Introduction
Osteosarcoma is the most commonly diagnosed primary
malignancy of bone, particularly among children and adolescents
(1,2). It is characterized by the
proliferation of malignant mesenchymal cells that are capable of
producing osteoid or immature bone (3), and is associated with high morbidity,
early metastasis and mortality (4–6).
Survival of osteosarcoma is poor despite the aggressive use of
surgery, chemotherapy, and/or radiotherapy (7). Furthermore, surgery cannot halt the
metastasis of the tumor and chemotherapy is restricted by the
development of resistance and various side effects. Currently,
traditional Chinese medicine (TCM) is commonly administered to
cancer patients as an adjunct to conventional therapies to improve
the quality of life by alleviating symptoms and side effects
(8). The active gradients of TCM
have been demonstrated to inhibit tumor cell proliferation and
induce tumor cell apoptosis (9,10).
Evodiamine is a natural alkaloid compound extracted
from a variety of TCMs, such as Evodia rutaecarpa (Juss.)
Benth., E. rutaecarpa (Juss.) Benth. var. officinalis (Dode)
Huang and E. rutaecarpa (Juss.) Benth. var. bodinieri (Dode)
Huang. Vasodilatation (11),
anti-inflammation (12), analgesia
(13) and anti-tumor (14) effects are the major efficacies of
evodiamine. Additionally, evodiamine has been shown to induce the
apoptosis of various tumors, such as cervical (15), prostatic (16,17)
and breast cancer (18), as well as
melanoma (19) and leukemia
(20), via the inhibition of
proliferation, blockade of the cell cycle, induction of apoptosis
and suppression of metastasis. However, to the best of our
knowledge, no studies been conducted that focus on the effects of
evodiamine in osteosarcoma.
In the present study, human osteosarcoma U2OS cells
were cultured with different concentrations of evodiamine to
explore its effect on U2OS cell proliferation and apoptosis.
Additionally, the expression levels of apoptosis-associated
proteins were determined to understand the underlying
mechanism.
Materials and methods
Reagents
Evodiamine was purchased from the National
Institutes for Food and Drug Control (Beijing, China), and dimethyl
sulfoxide, RPMI-1640 medium and fetal bovine serum (FBS) were
purchased for cell culture from Gibco-BRL (Carlsbad, CA, USA). An
annexin V-fluorescein isothiocyanate/propidium iodide (FITC/PI) kit
was purchased from Sigma-Aldrich (St. Louis, MO, USA) for cell
apoptosis detection, and a Cell Counting Kit 8 (CCK-8) was
purchased from Beyotime Institute of Biotechnology (Haimen, China)
for the cell viability assay. Furthermore, for western blot
analysis, monoclonal mouse anti-human B-cell lymphoma (Bcl-2;
1:1,000; cat. no. sc-130307) and polyclonal mouse anti-human
Bcl-2-associated X protein (Bax; 1:1,000; cat. no. sc-20067)
antibodies, and polyclonal peroxidase-conjugated goat anti-mouse
(1:1,000; cat. no. sc-2354) antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Monoclonal
mouse-anti-human caspase-3 (1:1,000; cat. no. 9668), mouse
anti-human β-actin (1:1,000; cat. no. 3700) and mouse anti-human
survivin (1:1,000; cat. no. 2802) antibodies were purchased from
Cell Signaling Technology (Danvers, MA, USA). The luminol-enhanced
chemiluminescence kit was obtained from GE Healthcare (Chalfont,
UK).
Cell line and culture conditions
Human osteosarcoma U2OS cells were purchased from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China)
and grown in RPMI-1640 medium with 10% (v/v) FBS, 100 U/ml
streptomycin and 100 U/ml penicillin. The cell cultures were
maintained in a 37°C incubator with a humidified atmosphere of 5%
CO2. U2OS cells were harvested in the logarithmic growth
phase and used in following experiments.
CCK-8 assay
The viability of the U2OS cells was assessed using a
CCK-8 assay. The U2OS cells (1×104/ml) were plated on a
96-well plate at 100 μl/well and incubated overnight. A total of 24
wells containing cultured cells of the 96-well plate were then
divided into four groups (n=6 wells in each group): control group
[treated with 0.1% DMSO (v/v)] and three evodiamine groups (treated
with 0.5, 2.5 and 12.5 μg/ml, respectively). After culturing with
and without evodiamine for 48 h, 10 μl CCK-8 solution was added to
each well, according to the manufacturer’s instructions. After 2 h
incubation at 37°C, the optical density value was measured at a
wavelength of 450 nm. The following formula was used to calculate
the cell survival rate: Cell survival rate (%) = 1 − {[(control
well) − (evodiamine well)] / (control well)} × 100%.
Annexin V-FITC/PI flow cytometry
analysis
U2OS cells (1×106/ml) were plated on a
96-well plate at 2,000 μl/well and incubated overnight. A total of
24 wells containing cultured cells of the 96-well plate were then
divided into four groups (n=6 wells in each group): control group
[treated with 0.1% DMSO (v/v)] and three evodiamine groups (treated
with 0.5, 2.5 and 12.5 μg/ml, respectively). After culturing with
and without evodiamine for 48 h, the cells were harvested, and the
percentages of apoptotic or necrotic cells were determined using
the Annexin V-FITC/PI Apoptosis Detection kit, according to the
manufacturer’s instructions, in a flow cytometer (FACSCalibur™; BD
Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
The expression levels of cellular proteins were
evaluated by performing western blot analysis. U2OS cells
(1×106/ml) were plated on a 96-well plate at 2,000
μl/well and incubated overnight. A total of 24 wells
containing cultured cells of the 96-well plate were then divided
into four groups (n=6 wells in each group): control group [treated
with 0.1% DMSO (v/v)] and three evodiamine groups (treated with
0.5, 2.5 and 12.5 μg/ml, respectively). Following treatment for 12
h, total proteins were extracted and the protein concentrations
were determined using a bicinchoninic acid protein assay. Equal
amounts of protein from each sample were separated by 12% SDS-PAGE.
Following electrophoresis, the proteins were electroblotted onto
polyvinylidene difluoride membranes for 1 h at room temperature.
Subsequently, the membranes were individually incubated with the
following primary antibodies (dilution, 1:1,000) overnight at 4°C:
Mouse anti-human Bcl-2 monoclonal antibody, mouse anti-human Bax
polyclonal antibody, mouse anti-human caspase-3 and survivin
monoclonal antibody. The membranes were washed three times and
incubated with the secondary peroxidase-conjugated antibody
(dilution, 1:1,000) for 1 h at room temperature. Following
incubation, the membranes were washed and the peroxidase activity
was visualized on X-ray film using the luminol-enhanced
chemiluminescence kit (GE Healthcare), according to the
manufacturer’s instructions. β-actin was used as a reference for
normalization. The protein bands were quantified using ImageJ
software (http://rsb.info.nih.gov/ij/)
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The data are expressed as the mean ± standard
deviation. Statistical correlation of data was checked for
significance by analysis of variance and Student’s t-test.
P<0.05 was considered to indicate a statistically significant
difference. These analyses were performed using SPSS software
(version 11; SPSS, Inc., Chicago, IL, USA).
Results
Evodiamine suppresses proliferation in
human osteosarcoma U2OS cells
CCK-8 assays were performed to detect the impact of
evodiamine on the proliferation of U2OS cells. The U2OS cell line
was incubated with 0, 0.5, 2.5 and 12.5 μg/ml evodiamine. After 48
h incubation, the U2OS cells treated with 0.5, 2.5 and 12.5 μg/ml
evodiamine exhibited reduced levels of cell viability, to
89.90±6.12, 66.65±8.01 and 46.22±6.23%, respectively, compared with
the control group (Fig. 1). These
results indicated that evodiamine treatment induced cell growth
inhibition in a concentration-dependent manner in the U2OS human
osteosarcoma cell line. The cells treated with 2.5 (P<0.05) and
12.5 μg/ml (P<0.05) evodiamine exhibited a statistically
significant reduced level of cell viability compared with the
control group.
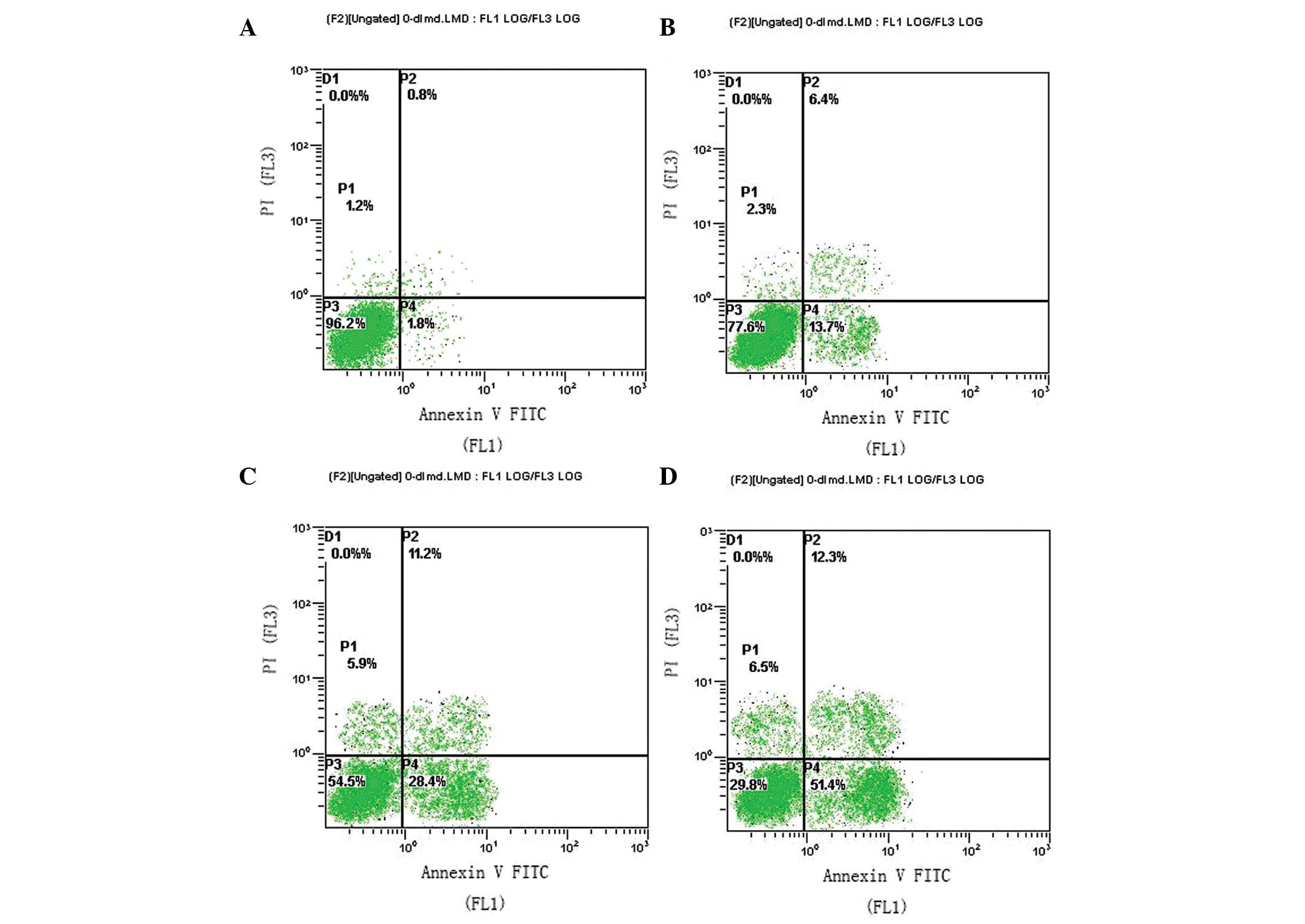
Evodiamine induces apoptosis in human
osteosarcoma U2OS cells
U2OS cells were incubated with 0, 0.5, 2.5 and 12.5
μg/ml evodiamine for 48 h and were analyzed by flow cytometry. The
proportions of early apoptotic cells were determined as 1.8, 13.7,
28.4 and 51.4%, respectively (Fig.
2). These results indicated that evodiamine treatment induced
cell apoptosis in a concentration-dependent manner in the U2OS
human osteosarcoma cell line.
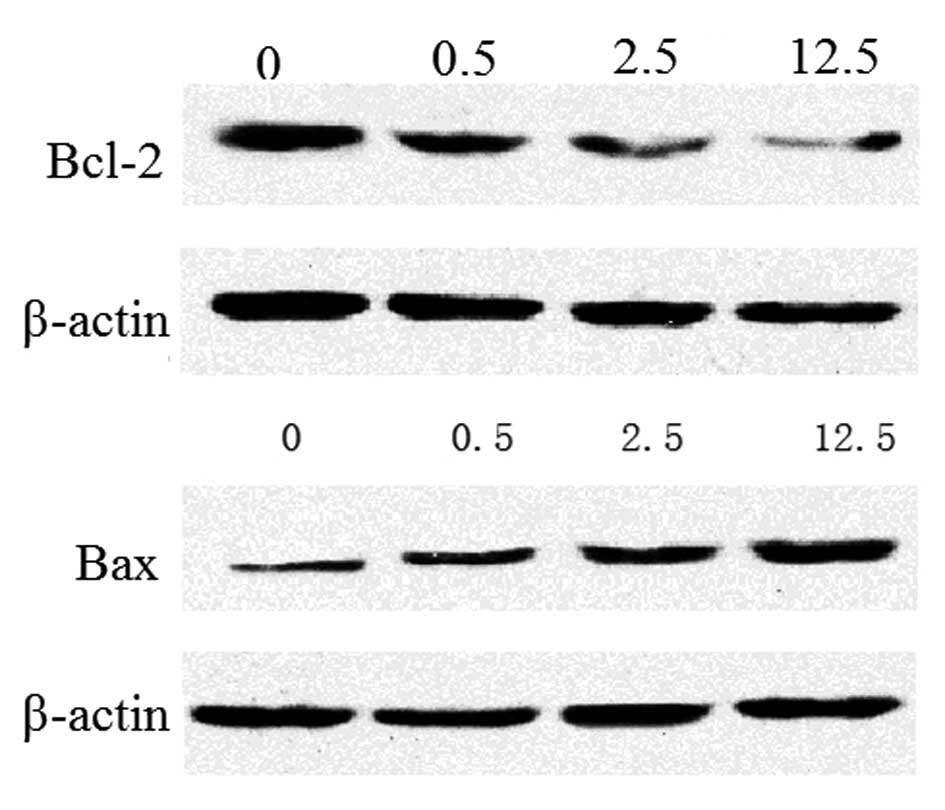
Effect of evodiamine on the expression
levels of Bcl-2 and Bax protein
To determine the molecular mechanism by which
evodiamine induces the apoptosis of U2OS cells, the protein
expression levels of Bcl-2 (an inhibitor of apoptosis) (21) and Bax (a pro-apoptotic member of the
Bcl-2 family) (22) were assessed
by performing western blot analysis. As indicated in Fig. 3, quantitative analysis revealed that
the protein expression levels of Bcl-2 significantly decreased
(P<0.05) and the protein expression levels of Bax significantly
increased (P<0.05) following treatment with evodiamine for 12 h,
compared with the control group. Therefore, evodiamine may induce
apoptosis of U2OS cells through the mitochondrial pathway.
Evodiamine decreases the expression
levels of caspase-3 in U2OS cells
The expression levels of caspase-3 (a pro-apoptotic
protein) was downregulated in a concentration-dependent manner as
the concentration of evodiamine increased (Fig. 4). The results indicated that the
apoptosis induced by evodiamine may involve the caspase
cascade.
Evodiamine decreases the expression
levels of survivin in U2OS cells
Survivin, an anti-apoptotic protein, exerts an
important role in the development of tumors. The expression levels
of survivin were downregulated in a concentration-dependent manner
as the concentration of evodiamine was increased (Fig. 5). The results indicated that
survivin may be a target of the apoptosis pathway induced by
evodiamine.
Discussion
Previous studies have demonstrated the
apoptosis-inducing and chemotherapy resistance-reversing effects of
TCMs and their active ingredients (8–10).
Evodiamine is one of the main constituents of Evodiae
fructus, and exhibits antitumor and antiproliferative
properties (16,23). In the present study, experimental
data demonstrated that evodiamine significantly inhibits the
proliferation and induces the apoptosis of U2OS cells in a
dose-dependent manner. Following 48 h co-culturing with 12.5 μg/ml
evodiamine, the cell viability was reduced to 46.22±6.23% and the
proportion of early apoptotic cells was 51.4%, which indicates that
evodiamine may efficiently inhibit the proliferation of U2OS cells.
Tumorigenesis is closely associated with the loss of control of
cell proliferation and diminished apoptosis, thus, evodiamine
administration may exert a curative effect on osteosarcoma
patients.
Programmed cell death, or apoptosis, is important
for the development and homeostasis of the majority of tissue types
(24). Apoptosis is regulated by
various factors and signaling pathways, such as the endoplasmic
reticulum pathway, the mitochondrial pathway and the death ligand
pathway (25). The mitochondrial
pathway is activated in response to the activation of the
anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax of
the Bcl-2 family, which promote the secretion of cytochrome
c (24) and activate
caspase-3 and -9 in the downstream signaling pathways of Bcl-2 and
Bax (26–28). However, Bcl-2 indirectly inhibits
caspase-3 activation in a variety of pro-apoptotic conditions
(29). Bcl-2 may prevent the
accumulation of cytochrome c, subsequently preserving
capase-3 in the inactive zymogen state, which leads to the
inhibition of the apoptotic cascade (30). A previous study demonstrated that
reduced Bcl-2 expression levels caused by evodiamine administration
resulted in increased mitochondrial cytochrome c release,
and an increased ratio of Bax/Bcl-2 expression was closely
associated with evodiamine-induced apoptosis (14). Survivin, an anti-apoptotic protein,
may facilitate apoptosis-resistance in specific cells, and is an
important target in current antitumor research (31–32).
Survivin specifically binds to members of the caspase family of
proteins and inhibits the activity of caspase-3 to block apoptosis.
Furthermore, survivin expression is positively associated with the
expression levels of Bcl-2, showing a synergistic effect, and is
negatively associated with the expression levels of Bax,
demonstrating an antagonistic effect (33–36).
Western blot analysis was performed to detect the
expression levels of apoptosis-associated proteins Bcl-2, Bax,
caspase-3 and survivin, and it was identified that the ratio of
Bax/Bcl-2 increased with increasing concentrations of evodiamine.
The differential expression of caspase-3 verified that evodiamine
may induce apoptosis of U2OS tumor cells via the mitochondrial
pathway. In addition, survivin is an independent index of
osteosarcoma prognosis (31,37,38);
in the present study, the expression levels of survivin decreased
with increasing concentrations of evodiamine. Thus, survivin served
as a target for the regulation of evodiamine-induced apoptosis in
osteosarcoma, and was associated with Bcl-2, Bax and caspase-3
expression levels.
In conclusion, the present study identified that the
mitochondrial pathway and induced survivin expression may be
mechanisms by which evodiamine inhibits proliferation and induces
apoptosis in osteosarcoma cells. Therefore, evodiamine may be used
as an adjuvant agent to chemotherapeutics in the treatment of
osteosarcoma. However, additional studies are required to explore
the toxicity of evodiamine in vivo, as well as its tolerance
and pharmacokinetic characteristics.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Osaki M, Takeshita F, Sugimoto Y, et al:
MicroRNA-143 regulates human osteosarcoma metastasis by regulating
matrix metalloprotease-13 expression. Mol Ther. 19:1123–1130. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Federman N, Bernthal N, Eilber FC and Tap
WD: The multidisciplinary management of osteosarcoma. Curr Treat
Option Oncol. 10:82–93. 2009. View Article : Google Scholar
|
|
4
|
Salah S, Ahmad R, Sultan I, et al:
Osteosarcoma with metastasis at initial diagnosis: Current outcomes
and prognostic factors in the context of a comprehensive cancer
center. Mol Clin Oncol. 2:811–816. 2014.PubMed/NCBI
|
|
5
|
Qu JT, Wang M, He HL, Tang Y and Ye XJ:
The prognostic value of elevated vascular endothelial growth factor
in patients with osteosarcoma: a meta-analysis and systemic review.
J Cancer Res Clin Oncol. 138:819–825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagarajan R, Kamruzzaman A, Ness KK, et
al: Twenty years of follow-up of survivors of childhood
osteosarcoma: a report from the Childhood Cancer Survivor Study.
Cancer. 117:625–634. 2011. View Article : Google Scholar :
|
|
7
|
Ta HT, Dass CR, Choong PF and Dunstan DE:
Osteosarcoma treatment: state of the art. Cancer Metastasis Rev.
28:247–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia L, Ma S, Hou X, et al: The synergistic
effects of traditional Chinese herbs and radiotherapy for cancer
treatment. Oncol Lett. 5:1439–1447. 2013.PubMed/NCBI
|
|
9
|
Shi RX, Ong CN and Shen HM: Luteolin
sensitizes tumor necrosis factor-α-induced apoptosis in human tumor
cells. Oncogene. 23:7712–7721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin SY, Liu JD, Chang HC, et al: Magnolol
suppresses proliferation of cultured human colon and liver cancer
cells by inhibiting DNA synthesis and activating apoptosis. J Cell
Biochem. 84:532–544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiou WF, Chou CJ, Shum AY and Chen CF:
The vasorelaxant effect of evodiamine in rat isolated mesenteric
arteries: mode of action. Eur J Pharmacol. 215:277–283. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi YH, Shin EM, Kim YS, Cai XF, Lee JJ
and Kim HP: Anti-inflammatory principles from the fruits of Evodia
rutaecarpa and their cellular action mechanisms. Arch Pharm Res.
29:293–297. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi Y: The nociceptive and
anti-nociceptive effects of evodiamine from fruits of Evodia
rutaecarpa in mice. Planta Med. 69:425–428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fei XF, Wang BX, Li TJ, et al: Evodiamine,
a constituent of Evodiae Fructus, induces anti-proliferating
effects in tumor cells. Cancer Sci. 94:92–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Wu LJ, Tashiro S, Onodera S and
Ikejima T: Evodiamine induces tumor cell death through different
pathways: apoptosis and necrosis. Acta Pharmacol Sin. 25:83–89.
2004.PubMed/NCBI
|
|
16
|
Kan SF, Yu CH, Pu HF, Hsu JM, Chen MJ and
Wang PS: Anti-proliferative effects of evodiamine on human prostate
cancer cell lines DU145 and PC3. J Cell Biochem. 101:44–56. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kan SF, Huang WJ, Lin LC and Wang PS:
Inhibitory effects of evodiamine on the growth of human prostate
cancer cell line LNCaP. Int J Cancer. 110:641–651. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao CH, Pan SL, Guh JH, et al: Antitumor
mechanism of evodiamine, a constituent from Chinese herb Evodiae
fructus, in human multiple-drug resistant breast cancer NCI/ADR-RES
cells in vitro and in vivo. Carcinogenesis. 26:968–975. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Wu LJ, Tashiro S, Onodera S and
Ikejima T: Evodiamine induces A375-S2 cell death through two
different pathways. Yao Xue Xue Bao. 38:650–653. 2003.(In
Chinese).
|
|
20
|
Huang YC, Guh JH and Teng CM: Induction of
mitotic arrest and apoptosis by evodiamine in human leukemic
T-lymphocytes. Life Sci. 75:35–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hockenbery DM, Oltvai ZN, Yin XM, et al:
Bcl-2 functions in an antioxidant pathway to prevent apoptosis.
Cell. 75:241–251. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Antonsson B: Bax and other pro-apoptotic
Bcl-2 family “killer-proteins” and their victim the mitochondrion.
Cell Tissue Res. 306:347–361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang J and Hu C: Evodiamine: a novel
anti-cancer alkaloid from Evodia rutaecarpa. Molecules.
14:1852–1859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fiandalo MV and Kyprianou N: Caspase
control: protagonists of cancer cell apoptosis. Exp Oncol.
34:165–175. 2012.PubMed/NCBI
|
|
26
|
Yang J, Liu X, Bhalla K, et al: Prevention
of apoptosis by Bcl-2: release of cytochrome c from mitochondria
blocked. Science. 275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Siu WP, Pun PB, Latchoumycandane C and
Boelsterli UA: Bax-mediated mitochondrial outer membrane
permeabilization (MOMP), distinct from the mitochondrial
permeability transition, is a key mechanism in diclofenac-induced
hepatocyte injury: Multiple protective roles of cyclosporin A.
Toxicol Appl Pharmacol. 227:451–461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohtsuka T, Buchsbaum D, Oliver P, Makhija
S, Kimberly R and Zhou T: Synergistic induction of tumor cell
apoptosis by death receptor antibody and chemotherapy agent through
JNK/p38 and mitochondrial death pathway. Oncogene. 22:2034–2044.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Swanton E, Savory P, Cosulich S, et al:
Bcl-2 regulates a caspase-3/caspase-2 apoptotic cascade in
cytosolic extracts. Oncogene. 18:1781–1787. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park JW, Choi YJ, Suh SI, et al: Bcl-2
overexpression attenuates resveratrol-induced apoptosis in U937
cells by inhibition of caspase-3 activity. Carcinogenesis.
22:1633–1639. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Osaka E, Suzuki T, Osaka S, et al:
Survivin expression levels as independent predictors of survival
for osteosarcoma patients. J Orthop Res. 25:116–121. 2007.
View Article : Google Scholar
|
|
32
|
Altieri DC: Survivin, versatile modulation
of cell division and apoptosis in cancer. Oncogene. 22:8581–8589.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu CD, Altieri DC and Tanigawa N:
Expression of a novel antiapoptosis gene, survivin, correlated with
tumor cell apoptosis and p53 accumulation in gastric carcinomas.
Cancer Res. 58:1808–1812. 1998.PubMed/NCBI
|
|
34
|
Tanaka K, Iwamoto S, Gon G, Nohara T,
Iwamoto M and Tanigawa N: Expression of survivin and its
relationship to loss of apoptosis in breast carcinomas. Clin Cancer
Res. 6:127–134. 2000.PubMed/NCBI
|
|
35
|
Kawasaki H, Altieri DC, Lu CD, Toyoda M,
Tenjo T and Tanigawa N: Inhibition of apoptosis by survivin
predicts shorter survival rates in colorectal cancer. Cancer Res.
58:5071–5074. 1998.PubMed/NCBI
|
|
36
|
Tamm I, Wang Y, Sausville E, et al:
IAP-family protein survivin inhibits caspase activity and apoptosis
induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer
Res. 58:5315–5320. 1998.PubMed/NCBI
|
|
37
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar
|
|
38
|
Suzuki A and Shiraki K: Tumor cell ‘dead
or alive’: caspase and survivin regulate cell death, cell cycle and
cell survival. Histol Histopathol. 16:583–593. 2001.PubMed/NCBI
|