Introduction
Osteosarcoma is a common type of bone cancer that
predominantly occurs during adolescence, and is clinically
characterized by local infiltration and early, distant,
hematogenous metastasis (1–3). Although the prognosis of osteosarcoma
patients has significantly improved since the implementation of a
comprehensive treatment strategy using surgery and adjuvant
chemotherapy (4–7), the prognosis remains poor for a number
of patients due to the development of acquired resistance.
Intrinsic resistance is a phenomenon that occurs prior to
chemotherapy and is not associated with the administration of
chemotherapeutic agents, whereas acquired resistance is induced by
chemotherapeutic agents (8,9). In clinical practice, adjuvant
chemotherapy for osteosarcoma generally includes the administration
of methotrexate (MTX), cisplatin [cis-diamminedichloroplatinum II
[DDP)], ifosfamide (IFO), doxorubicin, pirarubicin or a combination
of these agents. High-dose MTX is considered to be the key agent in
determining the chemotherapeutic outcome of osteosarcoma patients
(10); however, multidrug
resistance often develops in the late stage of treatment. In the
present study, shock treatment and a gradually increasing dose of
MTX were used to investigate acquired resistance in the
MTX-resistant osteosarcoma cell line, Saos-2/MTX4.4. Our previous
study demonstrated that MTX induces resistance in MTX-resistant
cell lines (11). This resistance
may be associated with the downregulation of folate carrier gene
expression levels, as well as the reduced cellular influx of MTX,
reducing the ability of MTX to competitively inhibit tumor cell DNA
synthesis (12–15). In the present study, the multidrug
resistance of the Saos-2/MTX4.4 cell line was investigated.
Furthermore, the association between multidrug resistance and
multidrug resistance gene 1 (MDR1) overexpression was determined in
the presence of substrates of P-glycoprotein (Pgp), a product of
the MDR1 gene. The aim of the present study was to investigate the
efficacy of verapamil (VER) in preventing Pgp pumping MTX out of
the cell, in order to overcome MTX resistance in osteosarcoma
treatment.
Materials and methods
Cell culture and MTT assay
Human osteosarcoma Saos-2 cells (Shanghai Institutes
for Biological Sciences, Chinese Academy of Sciences, Shanghai,
China) were exposed to shock therapy using gradually increasing
concentrations of MTX (1.1, 2.2 and 4.4 μM; Shanghai New Hualian
Pharmaceutical Co., Ltd., Shanghai, China) to create an
MTX-resistant cell line. The Saos-2 parent cells were cultured in
RPMI-1640 medium (Invitrogen Life Technologies, Carlsbad, CA, USA)
with 10% fetal bovine serum (HyClone, Logan, UT, USA) ) and 0.2%
penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA), at
37°C and 5% CO2. When the cells had reached 60–70%
confluence in the logarithmic growth phase, 4.4 μM MTX was added.
After 24 h, the cells were washed twice with 1X phosphate-buffered
saline (PBS) at 37°C, and an agent-free medium was added to the
cells. Once the cells had grown to 60–70% confluence in a
logarithmic phase, the process was repeated three times at each MTX
concentration. Following seven months of resistance-induction, the
MTX-resistant cell line, termed Saos-2/MTX4.4, was established and
compared with the primary Saos-2 cells.
An MTT assay (Sigma-Aldrich) was used to determine
the sensitivity of the Saos-2 and Saos-2/MTX4.4 cells to MTX, IFO
(Jiangsu Henrui Medicine Co., Ltd., Jiangsu, China), DDP (Haosen
Pharmaceutical Co., Ltd., Jiangsu, China), Adriamycin (ADM;
Zhejiang Haizheng Pharmaceuticals, Taizhou, China), epirubicin
(EPI; Pfizer, Inc., Madison, NJ, USA) and theprubicin (THP;
Shenzhen Wanle Pharmaceutical Co., Ltd., Shenzhen, China).
Logarithmic growth phase cells were suspended in RPMI-1640 medium,
seeded in a 96-well plate at 200 μl/well (1×104 cells)
and cultured for 24 h. Based on the peak plasma concentrations of
the clinical agents, seven concentration gradients (1000, 100, 10,
1, 0.1, 0.01 and 0.001 plasma protein concentration) of MTX, DDP,
IFO, EPI and THP were added. Untreated cells were used as the
control. After 24 h of incubation, 20 μl of 5% MTT was added to
each well, and after another 4 h of incubation, the culture
supernatant was removed, 150 μl dimethylsulfoxide was added to each
well and the cell mixture was agitated for 10 min at room
temperature. A Wellscan MK3 microplate reader (Labsystems Dragon,
Helsinki, Finland) measured the light absorption of the cells at a
wavelength of 490 nm. The inhibitory rate of the cells was
determined using the following formula: Inhibitory rate = (1 - mean
absorption value of resistance group/mean absorption value of
control group) × 100. The median inhibitory concentration
(IC50) was identified and the resistance index (RI) of
the two cell lines was determined using the following formula: RI =
IC50(Saos-2/MTX4.4)/IC50(Saos-2).
Rhodamine 123 (R123) efflux
The Pgp activity of the cell lines was determined by
evaluating R123 efflux. R123 is a Pgp-specific fluorescent
substrate and its intracellular accumulation is associated with the
efficiency of Pgp activity. The changing rate of R123 efflux was
determined using the following equation: Rate of efflux =
(fluorescence intensity of experimental group - fluorescence
intensity of control group)/fluorescence intensity of control group
× 100. Equal quantities of Saos-2 and Saos-2/MTX4.4 cells were
seeded on a 96-well plate (density, ~1×105 cells/well)
and cultured in RPMI-1640 medium for 24 h. The cells were divided
into four groups: Primary cells (Saos-2; group A), MTX-resistant
cells (Saos-2/MTX4.4; group B), primary cells treated with VER
(group C) and MTX-resistant cells treated with VER (group D). VER
was added to the wells containing group C and D cells at a final
concentration of 4.5 μM 1 h prior to the addition of R123. R123 was
added into all wells at a final concentration of 0.15 μg/ml. Each
group was replicated in six wells and incubated in an atmosphere of
5% CO2 and 100% humidity. Three wells from groups C and
D were harvested 30 min after incubation with VER and R123, and the
other three wells were harvested 60 min after incubation.
Subsequent to being harvested, these cells were rinsed twice with
PBS, resuspended in RPMI-1640 medium and observed using
fluorescence microscopy (IX17; Olympus Corporation, Tokyo, Japan).
Following a further 4 h of incubation, all the cells were
harvested, washed twice with D-Hank’s solution (Shanghai Jiaotong
University Laboratory, Shanghai, China) and digested at 4°C
overnight in a 50% ethanol solution containing 0.3 mol/l HCl. The
fluorescence intensity was measured using a multifunctional
microplate reader (Wellscan MK3) at an excitation wavelength of 485
nm and an emission wavelength of 538 nm.
Semiquantitative analysis of MDR1 mRNA
expression
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen Life Technologies) and was subjected to reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
using a PrimeScript RT reagent kit (Takara Bio, Inc., Shiga,
Japan), according to the manufacturer’s instructions. Gene-specific
primer pairs were designed based on the human MDR1 and β-actin
complementary DNA sequences (GenBank Assembly ID: GCA_000161855.1)
from Genbank (www.ncbi.nlm.nih.gov/genbank) and were synthesized by
Sangon Biotech Co., Ltd (Shanghai, China). The primer sequences
were as follows: Forward, 5′-GTT GCT GCT TAC ATT CAG GTT TC-3′ and
5′-ACC AGC CTA TCT CCT GTC GC-3′ for MDR1; and forward, 5′-AAC TGG
GAC GAC ATG GAG AAA ATC-3′ and reverse, 5′-AGG AAG GAA GGC TGG AAG
AGT GC-3′ for β-actin. PCR reactions were performed using GeneAmp
PCR System 9700 (Applied Biosystems Life Technologies, Foster City,
CA, USA), with pre-denaturation at 95°C for 5 min, followed by 35
cycles at 94°C for 30 sec, 64°C for 30 sec and 72°C for 60 sec.
Statistical analysis
All experiments were conducted in triplicate. Data
were analyzed using SPSS software (version 13.0; SPSS, Inc.,
Chicago, IL, USA), and are expressed as the mean ± standard
deviation. Comparisons between two groups were performed using
one-way analysis of variance, and P<0.05 was considered to
indicate a statistically significant difference.
Results
MTT assay
The IC50 of the Saos-2/MTX4.4 cells to
MTX was 12.73 times higher than that of the Saos-2 cells, giving an
RI of 12.73, indicating that Saos-2/MTX4.4 cells exhibit moderate
resistance to MTX. The Saos-2/MTX4.4 cells demonstrated lower
resistance to IFO, ADM, EPI and THP; however, the Saos-2/MTX4.4
cells exhibited no evident cross-resistance to DDP (Table I).
 | Table IResistance of Saos-2 and Saos-2/MTX4.4
cells to various chemotherapeutic agents [IC50 (μmol/l)
mean ± standard deviation]. |
Table I
Resistance of Saos-2 and Saos-2/MTX4.4
cells to various chemotherapeutic agents [IC50 (μmol/l)
mean ± standard deviation].
| Chemotherapeutic
agent | Saos-2 | Saos-2/MTX4.4 | RI |
|---|
| MTX | 25.78±0.29 | 328.24±0.29 | 12.73 |
| IFO | 583.23±0.14 | 2346.52±0.37 | 4.02 |
| DDP | 127.67±0.21 | 156.56±0.87 | 1.23 |
| ADM | 8.80±0.45 | 34.67±0.23 | 3.94 |
| EPI | 10.68±0.35 | 43.67±0.46 | 4.09 |
| THP | 12.29±0.72 | 38.72±0.25 | 3.15 |
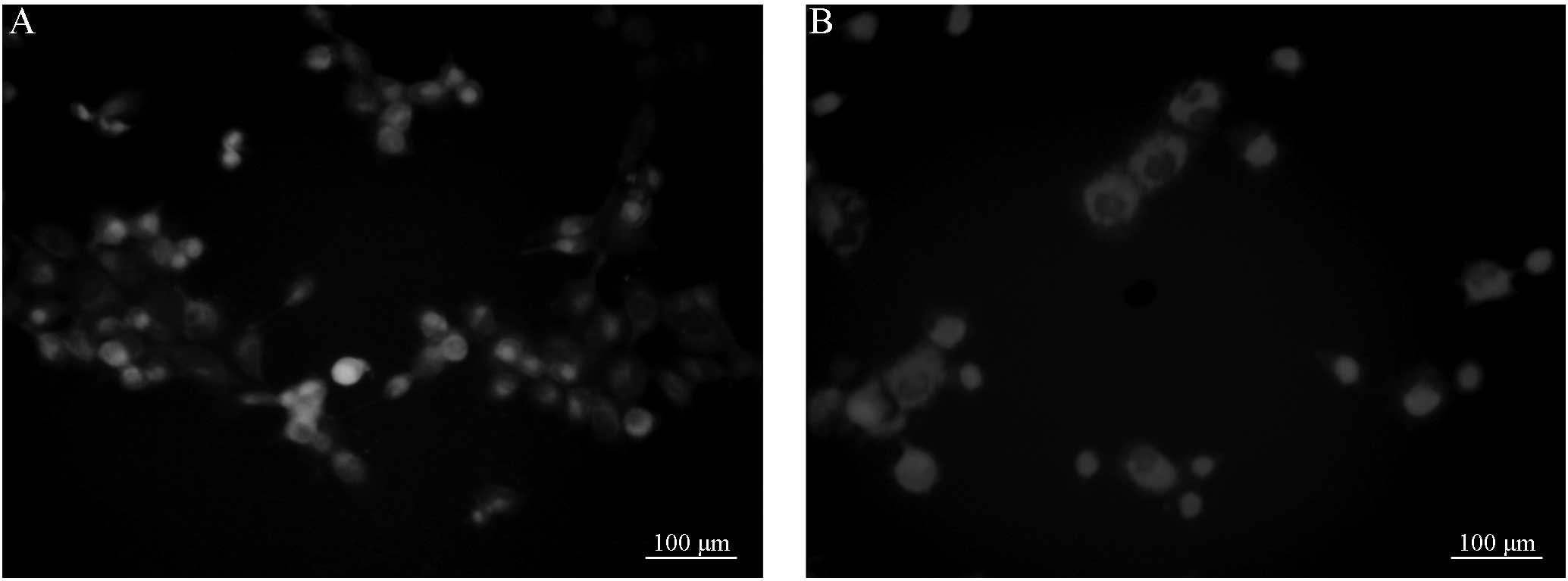
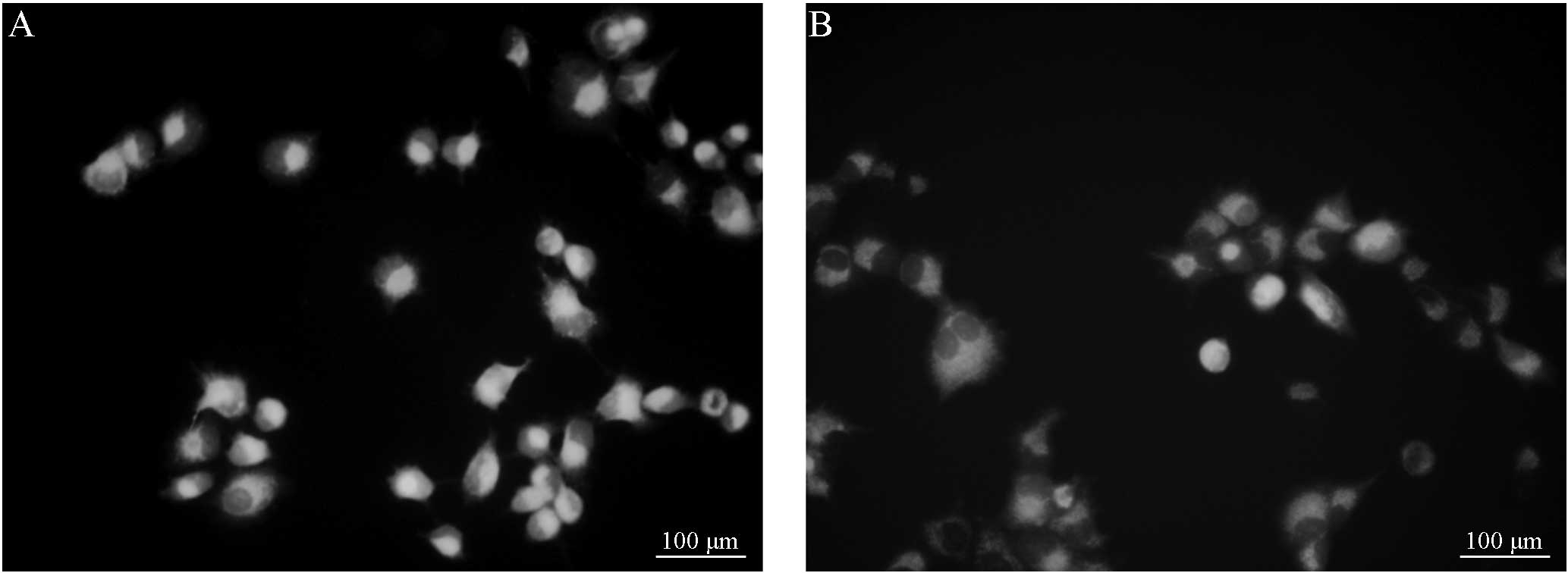
R123 efflux in the Saos-2/MTX4.4 and
Saos-2 cells
Fluorescence microscopy demonstrated that 30 or 60
min post-incubation, the cells in groups A and B exhibited full
shapes or multiple pseudopodia. In addition, 30 or 60 min
post-incubation, the cells in groups C and D also exhibited full
shapes or multiple pseudopodia. However, no significant differences
in cell morphology were observed between the groups. Furthermore,
the intracellular fluorescence intensity of the cells was apparent,
and no significant differences were identified between groups A and
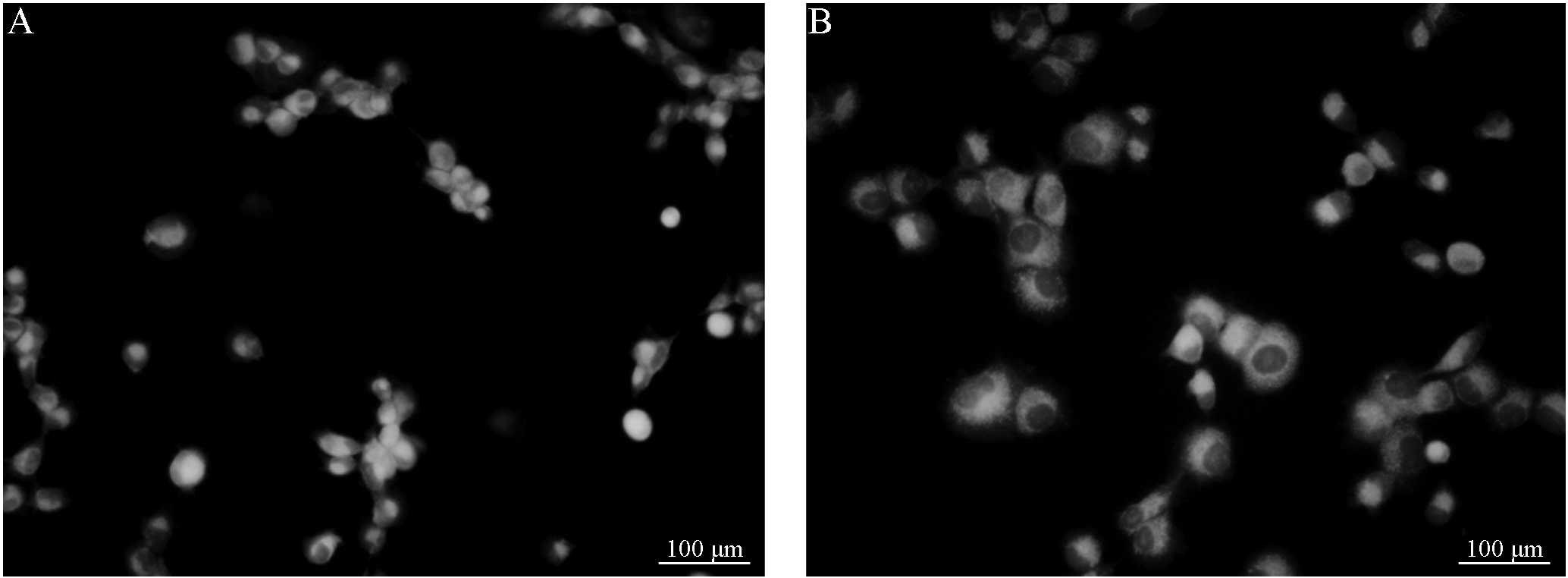
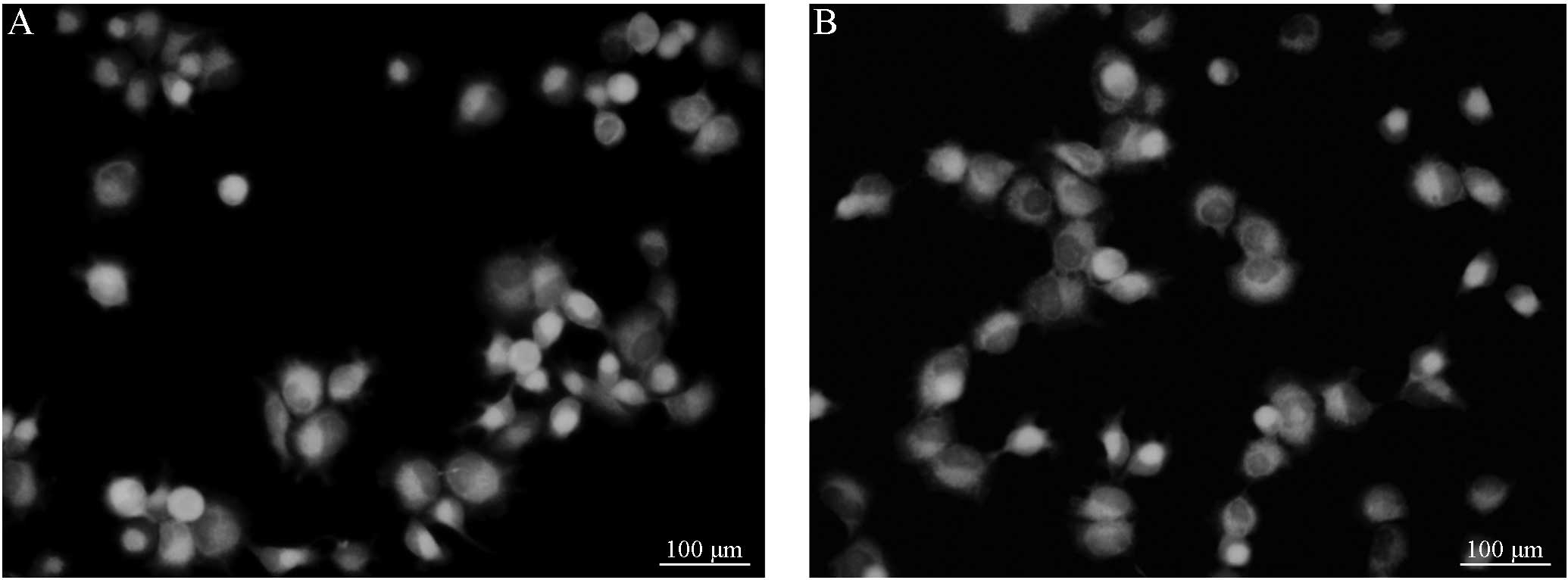
B (Figs. 1 and 2). Following treatment with VER, the
fluorescence intensity of the group C cells demonstrated no
significant difference from that of the group D cells (Figs. 3 and 4). However, the cells in groups C and D
demonstrated a marked difference in fluorescence intensity compared
with cells from groups A and group B, respectively. Considering
that the cells were digested and detected using a multifunctional
microplate reader, the results are consistent with the observations
obtained from fluorescence microscopy. No significant difference
was identified between the R123 fluorescence intensity of the
primary and resistant cells. However, significant differences were
observed prior to and following the addition of VER in the primary
and resistant cell groups (P<0.05; Table II).
 | Table IIRhodamine 123 fluorescence intensity
in four groups at various time-points [(arb. unit) mean ± standard
deviation]. |
Table II
Rhodamine 123 fluorescence intensity
in four groups at various time-points [(arb. unit) mean ± standard
deviation].
| Incubation period,
min |
|---|
|
|
|---|
| Group | 30 | 60 |
|---|
| A | 4.35±0.20 | 3.25±0.12 |
| B | 4.89±0.32 | 3.20±0.21 |
| C | 6.58±0.45 | 5.20±0.23 |
| D | 5.93±0.23 | 5.12±0.14a |
MDR1 mRNA expression in Saos-2/MTX4.4
cells
The MDR1 mRNA expression level is expressed as the
ratio of the optical density of MDR1 RT-qPCR products to the
optical density of the β-actin RT-qPCR products. The optical
density of the β-actin band in each lane was set to one and the
relative MDR1 mRNA expression was calculated using gel-imaging
system analysis software (Quantity One v4.6; Bio-Rad Laboratories,
Hercules, CA, USA). The MDR1 mRNA relative expression levels in the
Saos-2/MTX4.4 and Saos-2 cells were 0.4350±0.0354 and
0.3886±0.0456, respectively. A t-test identified no significant
difference between the MDR1 mRNA expression levels in the Saos-2
and Saos-2/MTX4.4 cells.
Discussion
The concept of the multidrug resistance of tumors
was initially proposed in 1970 (16) and stated that tumor cells exhibit a
cross-resistance to a variety of chemotherapeutic agents with
different structures and functions. Various basic and clinical
studies have indicated that, in the majority of cancers, multidrug
resistance is associated with the expression of the MDR1 gene. The
protein product of MDR1 is P-170, a membrane glycoprotein that
functions as an energy-dependent drug pump. P-170 actively exports
various anticancer drugs and hydrophobic compounds to reduce the
intracellular drug concentration, ultimately resulting in drug
resistance (17–24). Numerous studies (17–24)
have indicated that doxorubicin-resistant cell lines generally
exhibit increased MDR1 gene expression. In the present study, the
induced MTX-resistant cell line also showed multidrug resistance.
Schwartz et al (19) used an
immunohistochemical assay to investigate Pgp expression in biopsy
specimens from 685 osteosarcoma patients prior to treatment with
chemotherapy, and identified that Pgp expression was not correlated
with patient prognosis. However, Baldini et al (25) supported the finding that the Pgp
expression level predicts the clinical effects of doxorubicin-based
chemotherapy. Thus, Pgp cannot be used as a single predictor of
doxorubicin-resistance in the treatment of osteosarcoma. The
results of the present study are consistent with these findings.
Although the MTX-resistant cell line exhibited multidrug
resistance, no significant difference was identified in the
expression of the MDR1 gene between the primary and MTX-resistant
cells. Furthermore, similar results were identified for R123
efflux; no significant difference was identified in the rate of
R123 efflux between the primary and MTX-resistant cells. Thus, MDR1
gene expression may not be the primary cause of multidrug
resistance and the efflux of therapeutic agents via the Pgp pathway
may not be the primary route of multidrug resistance.
Previously, VER has been reported to reverse
multidrug resistance (26,27). Consistent with this, the present
study demonstrated that VER significantly increased the efflux of
R123, indicating that VER increases the quantity of soluble
intercellular agents by inhibiting the function of Pgp, thus
increasing the efficiency of chemotherapeutic agents. However,
patients are unable to tolerate treatment with VER due to its toxic
cardiovascular side-effects, thus limiting the application of VER
in clinical practice (28–31). In conclusion, further investigation
into the underlying mechanism of VER activity may provide
alternative means for the development of novel therapeutics.
References
|
1
|
Bielack SS, Kempf-Bielack B, Delling G, et
al: Prognostic factors in high-grade osteosarcoma of the
extremities or trunk: an analysis of 1,702 patients treated on
neoadjuvant cooperative osteosarcoma study group protocols. J Clin
Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bacci G, Longhi A, Versari M, Mercuri M,
Briccoli A and Picci P: Prognostic factors for osteosarcoma of the
extremity treated with neoadjuvant chemotherapy: 15-year experience
in 789 patients treated at a single institution. Cancer.
106:1154–1161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
4
|
Ferrari S, Smeland S, Mercuri M, et al:
Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose
methotrexate, cisplatin, and doxorubicin for patients with
localized osteosarcoma of the extremity: a joint study by the
Italian and Scandinavian Sarcoma Groups. J Clin Oncol.
23:8845–8852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lewis IJ, Nooij MA and Whelan J:
Improvement in histologic response but not survival in osteosarcoma
patients treated with intensified chemotherapy: a randomized phase
III trial of the European Osteosarcoma Intergroup. J Natl Cancer
Inst. 99:112–128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meyers PA, Schwartz CL, Krailo MD, et al;
Children’s Oncology Group. Osteosarcoma: the addition of muramyl
tripeptide to chemotherapy improves overall survival - a report
from the Children’s Oncology Group. J Clin Oncol. 26:633–638. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anninga JK, Gelderblom H, Fiocco M, et al:
Chemotherapeutic adjuvant treatment for osteosarcoma: where do we
stand? Eur J Canc. 47:2431–2445. 2011. View Article : Google Scholar
|
|
8
|
Le Deley MC, Guinebretière JM, Gentet JC,
et al; Société Française d’Oncologie Péediatrique (SFOP). SFOP
OS94: a randomised trial comparing preoperative high-dose
methotrexate plus doxorubicin to high-dose methotrexate plus
etoposide and ifosfamide in osteosarcoma patients. Eur J Cancer.
43:752–761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patiño-Garcia A, Zalacaín M, Marrodán L,
San-Julián M and Sierrasesúmaga L: Methotrexate in pediatric
osteosarcoma: response and toxicity in relation to genetic
polymorphisms and dihydrofolate reductase and reduced folate
carrier 1 expression. J Pediatr. 154:688–693. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Delepine N, Delepine G, Jasmin C, Desbois
JC, Cornille H and Mathé G: Importance of age and methotrexate
dosage: prognosis in children and young adults with high-grade
osteosarcomas. Biomed Pharmacother. 42:257–262. 1988.PubMed/NCBI
|
|
11
|
Wang JJ and Li GJ: Relationship between
RFC gene expression and intracellular drug concentration in
methotrexate-resistant osteosarcoma cells. Genet Mol Res.
13:5313–5321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laverdière C, Chiasson S, Costea I,
Moghrabi A and Krajinovic M: Polymorphism G80A in the reduced
folate carrier gene and its relationship to methotrexate plasma
levels and outcome of childhood acute lymphoblastic leukemia.
Blood. 100:3832–3834. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hattinger CM, Reverter-Branchat G,
Remondini D, et al: Genomic imbalances associated with methotrexate
resistance in human osteosarcoma cell lines detected by comparative
genomic hybridization-based techniques. Eur J Cell Biol.
82:483–493. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaufman Y, Drori S, Cole PD, et al:
Reduced folate carrier mutations are not the mechanism underlying
methotrexate resistance in childhood acute lymphoblastic leukemia.
Cancer. 100:773–782. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Serra M, Reverter-Branchat G, Maurici D,
et al: Analysis of dihydrofolate reductase and reduced folate
carrier gene status in relation to methotrexate resistance in
osteosarcoma cells. Ann Oncol. 15:151–160. 2004. View Article : Google Scholar
|
|
16
|
Biedler JL and Riehm H: Cellular
resistance to actinomycin D in Chinese hamster cells in vitro:
Cross-resistance, radioautographic and cytogenetic studies. Cancer
Res. 30:1174–1184. 1970.PubMed/NCBI
|
|
17
|
Stein U, Walther W and Wunderlich V: Point
mutations in the mdrl1 promoter of human osteosarcomas are
associated with in vitro responsiveness to multidrug resistance
relevant drugs. Eur J Cancer. 30A:1541–1545. 1994. View Article : Google Scholar
|
|
18
|
Fan K, Fan D, Cheng LF and Li C:
Expression of multidrug resistance-related markers in gastric
cancer. Anticancer Res. 20:4809–4814. 2000.
|
|
19
|
Schwartz CL, Gorlick R, Teot L, et al;
Children’s Oncology Group. Multiple drug resistance in osteogenic
sarcoma: INT0133 from the Children’s Oncology Group. J Clin Oncol.
25:2057–2062. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Benderra Z, Faussat AM, Sayada L, et al:
Breast cancer resistance protein and P-glycoprotein in 149 adult
acute myeloid leukemias. Clin Cancer Res. 10:7896–7902. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim DH, Lee NY, Sung WJ, et al: Multidrug
resistance as a potential prognostic indicator in acute myeloid
leukemia with normal karyotypes. Acta Haematol. 114:78–83. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marsh S, Paul J, King CR, Gifford G,
McLeod HL and Brown R: Pharmacogenetic assessment of toxicity and
outcome after platinum plus taxane chemotherapy in ovarian cancer:
the Scottish Randomised Trial in Ovarian Cancer. J Clin Oncol.
25:4528–4535. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Müller PJ, Dally H, Klappenecker CN, et
al: Polymorphisms in ABCG2, ABCC3 and CNT1 genes and their possible
impact on chemotherapy outcome of lung cancer patients. Int J
Cancer. 124:1669–1674. 2009. View Article : Google Scholar
|
|
24
|
Sun N, Sun X, Chen B, et al: MRP2 and
GSTP1 polymorphisms and chemotherapy response in advanced non-small
cell lung cancer. Cancer Chemother Pharmacol. 65:437–446. 2010.
View Article : Google Scholar
|
|
25
|
Baldini N, Scotlandi K, Serra M, et al:
P-glycoprotein expression in osteosarcoma: a basis for risk-adapted
adjuvant chemotherapy. J Orthop Res. 17:629–632. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith MA, Merry S, Smith JG and Kaye SB:
Clinically relevant concentrations of verapamil do not enhance the
sensitivity of human bone marrow CFU-GM to adriamycin and VP16. Br
J Cancer. 57:576–578. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shudo N, Mizoguchi T, Kiyosue T, Arita M,
Yoshimura A, Seto K, Sakoda R and Akiyama S: Two pyridine analogues
with more effective ability to reverse multidrug resistance and
with lower calcium channel blocking activity than their
dihydropyridine counterparts. Cancer Res. 50:3055–3061.
1990.PubMed/NCBI
|
|
28
|
Mahmood M, Mustafa T, Xu Y, Nima Z,
Kannarpady G, Bourdo S, Casciano D and Biris AS: Calcium-channel
blocking and nanoparticles-based drug delivery for treatment of
drug-resistant human cancers. Ther Deliv. 5:763–780. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Othman RT, Kimishi I, Bradshaw TD, Storer
LC, Korshunov A, Pfister SM, Grundy RG, Kerr ID and Coyle B:
Overcoming multiple drug resistance mechanisms in medulloblastoma.
Acta Neuropathol Commun. 2:572014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zu Y, Yang Z, Tang S, Han Y and Ma J:
Effects of P-glycoprotein and its inhibitors on apoptosis in K562
cells. Molecules. 19:13061–13075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim SS, Seong S and Kim SY: Synergistic
effect of ginsenoside Rg3 with verapamil on the modulation of
multidrug resistance in human acute myeloid leukemia cells. Oncol
Lett. 7:1265–1269. 2014.PubMed/NCBI
|