Introduction
Lung cancer is one of the most commonly diagnosed
malignant tumors in China. Non-small cell lung cancer (NSCLC)
demonstrates the highest incidence and accounts for 80% of all lung
cancer cases. Lung cancer exhibits a high rate of mortality and is
often not diagnosed at an early stage. Therefore, 75% of NSCLS
patients are ineligible for surgical resection at the time of
diagnosis (1). The principal
therapeutic modality for the treatment of advanced-stage lung
cancer is chemotherapy, which usually consists of a combination of
third-generation cytotoxic drugs and platinum (2). However, a limitation of this therapy
is a reduction in the quality of life of the patients due to the
side-effects of the drugs. In addition, drug resistance is common
in cases of NSCLC, and therefore relapse rates are extremely
high.
The growth and metastasis of a tumor depends upon
the steady supply of nutrients and oxygen, which are delivered via
the vascular system. All solid malignant tumors develop a network
of blood vessels. For this reason, anti-angiogenic ‘hunger
therapies’ are used to limit the development of the blood vessels
(3). Anti-angiogenic therapies are
able to work synergistically with conventional chemotherapy
treatments (4–6). However, certain studies have reported
negative results with the combined use of anti-angiogenic and
chemotherapy drugs (7, 8). Hypotheses, such as the ‘time window’
(9) and ‘vascular normalization’
(10), have been proposed in order
to explain these negative findings. A study by Weichselbaum
(11) revealed that γ-ray treatment
of U87-MG xenografts was more effective in decreasing tumor size
when the tumors were treated with the vascular endothelial growth
factor inhibitor, DC101, for 4–6 days prior to radiation treatment
(11). Other studies suggested that
anti-angiogenic drugs combined with chemotherapy may exhibit
optimal efficacy when administered successively, and that a short
‘time window’ for optimal results may exist (12–14).
However, certain evidence exists that contradicts this hypothesis
(15–17). Therefore, further research to
address which optimal combination and administration regime of
anti-angiogenic and antitumor drugs, whether it may be simultaneous
or sequential, is required.
Cisplatin, a non-specific cell cycle-dependent
agent, is the primary chemotherapeutic drug used to treat cases of
NSCLC. The recombinant human endostatin, Endostar, is the first
anti-angiogenic drug to be developed in China, and has been
reported to be more efficient in blocking angiogenesis and
suppressing the growth of primary tumors and metastases compared
with other endostatin preparations (18). Endostar, in combination with
vinorelbine and cisplatin, has been approved for use in the
treatment of advanced NSCLC (19).
The present study aimed to identify the optimal treatment regimen
for the combination of Endostar and cisplatin in a murine tumor
model, and to define a treatment schedule in order to guide future
clinical therapies more efficiently than existing protocols.
Materials and methods
Experimental animal and tumor models
C57/BL/6 mice, 6–8 weeks old, were purchased from
Tengxin Biotechnology Co., Ltd., (Chongqing, China) and housed in
the animal research facility at The Affiliated Hospital of Luzhou
Medical College (Luzhou, China). The mice were kept in groups of
between three and five animals per cage, and fed with clean food
and water. The animals were acclimatized to laboratory procedures
for at least a week under the standard conditions of 24±2°C and
50±10% relative humidity. The murine Lewis lung carcinoma (LLC)
cell line was obtained from The Experimental Center of Sichuan
University (Chengdu, China) and maintained in RPMI-1640. In total,
~1×106 LLC cells were suspended in 0.1 ml phosphate
buffered saline (PBS; pH, 7.0) and then injected subcutaneously
into the right lumbar region of each mouse. Following the
development of tumors, the tumor tissue blocks were resected and
then implanted into the right lumbar region of another mouse. The
tumor cells were passaged in this way for three generations in
order to adapt them to the in vivo environment. The tumor
growth was evaluated every other day by the measurement of the
tumor diameter. The volume of each tumor was determined using the
following formula: Tumor volume (cm3) = length ×
width2 × 0.5. All animal studies were approved by the
Institutional Animal Care and Treatment Committee of Luzhou Medical
College.
Chemotherapy
Cisplatin was purchased from Gejiu Bio-Medicine
Industry Ltd. (Yunnan, China). According to the dose conversion
table for animal and human body weights, which uses the Du Bois
formula to calculate the body surface area (BSA) of the patient
(m2): 0.007184 × (patient height in cm)0.725
× (patient weight in kg)0.425, the maximum daily dose of
cisplatin for mice is 6.15 mg/kg. Therefore, doses of 6, 5, 4 and 3
mg/kg/day were selected for the preliminary experiments. Cisplatin
was administered intraperitoneally (i.p.). At 4 mg/kg/day,
cisplatin exerted the maximum antitumor effect, therefore, this
dose was selected for use in the combination study with Endostar,
which was diluted in 0.2 ml sterile 0.9% normal saline (NS).
Design and grouping of experiments
The recombinant human endostatin, Endostar, was
provided by Shandong Simcere Medgenn Biopharmaceutical Co., Ltd
(Yantai, Shandong, China) and stored at 4°C until required.
According to the protocols adopted in the preliminary experiments,
Endostar was dissolved in 0.2 ml 0.9% NS and administered to each
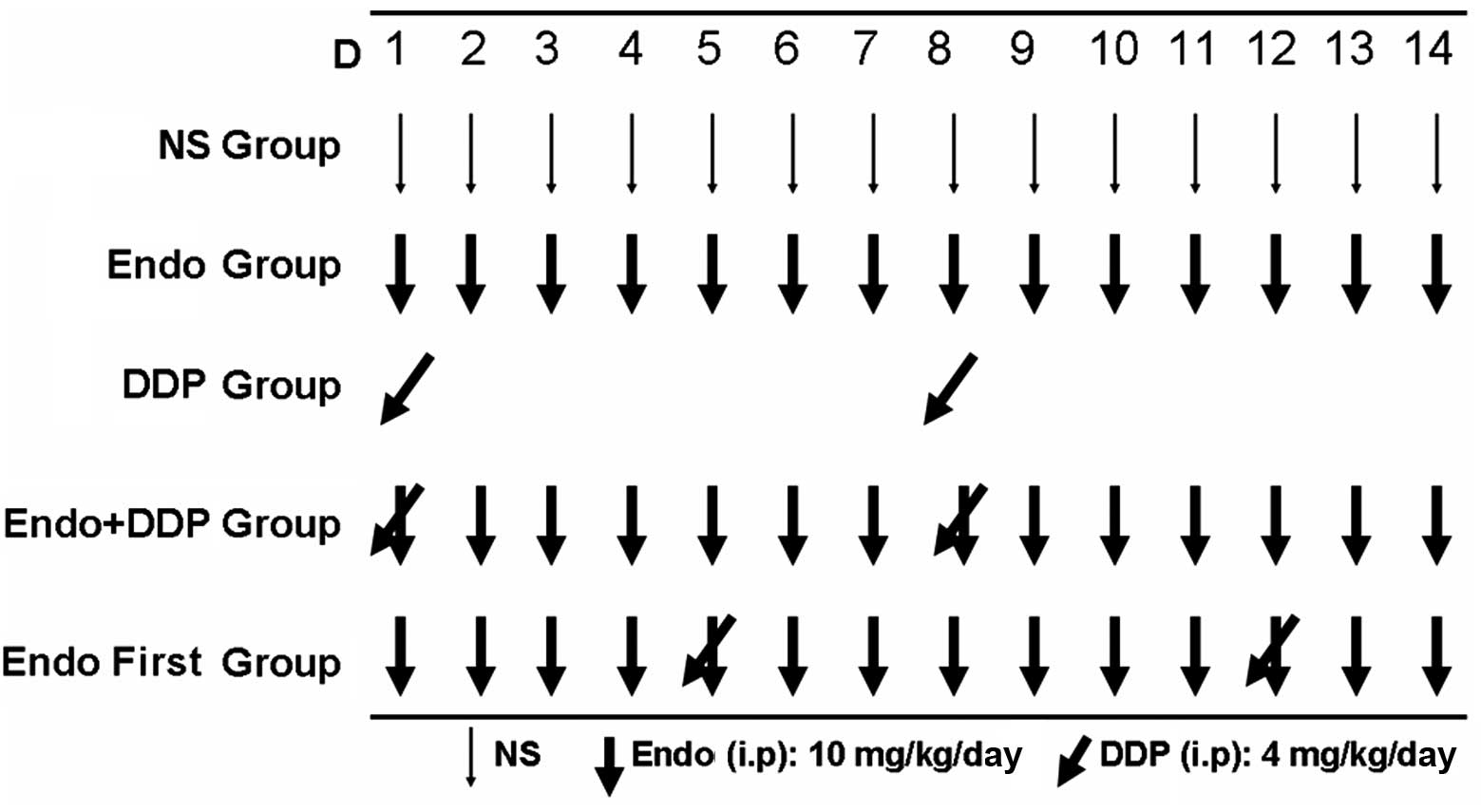
animal at a dose of 10 mg/kg/day. All drugs were administrated via
i.p. injection according to the regimen shown in Fig. 1. When the diameter of the tumors had
reached between 8 and 10 mm, the animals were randomized into five
groups (n=7 in each group) and treated for 14 consecutive days as
follows: i) NS group (negative control); ii) Endostar group (10
mg/kg/day); iii) cisplatin group (4 mg/kg/day administered on days
1 and 8); iv) Endostar + cisplatin group (drugs administered
simultaneously according to the same dose and regimen as the
Endostar and cisplatin groups; and v) Endostar first group
(Endostar administered on days 1 to 14 and cisplatin administered
on days 5 and 12). In order to assess the tumor growth inhibition
rate and analyze any histological changes, the animals were
sacrificed on day 15 by cervical dislocation. The tumors were then
excised and weighed. A section of each tumor was fixed in 10%
neutral formaldehyde solution in preparation for the histological
analysis, and another section was fixed with 70% ethanol for the
flow cytometry analysis. The tumor growth inhibition rate was
determined using the following formula: Inhibition rate (%) = (1 −
Wep/WNS) × 100, where Wep is the average tumor weight of the
experimental group, and WNS is the mean tumor weight of the control
group. The co-operation index (q) was calculated as follows: q = Ea
+ b/Ea + Eb − Ea × Eb, where Ea + b represents the inhibition rate
of the drugs combined, and Ea and Eb represent the inhibition rate
of the drugs used alone, respectively. In order to measure the
cisplatin serum and tissue concentrations, the animals were
randomized into three groups (n=20 in each group) as follows: i)
The cisplatin group; ii) the Endostar + cisplatin group; and iii)
the Endostar first group. Each group was treated using the same
doses and regimens as used in the aforementioned experiment. Blood
samples were collected by retro-orbital bleeding on days 1 (20 min
after the first injection of cisplatin), 3, 5 and 8 (20 min after
the second injection of cisplatin). The mice were then sacrificed
and the collected blood samples and excised tumors were stored in
preparation for the high-performance liquid chromatography (HPLC)
analysis. A section of tumor tissue was also fixed using 10%
neutral formaldehyde for use in the immunohistochemical
analysis.
Flow cytometry analysis
The tumor tissues were fixed with 70% cold ethanol
and prepared into a single cell suspension. Next, the suspension
was precipitated following centrifugation at 95 × g for 10 min. The
cells were then rinsed twice in PBS (pH 7.4) for 10 min each. Next,
the samples were stained with propidium iodide (Beckman Coulter
Inc., Brea, CA, USA) for the cell cycle analysis. The proliferation
index (PI) was calculated using the following equation: PI = 100 ×
(S + G2M)/(G0/G1 + S +
G2M).
Immunohistochemistry
The tumors were fixed in 10% neutral formaldehyde,
embedded in paraffin and then cut into 3-μm sections. Next, the
sections were stained with hematoxylin and eosin. The sections were
then labeled with a rabbit anti-mouse anti-cluster of
differentiation 31 (CD31) antibody (1:10 dilution; Bio-World,
Dublin, OH, USA) and visualized using a biotinylated goat
anti-rabbit anti-immunoglobulin G (IgG; 1:10 dilution) and
streptavidin peroxidase (SP)-conjugated antibody (1:10 dilution;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) to assess the
proliferation of microvessels. The sections were deparaffinized in
xylene and then rehydrated in a graded alcohol series as follows:
Xylene for 10 min (x2), 95% ethanol for 5 min (x2) and 100% ethanol
for 5 min (x2). Following high pressure saturated steaming (126°C,
1.6 bar, 23 psi), antigen retrieval was performed by autoclaving
the tumor sections in retrieval buffer (EDTA buffer, pH 6.0) in the
saturated steam for 10 min. The endogenous peroxidase activity was
blocked with 3% hydrogen peroxide in methanol for 10 min at room
temperature. Next, the sections were rinsed twice with PBS and then
incubated with the rabbit anti-mouse anti-CD31 (1:10 dilution)
antibody for 1 h at room temperature, followed by incubation with
the biotinylated goat anti-rabbit anti-IgG antibody [diluted
1:2,000 in BLOTTO (Thermo Fisher Scientific, Waltham, MA, USA]) at
room temperature for 1 h and staining with 3,3′-diaminobenzidine
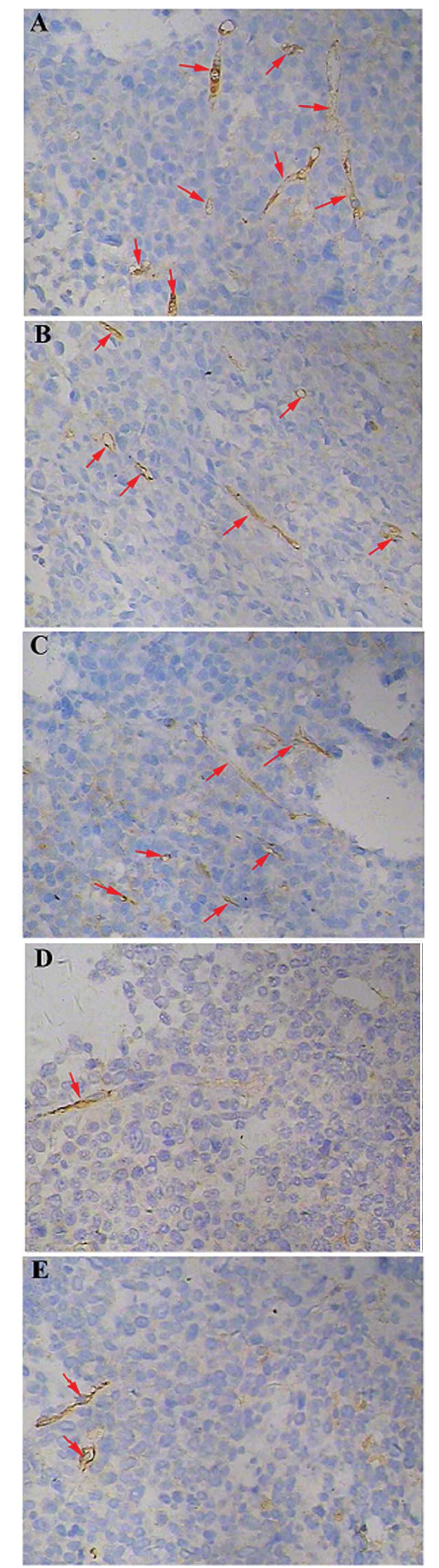
for brown color development. Quantification of the microvessel
density (MVD) was performed according to the method used by Weidner
et al (20). First, the
sections were screened at a low magnification (×100) in order to
identify the region of the tumor that exhibited the densest
vascularization, designated as a ‘hot spot’. Within the hot spot,
stained microvessels were counted in a single high-power field
(×400). The MVD was expressed as the number of microvessels counted
per field. Any CD31-stained endothelial cells, or endothelial cell
clusters that were clearly separated from adjacent microvessels,
tumor cells or connective tissue were considered to be a single
microvessel.
HPLC
In order to measure the cisplatin concentration
within the tumors, the tumor tissue was homogenized and centrifuged
at 16,060 × g for 10 min. The homogenate was then collected, and
500 μl methanol was added to the cell suspension. In order to
measure the plasma concentration of cisplatin, 500 μl methanol was
added to the blood plasma to precipitate the proteins. Next, the
suspension was centrifuged at 16,060 × g for 10 min, and 50 μl 5%
DDTC was added. The mixtures were then incubated at 37°C for 30
min. The protein precipitate was extracted using 500 μl chloroform,
vortexed for 2 min and then centrifuged at 16,060 × g for 15 min.
The chloroform phase was transferred to another vial and evaporated
under dry air. The precipitate was then re-dissolved in 100 μl
chloroform and vortexed for a further 30 sec. In total, 15 μl
precipitate was injected into the HPLC device. The HPLC device
(Ultimate 3000; Dionex, Sunnyville, CA, USA) was equipped with an
Ultimate 3000 array detector, which uses UV light at 254 nm at room
temperature and a narrow-bore column (Hypersil C18 column; 250×4.6
mm; 5-μm particle size). The mobile phase used methanol/water
(75/25, v/v) at a flow rate of 0.1 ml/min. A standard curve was
used for the quantification of cisplatin in the blood and tumor
tissue.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Comparisons between multiple groups were performed using
a one-way analysis of variance, followed by Dunnet’s test. The
statistical analyses of the results were performed using SPSS
software version 19.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of cisplatin and Endostar on LLC
tumors
In order to determine the optimal treatment regimen,
the present study investigated the effect of the combination of
Endostar and cisplatin on the growth of LLC tumors in C57/BL/6
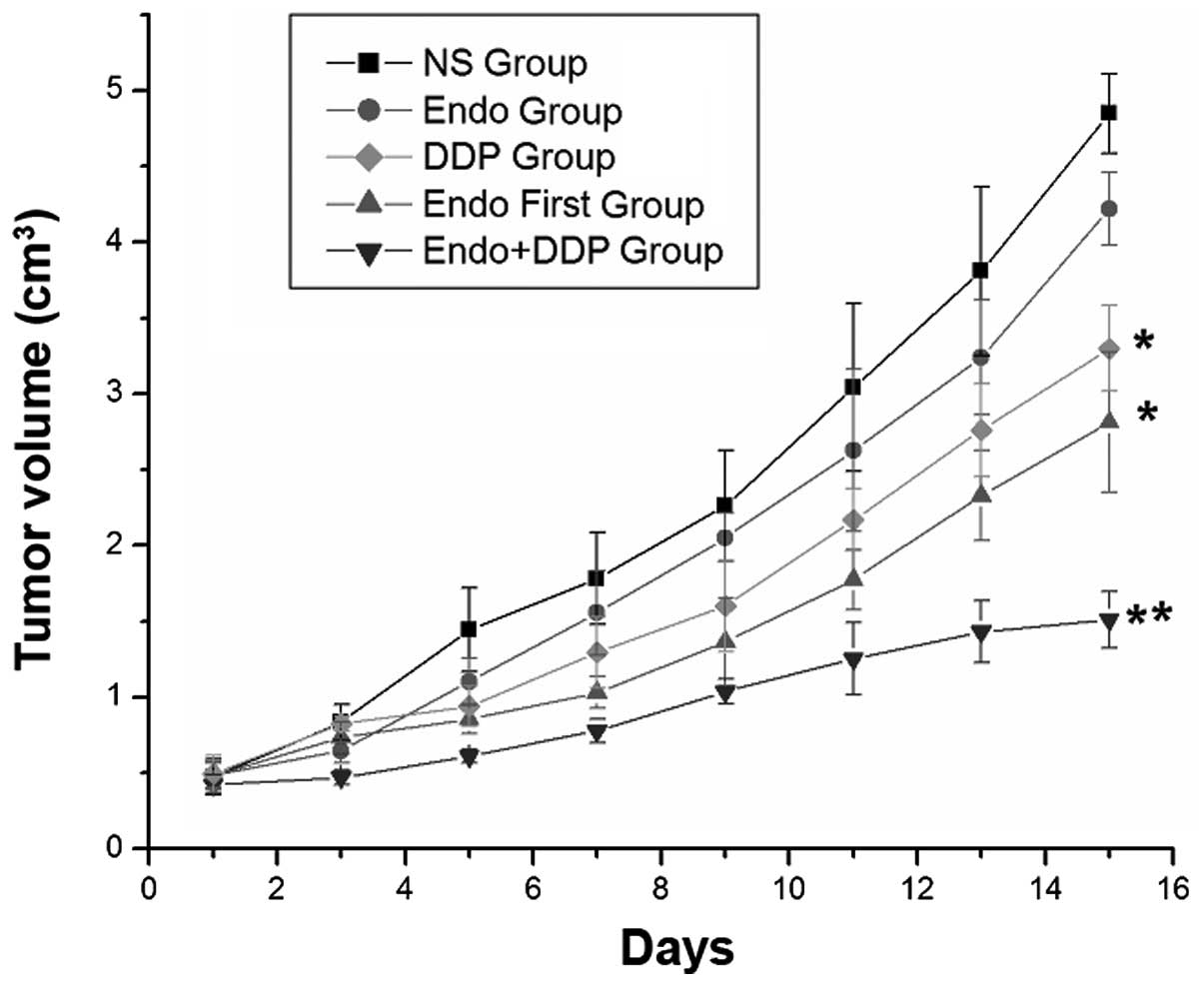
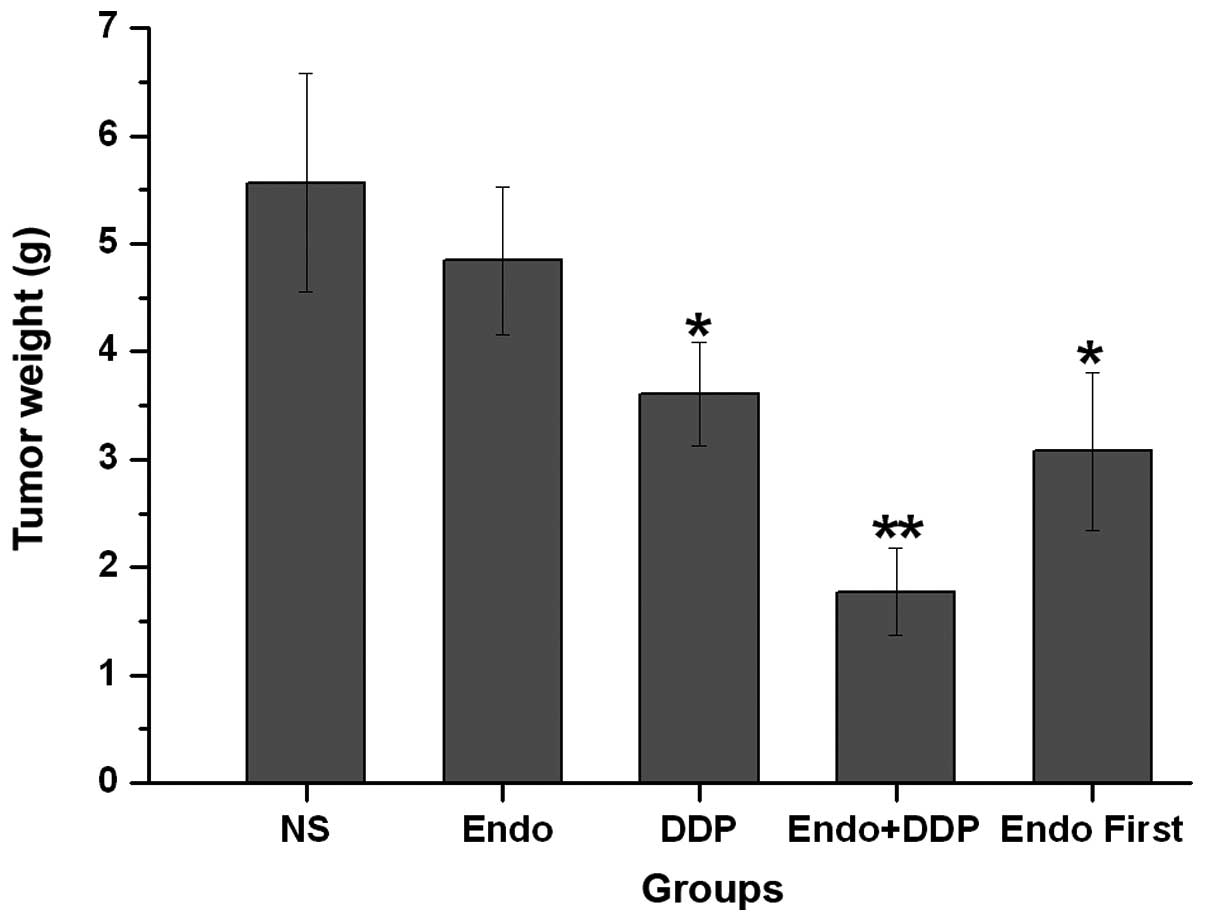
mice. Treatment with cisplatin demonstrated significant inhibition
of tumor growth compared with NS or Endostar alone. Compared with
all other groups, the cisplatin + Endostar group demonstrated the
most significant inhibition of tumor growth, as determined by tumor
volume and weight on day 14 (P<0.001). This comparison included
the Endostar first group, which also received a cisplatin and
Endostar combination treatment, but one in which the Endostar
treatment was started prior to the cisplatin treatment (Figs. 2 and 3). The tumor growth rate was significantly
lower in the cisplatin, cisplatin + Endostar and Endostar first
groups compared with the control group. The q index of the
cisplatin + Endostar group was 1.564, which indicated a synergetic
effect when the drugs were administered simultaneously. However,
the q value of the Endostar first group was 1.095, which suggested
that the effects of the drugs were additive when administered
successively.
Effects of cisplatin and Endostar on the
distribution patterns of the cell cycle
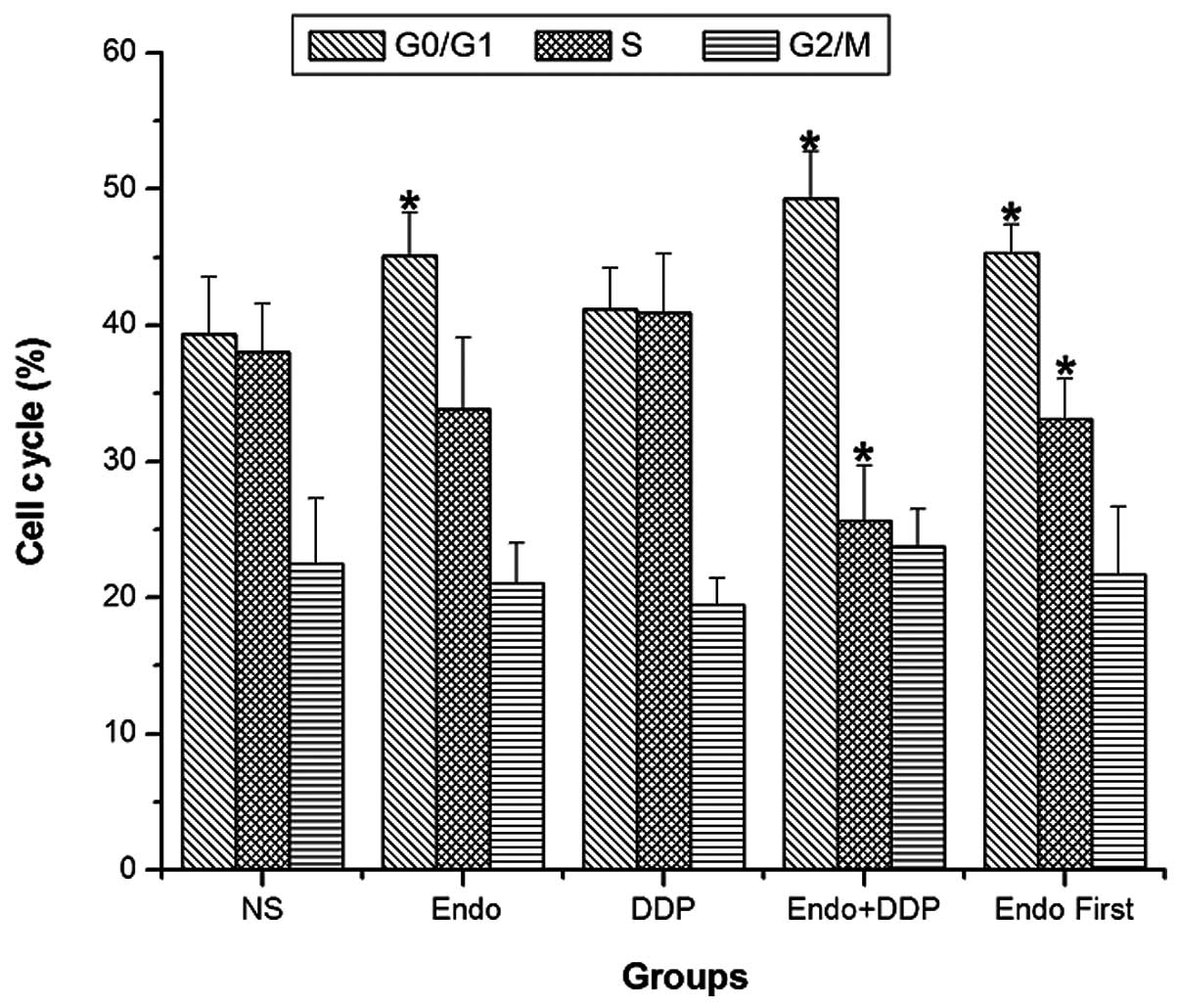
After 14 days of treatment, cell cycle analysis of
the tumor cells was performed using flow cytometry. The PIs were
calculated for each treatment group as described in the Materials
and methods section. The PI values of the NS, Endostar, cisplatin,
Endostar + cisplatin and Endostar first groups were 60.514±4.245,
55.600±4.494, 60.371±5.033, 49.386±2.149 and 54.386±2.812,
respectively. The proportion of tumor cells in the
G0/G1 phase was significantly higher in the
Endostar, cisplatin + Endostar and Endostar first groups compared
with the control group. These groups also demonstrated a decreased
PI compared with the control group. The difference in the
proliferation index was most significant when the two drugs were
administered simultaneously (P<0.05). The PI of the cisplatin +
Endostar group was significantly lower compared with that of the
Endostar first group (P<0.05) (Fig.
4).
Inhibition of tumor-induced
angiogenesis
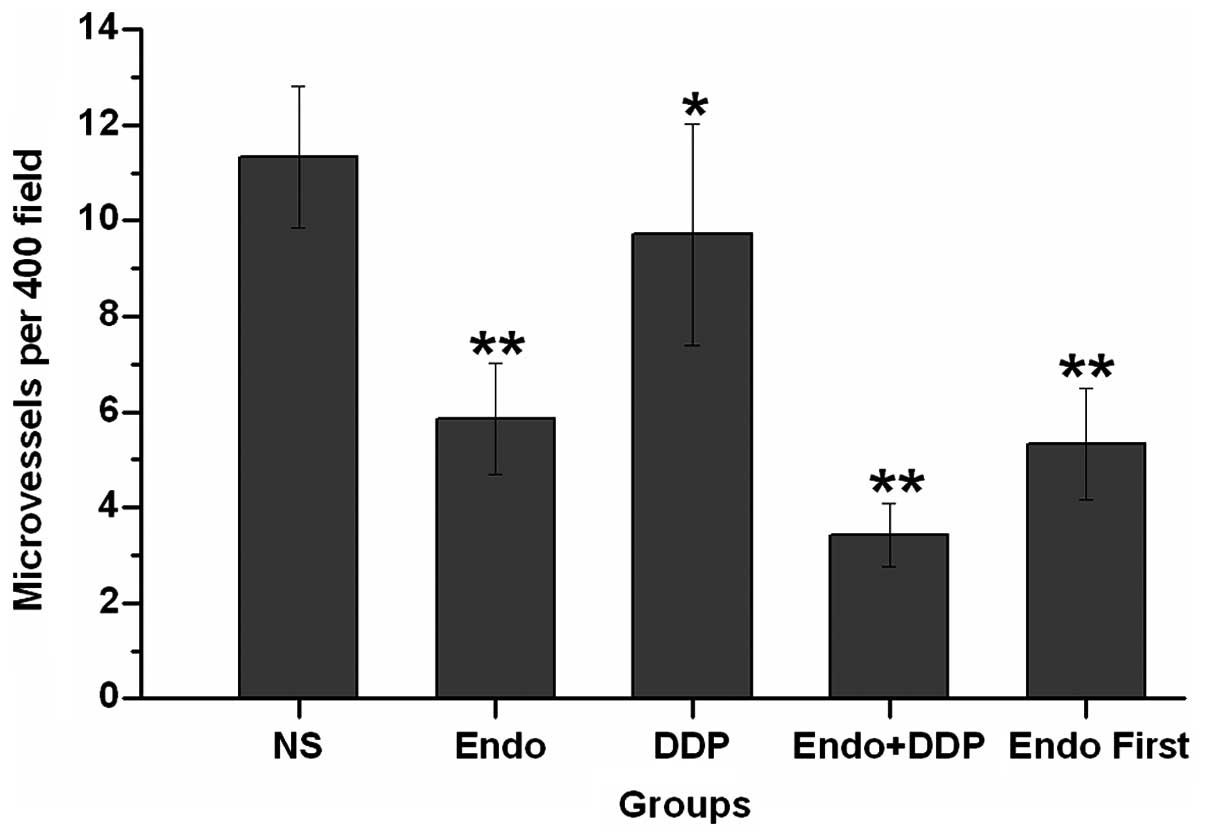
Angiogenesis within the tumor tissues was estimated
by the measurement of the MVD, which was performed by counting the
number of microvessels in a given area. CD31 was used as a marker
of microvessels within the tumor tissues. The tumor microvessel
count in the control group was higher than that in all other
groups. The difference in the MVD was particularly notable when
comparing the tumors from animals simultaneously treated with
cisplatin and Endostar with those in the control group (P<0.05).
Furthermore, the microvessel densities in the animals from the
simultaneously treated group were lower than those observed in the
Endostar first group (P=0.21) (Figs.
5 and 6).
Tumor tissue and blood concentrations of
cisplatin
The cisplatin concentrations in the blood and tumor
tissue were measured by HPLC at various time-points. The HPLC
analysis revealed a cisplatin peak in the blood at 10 min, which
was distinct from an endogenous impurity peak. The cisplatin
concentration in the blood was higher than that in the tumor tissue
in all groups (P<0.05). Overall, no statistically significant
differences were identified between the cisplatin concentrations of
the three groups on the first day. On day 3, the group that
received the two drugs simultaneously exhibited higher cisplatin
concentrations in the tumor tissue than in the blood (P<0.05).
This was not observed in the other drug-treated groups. Similar
results were observed on day 5 (P<0.05). On day 8, the cisplatin
concentrations in the blood and tumor tissues increased
significantly in all groups when compared with the earlier
time-points. There were no statistically significant differences
identified in the blood concentrations between the groups
(P>0.05). As on days 3 and 5, the concentration of cisplatin in
the tumor tissues from the mice simultaneously treated with
cisplatin and Endostar was higher than that in the blood. The
cisplatin concentrations were also higher in the blood than in the
tumor tissues in the Endostar first and cisplatin alone groups
(P>0.05). The cisplatin concentrations in the blood and tumor
tissue in the Endostar first and cisplatin groups were not
statistically different (Table I).
The MVD in the tumor tissues from the three drug-treated groups
demonstrated an association with the concentration of cisplatin.
Overall, there were no statistical differences identified in the
MVD between the three groups on the first and third days of
treatment. However, the MVD of the tumor tissue from the
simultaneously-treated group was lower than that of the
cisplatin-only group on day 5 (P>0.05). On day 8, the MVDs of
the combination drug groups were lower compared with the
cisplatin-only group (P>0.01) (Table II).
 | Table IMean concentration of cisplatin in the
tumor tissues and blood at various time-points following treatment
with different drug schedules, as determined by high-performance
liquid chromatography. |
Table I
Mean concentration of cisplatin in the
tumor tissues and blood at various time-points following treatment
with different drug schedules, as determined by high-performance
liquid chromatography.
| Concentration of DDP,
μg/ml |
|---|
|
|
|---|
| Day 1 | Day 3 | Day 5 | Day 8 |
|---|
|
|
|
|
|
|---|
| Groups | Tissue | Blood | Tissue | Blood | Tissue | Blood | Tissue | Blood |
|---|
| DDP | 1.073±0.116 | 2.077±0.274 | 1.027±0.024 | 2.019±0.319 | 0.845±0.211 | 0.964±0.189 | 1.680±0.323 | 2.174±0.214 |
| Endo+DDP | 1.173±0.119 | 2.274±0.255 | 2.666±0.255 | 2.219±0.355 | 1.357±0.153 | 0.736±0.175 | 3.794±0.210 | 2.932±0.341 |
| Endo first | 1.078±0.210 | 2.433±0.276 | 1.047±0.260 | 2.235±0.302 | 1.028±0.219 | 1.173±0.192 | 1.307±0.293 | 2.710±0.410 |
 | Table IIMean tumor MVD at various time-points
following treatment with different drug schedules. |
Table II
Mean tumor MVD at various time-points
following treatment with different drug schedules.
| MVD (×400) |
|---|
|
|
|---|
| Groups | Day 1 | Day 3 | Day 5 | Day 8 |
|---|
| DDP | 5.50±0.71 | 6.83±0.29 | 8.10±1.15 | 10.00±1.02 |
| Endo+DDP | 5.33±0.58 | 6.33±0.58 | 5.50±0.71 | 4.23±0.40 |
| Endo first | 4.67±0.58 | 6.43±0.51 | 6.00±1.00 | 5.57±0.58 |
Discussion
The growth of primary tumors and metastases depends
upon a network of blood vessels that receive nutrients and oxygen.
Preclinical and clinical studies have revealed that anti-angiogenic
agents are able to normalize the tumor vasculature (21–23).
The identification of the optimal ‘time window’ of treatment is a
key factor to ensure the normalization of blood vessels. An
excessive or prolonged treatment period with anti-angiogenic drugs
can damage and degrade normal blood vessels. Weichselbaum (11) estimated the duration of this optimal
time window of treatment to be between four and six days. A study
by Peng et al investigated whether Endostar could create a
‘vascular normalization window’ to alleviate hypoxia and enhance
the inhibitory effects of radiation therapy in human nasopharyngeal
carcinoma xenograft models (24).
The results of the study revealed that when Endostar was
administered for three or five days, it alleviated hypoxia and
significantly sensitized the tumor tissue to radiation. These
results suggested that the optimal time window for Endostar
treatment combined with radiation was between three and five days,
which was consistent with the results described by Weichselbaum
(11) Another study reported an
optimal treatment time of between four and six days for Endostar
combined with chemotherapy (25).
Therefore, in the present study the mice were first treated with
Endostar for four days prior to initiating cisplatin treatment in
the group that received Endostar and cisplatin in succession.
However, the successive treatment of cisplatin and Endostar was not
as effective as the simultaneously administered combination.
Although the sequential administration of cisplatin and Endostar
exhibited increased tumor inhibition compared with the tumors
treated with Endostar or cisplatin alone, the sequential treatment
was less effective than the simultaneous treatment and demonstrated
only additive effects of the drugs.
The results of the present study suggest that the
combination of cytotoxic chemotherapy agents and angiogenesis
inhibitors may produce a synergistic therapeutic effect in the
treatment of cancer. When combined with chemotherapy,
anti-angiogenic therapy could result in the normalization of the
vascular structure and in the inhibition of tumor growth. The
hydrostatic pressure of the tumor tissue could potentially be
lowered by the anti-angiogenic agents, which would allow the
chemotherapy drugs to easily enter the core of the tumor tissue.
Another proposed mechanism is that a reduction in the extent of
angiogenesis in solid tumors creates a hypoxic environment, which
causes the tumor cells to become more sensitive to the chemotherapy
treatment (26). In the present
study, the concentration of cisplatin in the blood and tumor
tissues was measured by HPLC, and the MVD was analyzed using
immunohistochemistry. The MVD is the most common indicator used to
measure the degree of angiogenesis, and is correlated with the
metastatic activity of tumors (27). The results of the present study
revealed that the combination of Endostar and cisplatin resulted in
a higher cisplatin concentration in the tumor tissue. The
synergistic administration of Endostar and cisplatin also resulted
in a reduced MVD. Cytotoxic drugs have been demonstrated to
effectively inhibit the formation of blood vessels over a large
dose range (28). The MVD of the
cisplatin-treated mice was significantly lower than that of the
NS-treated mice (P=0.048) in the present study. In theory, if
angiogenesis inhibitors are active along with chemotherapy drugs,
the two can produce a synergistic anti-angiogenic effect. However,
the curative effect observed in the Endostar first group was less
than that in the Endostar + cisplatin group. Therefore, it is
likely that treatment with Endostar resulted in partial
normalization of the tumor vasculature in the first four days of
treatment, and that the cisplatin administered thereafter may have
initiated degeneration of the vascular network due to its own
anti-angiogenic effects.
In the clinical setting, cell cycle analyses are
used to guide the treatment of malignant tumors. Studies have
revealed that Endostar acts at a slower rate on tumor tissues than
cytotoxic drugs, and is predominantly effective in the
G0/G1 phase of the cell cycle where it
induces cellular apoptosis (29,30).
The rate of apoptosis is usually increased when Endostar is
administered in combination with chemotherapy drugs (16,31).
As a non-specific cytotoxic drug, cisplatin has exhibited a minimal
effect upon the proportion of cells in a specific phase of the cell
cycle. However, when combined with Endostar in the present study,
cisplatin synergistically altered the cell cycle distribution of
the tumor cells. This drug combination increased the number of
cells undergoing G0/G1 cell cycle arrest,
decreased the number of cells undergoing G2/S arrest and
lowered the PI. When the two drugs were used simultaneously, the
synergistic effect was more notable than when the drugs were
administered sequentially.
The present study did not determine an optimal time
window of treatment for the co-administration of cisplatin with
Endostar. Instead, the results demonstrated that cisplatin and
Endostar may exhibit synergistic effects, which prevent the
proliferation of solid tumors by reducing the density of
microvessels and allowing greater penetration of cisplatin into the
tumor tissue. This may be due to the fact that traditional
cytotoxic drugs exhibit an anti-angiogenic effect at low doses.
Following treatment with Endostar for four days, a region of the
tumor vascular became normalized. The subsequent addition of
cisplatin may have lead to excessive degradation of the normalized
vasculature, which caused inefficient tumor penetration by
cisplatin. Further research to determine the mechanism of Endostar
and cisplatin synergy is required to clarify this point.
In conclusion, the results of the present study
demonstrate that the simultaneous treatment of solid tumors with
cisplatin and Endostar can effectively inhibit the growth of LLC
xenografts, improve cell cycle distribution, increase cisplatin
concentration in the tumor tissue and improve the vascular
structure of the tumor. Therefore, the simultaneous, rather than
the sequential administration of cisplatin and Endostar may be more
effective for the treatment of tumors.
Acknowledgements
The authors would like to thank the members of the
Department of Pathology and Nuclear Medicine, The Center Laboratory
of Lu Zhou Medical College, for providing assistance throughout the
duration of the study. This study was financially supported by the
Fundamental Research Project of the Affiliated Hospital of Luzhou
Medical College (grant no. 2011-45) and the Union Project of Luzhou
City and Luzhou Medical College (grant no. 2013LZLY-J40).
References
|
1
|
Laskin JJ and Sandler AB: First-line
treatment for advanced non-small-cell lung cancer. Oncology
(Williston Park). 19:1671–1676; discussion 1678–1680. 2005.
|
|
2
|
Le Chevalier T, Scagliotti G, Natale R, et
al: Efficacy of gemcitabine plus platinum chemotherapy compared
with other platinum containing regimens in advanced non-small-cell
lung cancer: a meta-analysis of survival outcomes. Lung Cancer.
47:69–80. 2005. View Article : Google Scholar
|
|
3
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Browder T, Butterfield CE, Kräling BM, et
al: Antiangiogenic scheduling of chemotherapy improves efficacy
against experimental drug-resistant cancer. Cancer Res.
60:1878–1886. 2000.PubMed/NCBI
|
|
5
|
Bello L, Carrabba G, Giussani C, et al:
Low-dose chemotherapy combined with an antiangiogenic drug reduces
human glioma growth in vivo. Cancer Res. 61:7501–7506.
2001.PubMed/NCBI
|
|
6
|
Ramalingam SS, Dahlberg SE, Langer CJ, et
al; Eastern Cooperative Oncology Group. Outcomes for elderly,
advanced-stage non small-cell lung cancer patients treated with
bevacizumab in combination with carboplatin and paclitaxel:
analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin
Oncol. 26:60–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murata R, Nishimura Y and Hiraoka M: An
antiangiogenic agent (TNP-470) inhibited reoxygenation during
fractionated radiotherapy of murine mammary carcinoma. Int J Radiat
Oncol Biol Phys. 37:1107–1113. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma J, Pulfer S, Li S, Chu J, Reed K and
Gallo JM: Pharmacodynamic-mediated reduction of temozolomide tumor
concentrations by the angiogenesis inhibitor TNP-470. Cancer Res.
61:5491–5498. 2001.PubMed/NCBI
|
|
9
|
Lin MI and Sessa WC: Antiangiogenic
therapy: creating a unique ‘window’ of opportunity. Cancer Cell.
6:529–531. 2004.PubMed/NCBI
|
|
10
|
Jain RK: Normalization of tumor
vasculature: an emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weichselbaum RR: How does antiangiogenic
therapy affect brain tumor response to radiation. Nat Clin Pract
Oncol. 2:232–233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou ZT, Zhou FX, Wei Q, Zou LY, Qin BF
and Peng XS: Phase II study of cisplatin/etoposide and endostar for
extensive-stage small-cell lung cancer. Cancer Chemother Pharmacol.
68:1027–1032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma X, Yao Y, Yuan D, Liu H, Wang S, Zhou C
and Song Y: Recombinant human endostatin endostar suppresses
angiogenesis and lymphangiogenesis of malignant pleural effusion in
mice. PLoS One. 7:e534492012. View Article : Google Scholar
|
|
14
|
Lignet F, Benzekry S, Wilson S, et al:
Theoretical investigation of the efficacy of antiangiogenic drugs
combined to chemotherapy in xenografted mice. J Theor Biol.
320:86–99. 2013. View Article : Google Scholar
|
|
15
|
Mabuchi S, Terai Y, Morishige K, et al:
Maintenance treatment with bevacizumab prolongs survival in an in
vivo ovarian cancer model. Clin Cancer Res. 14:7781–7789. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan J, Wu CW, Liu ZJ, Wei XY and Li K:
Observation of the antitumor effect of endostar combined with
docetaxel under different administration sequences. Zhonghua Zhong
Liu Za Zhi. 32:580–585. 2010.(In Chinese). PubMed/NCBI
|
|
17
|
Peng XC, Qiu M, Wei M, et al: Different
combination schedules of gemcitabine with endostar affect antitumor
efficacy. Cancer Chemother Pharmacol. 69:239–246. 2012. View Article : Google Scholar
|
|
18
|
Ling Y, Yang Y, Lu N, et al: Endostar, a
novel recombinant human endostatin, exerts antiangiogenic effect
via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of
endothelial cells. Biochem Biophys Res Commun. 361:79–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Sun Y, Liu Y, et al: Results of
randomized, multicenter, double-blind phase III trial of
rh-endostatin (YH-16) in treatment of advanced non-small cell lung
cancer patients. Zhongguo Fei Ai Za Zhi. 8:283–290. 2005.(In
Chinese). PubMed/NCBI
|
|
20
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. New Engl J Med. 324:1–8. 1991. View Article : Google Scholar
|
|
21
|
Winkler F, Kozin SV, Tong RT, et al:
Kinetics of vascular normalization by VEGFR2 blockade governs brain
tumor response to radiation: role of oxygenation, angiopoietin-1,
and matrix metalloproteinases. Cancer Cell. 6:553–563.
2004.PubMed/NCBI
|
|
22
|
Dings RP, Loren M, Heun H, McNiel E,
Griffioen AW, Mayo KH and Griffin RJ: Scheduling of radiation with
angiogenesis inhibitors anginex and Avastin improves therapeutic
outcome via vessel normalization. Clin Cancer Res. 13:3395–3402.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohta M, Kawabata T, Yamamoto M, et al:
TSU68, an antiangiogenic receptor tyrosine kinase inhibitor,
induces tumor vascular normalization in a human cancer xenograft
nude mouse model. Surg Today. 39:1046–1053. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng F, Xu Z, Wang J, et al: Recombinant
human endostatin normalizes tumor vasculature and enhances
radiation response in xenografted human nasopharyngeal carcinoma
models. PLoS One. 7:e346462012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xin G, Du J, Zhu L, Yu YH, Li Y and Liu
PS: Differential anti-tumor effects for various regimens of
endostar plus cisplatin in ovarian cancer. Zhonghua Yi Xue Za Zhi.
91:3367–3370. 2011.(In Chinese).
|
|
26
|
Tong RT, Boucher Y, Kozin SV, Winkler F,
Hicklin DJ and Jain RK: Vascular normalization by vascular
endothelial growth factor receptor 2 blockade induces a pressure
gradient across the vasculature and improves drug penetration in
tumors. Cancer Res. 64:3731–3736. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guşet G, Costi S, Lazăr E, Dema A,
Cornianu M, Vernic C and Păiuşan L: Expression of vascular
endothelial growth factor (VEGF) and assessment of microvascular
density with CD34 as prognostic markers for endometrial carcinoma.
Rom J Morphol Embryol. 51:677–682. 2010.
|
|
28
|
Bocci G, Nicoluaou KC and Kerbel RS:
Prutracted low-dose effects on human endothelial cell proliferation
and survival in vitro reveal a selective antiangiogenic window for
various chemotherapeutic drugs. Cancer Res. 62:6938–6943.
2002.PubMed/NCBI
|
|
29
|
Wu SM, Zhao YA, Yang L, Wang L, et al:
Effects of recombinant human angiostatin on vascular endothelial
cell. Chin J Aesthetic Med. 17:685–688. 2008.
|
|
30
|
You ZY, Zhao Y, Liu F, Zhang YD and Wang
JJ: The radiosensitization effects of Endostar on human lung
squamous cancer cells H-520. Cancer Cell Int. 10:172010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu WJ, Huang C, Wang J, et al: Comparison
of the effects of recombinant human endostatin and docetaxel on
human umbilical vein endothelial cells in different growth states.
Chin Med J (Engl). 124:2883–2889. 2011.
|