAK and Tyr-416-phosphoryated Src. The
activity of Src and FAK decreased in response to treatment with
shikonin in a dose-dependent manner. Additionally, the expression
of Src and FAK was decreased in the SKOV-3 cells in response to
treatment with shikonin (Fig. 5).
These results indicate that shikonin may be crucial in the
downregulation of FAK expression.
Discussion
Shikonin, a novel compound isolated from the Chinese
herbal therapeutic agent Zicao, has been demonstrated to exhibit
anticancer activity (16,17). Previous studies have indicated that
shikonin inhibits tumor formation, carcinogenesis and metastasis,
predominantly by inhibiting the proliferation and induction of
apoptosis in tumor cell lines (18,19).
Various molecular targets have been associated with
shikonin-induced apoptotic cell death (20,21),
however, the mechanism by which shikonin exhibits its anticancer
activity is poorly understood.
In the present study, a potential therapeutic
pathway was identified by which shikonin appears to target ovarian
cancer cells by inhibiting growth and inducing apoptosis. Shikonin
has previously been demonstrated to exhibit an inhibitory effect on
human colorectal carcinoma COLO 205 cells via the induction of
apoptotic cell death, accompanied by the upregulation of p27 and
p53, and the downregulation of B-cell lymphoma (Bcl)-2 and
Bcl-extra large (22). Furthermore,
shikonin induces HeLa cell death via caspase-3 activation (23). Similarly, the present study detected
caspase activation in shikonin-induced ovarian cell apoptosis.
Furthermore, the extrinsic death receptor pathway and the intrinsic
mitochondrial pathway appears to be involved in shikonin-induced
apoptosis, indicating that the two pathways may interact with and
amplify each other in the process of activating effector caspases,
such as caspase-3 (23).
Additionally, the present study identified that
shikonin decreases ovarian cancer cell migration and invasion; this
was demonstrated by the treatment of SKOV-3 cells with various
concentrations of shikonin, causing a reduction in the motility of
the ovarian cancer cells. A shikonin derivative,
β-hydroxyisovalerylshikonin, inhibits the PTK activities of
epidermal growth factor and viral-Src receptors (24). Src and Src-family PTKs are
regulatory proteins that play key roles in cell differentiation,
motility, proliferation and survival (25). Src protein inhibition occurs via
targeting of its phosphorylation sites; the Src phosphorylation
sites initially described include the autophosphorylated activation
site at Tyr 416. In agreement with the aforementioned previous
studies, the present study demonstrated that shikonin inhibits Src
activity in SKOV-3 cells.
FAK, a non-receptor PTK, is a key component of focal
adhesion sites, particularly in the promotion, spread, migration
and transmission of anchorage-dependent anti-apoptotic signals
(26). FAK is activated following
the engagement of adjacent integrin molecules or the stimulation of
transmembrane receptors (27,28);
and this activation occurs via tyrosine autophosphorylation, as
well as via phosphorylation by other PTKs, including Src family
kinases (29,30) and the insulin receptor (31). Tyr-397 autophosphorylation is an
important step for promoting the biological function of FAK, and
Tyr-576 and -577 phosphorylation by Src increases FAK activity
(32). Previously, increased
protein expression levels of FAK have been identified in various
types of ovarian cancer, rendering FAK a potentially valuable
target for therapeutic intervention (15). Furthermore, increased FAK expression
and activity have previously been correlated with malignant or
metastatic disease and poor patient prognosis (33). FAK is regulated by growth factor
receptor stimulation and acts as a signaling intermediate that is
recruited to focal adhesions immediately following integrin
activation, therefore, the present study determined FAK activity in
response to the presence of various concentrations of shikonin by
analyzing the level of FAK autophosphorylation. In the SKOV-3
cells, shikonin decreased FAK activity in a dose-dependent manner.
The present study additionally reported that shikonin appears to
inhibit the metastasis of ovarian cancer cells; it is proposed that
shikonin may mediate this metastatic effect by decreasing the
activity and expression of FAK.
In conclusion, shikonin was shown to induce
apoptosis and decrease cellular migration in ovarian cancer cells.
In addition, shikonin decreased FAK activity in a dose-dependent
manner. Therefore, shikonin requires consideration as a candidate
agent for the prevention and treatment of human ovarian cancer.
Acknowledgements
This study was supported by the China National
Science Foundation (grant no. 81272537) and the Jiangsu Traditional
Chinese Medicine Science and Technology Project Foundation for 2013
(grant no. LZ13206).
References
|
1
|
Ishida T and Sakaguchi I: Protection of
human keratinocytes from UVB-induced inflammation using root
extract of Lithospermum erythrorhizon. Biol Pharm Bull. 30:928–934.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeh CC, Kuo HM, Li TM, et al:
Shikonin-induced apoptosis involves caspase-3 activity in a human
bladder cancer cell line (T24). In Vivo. 21:1011–1019. 2007.
|
|
3
|
Wu Z, Wu LJ, Tashiro S, et al:
Phosphorylated extracellular signal-regulated kinase up-regulated
p53 expression in shikonin-induced HeLa cell apoptosis. Chin Med J
(Engl). 118:671–677. 2005.
|
|
4
|
Li W, Liu J, Jackson K, Shi R and Zhao Y:
Sensitizing the therapeutic efficacy of taxol with shikonin in
human breast cancer cells. PloS One. 9:e940792014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jang SY, Jang EH, Jeong SY and Kim JH:
Shikonin inhibits the growth of human prostate cancer cells via
modulation of the androgen receptor. Int J Oncol. 44:1455–1460.
2014.PubMed/NCBI
|
|
6
|
Wang W, Dai M, Zhu C, et al: Synthesis and
biological activity of novel shikonin analogues. Bioorg Med Chem
Lett. 19:735–737. 2009. View Article : Google Scholar
|
|
7
|
Al-Obeidi FA and Lam KS: Development of
inhibitors for protein tyrosine kinases. Oncogene. 19:5690–5701.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hunter T: Protein kinases and
phosphatases: the yin and yang of protein phosphorylation and
signaling. Cell. 80:225–236. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wheeler DL, Iida M and Dunn EF: The role
of Src in solid tumors. Oncologist. 14:667–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wiener JR, Windham TC, Estrella VC, et al:
Activated SRC protein tyrosine kinase is overexpressed in
late-stage human ovarian cancers. Gynecol Oncol. 88:73–79. 2003.
View Article : Google Scholar
|
|
11
|
Parsons JT: Focal adhesion kinase: the
first ten years. J Cell Sci. 116:1409–1416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Westhoff MA, Serrels B, Fincham VJ, et al:
SRC-mediated phosphorylation of focal adhesion kinase couples actin
and adhesion dynamics to survival signaling. Mol Cell Biol.
24:8113–8133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schlaepfer DD and Mitra SK: Multiple
connections link FAK to cell motility and invasion. Curr Opin Genet
Dev. 14:92–101. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng X, Ueda H, Zhou H, et al:
Overexpression of focal adhesion kinase in vascular endothelial
cells promotes angiogenesis in transgenic mice. Cardiovasc Res.
64:421–430. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Judson PL, He X, Cance WG and Van Le L:
Overexpression of focal adhesion kinase, a protein tyrosine kinase,
in ovarian carcinoma. Cancer. 86:1551–1556. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sankawa U, Ebizuka Y, Miyazaki T, Isomura
Y and Otsuka H: Antitumor activity of shikonin and its derivatives.
Chem Pharm Bull (Tokyo). 25:2392–2395. 1977. View Article : Google Scholar
|
|
17
|
Sankawa U, Otsuka H, Kataoka Y, Iitaka Y,
Hoshi A and Kuretani K: Antitumor activity of shikonin, alkannin
and their derivatives. II X-ray analysis of cyclo-alkannin
leucoacetate, tautomerism of alkannin and cyclo-alkannin and
antitumor activity of alkannin derivatives. Chem Pharm Bull
(Tokyo). 29:116–122. 1981. View Article : Google Scholar
|
|
18
|
Yang H, Zhou P, Huang H, et al: Shikonin
exerts antitumor activity via proteasome inhibition and cell death
induction in vitro and in vivo. Int J Cancer. 124:2450–2459. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HJ, Lee HJ, Magesh V, Nam D, et al:
Shikonin, acetylshikonin and isobutyroylshikonin inhibit
VEGF-induced angiogenesis and suppress tumor growth in lewis lung
carcinoma-bearing mice. Yakugaku Zasshi. 128:1681–1688. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Z, Wu L, Li L, Tashiro S, Onodera S and
Ikejima T: p53-mediated cell cycle arrest and apoptosis induced by
shikonin via a caspase-9-dependent mechanism in human malignant
melanoma A375-S2 cells. J Pharmacol Sci. 94:166–176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han W, Li L, Qiu S, et al: Shikonin
circumvents cancer drug resistance by induction of a necroptotic
death. Mol Cancer Ther. 6:1641–1649. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu PC, Huang YT, Tsai ML, Wang YJ, Lin JK
and Pan MH: Induction of apoptosis by shikonin through coordinative
modulation of the Bcl-2 family, p27 and p53, release of cytochrome
c and sequential activation of caspases in human colorectal
carcinoma cells. J Agric Food Chem. 52:6330–6337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Z, Wu LJ, Li LH, Tashiro S, Onodera S
and Ikejima T: Shikonin regulates HeLa cell death via caspase-3
activation and blockage of DNA synthesis. J Asian Nat Prod Res.
6:155–166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakaya K and Miyasaka T: A shikonin
derivative, beta-hydroxyisovalerylshikonin, is an
ATP-non-competitive inhibitor of protein tyrosine kinases.
Anticancer Drugs. 14:683–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roskoski R Jr: Src kinase regulation by
phosphorylation and dephosphorylation. Biochem Biophys Res Commun.
331:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schlaepfer DD and Hunter T: Integrin
signalling and tyrosine phosphorylation: just the FAKs? Trends Cell
Biol. 8:151–157. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hanks SK, Calalb MB, Harper MC and Patel
SK: Focal adhesion protein-tyrosine kinase phosphorylated in
response to cell attachment to fibronectin. Proc Natl Acad Sci USA.
89:8487–8491. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schaller MD, Borgman CA, Cobb BS, Vines
RR, Reynolds AB and Parsons JT: pp125FAK a structurally distinctive
protein-tyrosine kinase associated with focal adhesions. Proc Natl
Acad Sci USA. 89:5192–5196. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Calalb MB, Polte TR and Hanks SK: Tyrosine
phosphorylation of focal adhesion kinase at sites in the catalytic
domain regulates kinase activity: a role for Src family kinases.
Mol Cell Biol. 15:954–963. 1995.PubMed/NCBI
|
|
30
|
Cobb BS, Schaller MD, Leu TH and Parsons
JT: Stable association of pp60src and pp59fyn with the focal
adhesion-associated protein tyrosine kinase, pp125FAK. Mol Cell
Biol. 14:147–155. 1994.PubMed/NCBI
|
|
31
|
Baron V, Calléja V, Ferrari P, Alengrin F
and Van Obberghen E: p125Fak focal adhesion kinase is a substrate
for the insulin and insulin-like growth factor-I tyrosine kinase
receptors. J Biol Chem. 273:7162–7168. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schlaepfer DD, Hanks SK, Hunter T and van
der Geer P: Integrin-mediated signal transduction linked to Ras
pathway by GRB2 binding to focal adhesion kinase. Nature.
372:786–791. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Recher C, Ysebaert L, Beyne-Rauzy O, et
al: Expression of focal adhesion kinase in acute myeloid leukemia
is associated with enhanced blast migration, increased cellularity
and poor prognosis. Cancer Res. 64:3191–3197. 2004. View Article : Google Scholar : PubMed/NCBI
|