1. Introduction
Colorectal cancer (CRC) is currently the third most
common malignancy worldwide (1).
The development of CRC is a stepwise progression from benign polyps
to invasive adenocarcinomas and distant metastases. Although
advancements have been made with regard to the available treatment
options, improvements in the survival rates of patients with CRC
have been restricted due to the lack of early detection and optimal
prognostic predictions, which require the exploration of
corresponding biomarkers and an understanding of the molecular
mechanisms of CRC.
Over the last 10 years, data from a number of
high-throughput genomic platforms has indicated that the evolution
of the developmental processes regulating complex organisms may be
attributed to not only the protein-coding regions of the genome,
but the non-coding regions as well (2). Non-coding RNAs (ncRNAs), which are
transcribed from non-coding regions, lack an open reading frame and
therefore have no apparent protein-coding capacity (3). Regulatory ncRNAs are classified
empirically as small (18–200 nt) or long ncRNAs (lncRNAs; between
200 nt and >100 kb) based on the size of the functional RNA
molecule (4).
In contrast to small ncRNAs, such as microRNAs
(miRs), which have been extensively studied for their biological
roles in cancer processes (5),
lncRNAs are relatively less well described. However, the inherent
biology of lncRNAs, often referred to as the dark matter of the
genome, is gradually being elucidated (3). Previous studies have revealed that a
large number of lncRNAs play significant roles in regulating
cellular development and differentiation, processes that are
frequently deregulated in cancer (6).
The present mini-review introduces all the
CRC-associated lncRNAs known to date and concentrates on the
potential utility of lncRNAs as diagnostic and prognostic tools in
CRC. The aim of this review is to improve the understanding of the
role of lncRNAs in CRC, which could lead to novel prevention
strategies and early detection.
2. Classical lncRNAs associated with
CRC
The H19-lncRNA is a paternally imprinted (maternally
expressed) oncofetal gene that is abundantly expressed in a number
of types of cancer. H19-derived miR-675 promotes human CRC cell
growth and malignant transformation by targeting the tumor
suppressor retinoblastoma protein (RB) (7). However, one study found that the
depletion of H19 resulted in an increased polyp count in a mouse
model of CRC (8). The cellular
environment of the tumor type may determine this dual role as an
oncogene and tumor suppressor. Although the hypermethylation of a
differentially-methylated region (DMR) upstream of the H19 gene may
result in activation of the normally silent maternal allele of the
insulin-like growth factor-II gene (IGF2) (9), hypomethylation of the H19 DMR and a
DMR upstream of IGF2, is observed in the CRC and normal mucosa of a
single patient (10). This finding
suggests that the favored IGF2-H19 enhancer competition model for
IGF2 imprinting is not applicable in CRC. High H19 expression has
been observed in liver metastases (LMs) derived from primary CRC
alone (11). Ohana et al
(12) and Sorin et al
(13) developed vectors carrying
the diphtheria toxin A (DTA) chain gene driven by H19 regulatory
sequences and administered these plasmids intra-arterially in the
CC531 rat colorectal LM (CLM) model. The results showed that the
DTA-H19 plasmid significantly delayed tumor growth, indicating that
lncRNA could be a therapeutic target in CRC.
A previous study reported that HOX transcript
antisense RNA (HOTAIR) reprograms chromatin organization and
promotes breast cancer metastasis (14). Pádua Alves et al (15) observed that the colon cancer stem
cell subpopulation (CD133+/CD44+) exhibits
higher HOTAIR levels compared with the non-stem cell subpopulation.
This result indicates that the role of the HOTAIR-lncRNA in CRC
progression is associated with the acquisition of stemness. HOTAIR
expression levels were also found to be higher in CRC tissues
compared with the corresponding normal tissues, and high HOTAIR
expression correlated with the presence of LMs (16). Furthermore, patients with high
expression levels of HOTAIR exhibited a relatively poor prognosis.
Using cDNA array data, a gene set enrichment analysis of a subset
of 32 CRC specimens revealed a close correlation between HOTAIR
expression and members of polycomb repressive complex 2 (PRC2)
(16). This finding suggested that
HOTAIR expression is associated with a genome-wide reprogramming of
PRC2 function in CRC.
Metastasis-associated lung adenocarcinoma transcript
1 (MALAT1)-lncRNA was first identified in metastatic non-small cell
lung carcinoma (17), and its high
expression was subsequently found to be associated with CRC
metastasis (18). MALAT1 mutations
occur in CRC cell lines or tissues, with one MALAT1 fragment
(spanning nucleotides 6918–8441) being an important biological
motif in metastasis (19).
Furthermore, a study indicated that the downregulation of MALAT1 by
resveratrol could decrease the nuclear localization of β-catenin
and attenuate Wnt/β-catenin signaling, thereby inhibiting CRC
invasion and metastasis (20). This
study indicated the potential use of MALAT1 as a marker for early
metastasis in patients with CRC.
Highly upregulated in liver cancer (HULC)-lncRNA was
first identified in a screen for deregulated genes in a
hepatocellular carcinoma-specific gene library (21). The upregulation of HULC was also
detected in LMs from CRC, although no HULC was detected in the
primary CRC samples (22).
Furthermore, a lack of HCLC gene expression was found in
vitro in the CRC HT-29 cell line, as well as in tumors induced
by the direct administration of HT-29 cells into the liver of
athymic mice over the course of two weeks (22). This finding may indicate that the
liver microenvironment is responsible for increased HULC expression
in CLM. Furthermore, in a pilot experiment, HULC was detected in
peripheral blood cells obtained from healthy volunteers by reverse
transcription-quantitative polymerase chain reaction (PCR)
(23), indicating that lncRNA may
be useful as a circulating biomarker in CRC.
As an imprinted gene, maternally-expressed gene 3
(MEG3) (24) is expressed in normal
intestinal mucosa, whereas its expression is lost in CRC cells
(including HT29 and HCT116 cells) (25). In culture and colony formation, the
re-activation of MEG3 expression inhibits tumor cell proliferation.
This inhibition of growth is partly a consequence of the apoptosis
induced by MEG3. MEG3 induces p53 protein accumulation and
stimulates transcription from a p53-dependent promoter (26). The aforementioned data suggests that
MEG3-lncRNA functions as a tumor suppressor in CRC.
3. CRC-specific associated lncRNAs
CCAT1 (CRC-associated transcript 1) was identified
in a study by Nissan et al (27), which reported its high expression in
CRC, but not in normal tissues. Furthermore, this lncRNA was also
found to be significantly upregulated in metastatic tissue.
Analysis of RNA obtained from five patients with CRC metastases to
either the liver or the peritoneal cavity revealed the >100-fold
upregulation of CCAT1 compared with normal colon tissue, with four
samples recorded with >450-fold upregulation. Significantly,
CCAT1 overexpression was also reported in 40.0% of peripheral blood
samples from CRC patients, but not from the samples of healthy
controls (27). Thus, it has been
suggested that testing for CCAT1 expression can detect small
numbers of CRC cells. Additionally, a CCAT1-specific peptide
nucleic acid-based molecular beacon was used to detect CRC
(28), and the results showed CCAT1
expression in all (4/4) subjects with pre-cancerous adenomas and in
all (8/8) patients with invasive CRC, which further proved that
CCAT1 is a potential biomarker for early CRC diagnosis.
Recently, CCAT2, which encompasses the rs6983267
single nucleotide polymorphism (SNP), was also reported to be
highly overexpressed in microsatellite-stable CRC, and to promote
tumor growth and metastasis (29).
This lncRNA may regulate Myc and Wnt in CRC pathogenesis and
provide an alternative explanation for SNP-conferred cancer risk
(29).
Colorectal neoplasia differentially expressed
(CRNDE) is an lncRNA gene that is overexpressed in >90% of CRC
tissues relative to paired healthy tissues (30). CRNDE expresses multiple splice
variants, and the expression levels of CRNDE-h demonstrate a
sensitivity of 95% and specificity of 96% for colon adenoma versus
normal tissue. The study by Graham et al (30) showed that the level of
CRNDE-h-lncRNA in plasma was positive for 87% of patients with CRC,
but only 7% of healthy individuals. Thereafter, CRNDE was proven to
be upregulated in gliomas, and its different splice forms are known
to provide specific functional scaffolds for regulatory complexes
(31). Recently, another study
showed that CRNDE is regulated by insulin/IGFs and promotes the
metabolic changes by which cancer cells evoke the Warburg effect
(32), indicating CRNDE
upregulation in CRC. Therefore, CRNDE may serve as an ideal
biomarker for the early diagnosis of CRC.
In one recent study, low LOC285194-lncRNA expression
was shown to be correlated with more distant metastasis in patients
with CRC (P=0.046) (33), which
indicated that this lncRNA plays a role as a tumor suppressor in
the CLM process.
The overexpressed in colon carcinoma-1 (OCC-1) gene
is transcribed as two regulatory lncRNAs. Elevated OCC-1-lncRNA
levels were present in three out of eight CRC samples compared with
the normal mucosa of the same patient, even though the same
characteristics were present in each tumor (34). This data indicates that OCC-1-lncRNA
overexpression may be a hallmark of CRC.
4. Other lncRNAs associated with CRC
Ultraconserved region transcripts (T-UCR) are a
novel class of lncRNAs transcribed from UCRs (35,36).
UCRs are frequently located in the intra- and intergenic regions
(37), and aberrant T-UCR
expression is also involved in CRC progression. The expression of
uc.73A(P) was found to be significantly upregulated in CRC
(37), as a decrease in its
overexpression induced apoptosis and anti-proliferative effects in
CRC cells abnormally expressing this T-UCR. However, the expression
of uc.388 was reported to be significantly decreased in CRC samples
and was associated with the distant metastasis of CRC and other
effects (38). By screening genomic
DNA for sequence variations in UCR loci in patients with CRC,
Wojcik et al (39) found UCR
mutations in CRC and created a catalog of DNA sequence variations
in UCRs in human cancers. These findings indicated that further
investigation of the genetic variations in ncRNAs may aid in the
identification of additional biomarkers for CLM.
As one type of lncRNA, pseudogenes have long been
labeled as ‘noise DNA’, inactive copies of genes that arise during
genome evolution (40). However,
recent results showed that pseudogene transcripts are often
deregulated between cancer and normal tissue (41), indicating their involvement in tumor
progression. The POU5F1 transcript, also known as octamer binding
transcription factor 4, is believed to be one of the key regulators
of cellular pluripotency (42). The
POU5F1 pseudogene, POU5F1P1, is not only overexpressed in prostate
cancer (43), but is also strongly
associated with an increased risk of CRC. Furthermore, a
genome-wide association study showed that the rs6983267 SNP in the
POU5F1P1 region was significantly associated with decreased
survival time in patients with stage III CRC (44). The tumor suppressor phosphatase and
tensin homolog (PTEN) is significantly correlated with CRC
(45), and its pseudogene, PTENP1
(also known as PTENpg1), can parallel PTEN and play a
growth-suppressive role in CRC cells, although the PTENP1 locus is
selectively lost in CRC (46,47).
Myosin light chain kinase pseudogene 1 (MYLKP1) is partially
duplicated from the original MYLK gene, which encodes non-muscle
and smooth muscle myosin light chain kinase (smMLCK) isoforms
(48). MYLKP1 overexpression can
inhibit the expression of smMLCK in CRC cells by decreasing RNA
stability, resulting in the increased proliferation of cells;
accordingly, smMLCK is markedly decreased in CRC tissues compared
with normal colon tissues (49).
These studies suggest a novel biological role for pseudogene
expression in CRC.
Plasmacytoma variant translocation 1 (PVT1), a
>300-kb locus found downstream of c-Myc on chromosome 8q24
(50), produces a wide range of
spliced lncRNAs. Compared with healthy tissues, PVT1-lncRNA is
overexpressed in breast and ovarian cancer (51), indicating that PVT1 may be an
oncogene. PVT1 small interfering RNA-transfected CRC cells exhibit
a significant loss in their abilities of proliferation and
invasion. Additionally, multivariate analysis found that the level
of PVT1 expression was an independent risk factor for overall
survival in patients with CRC (52). Unexpectedly, transcription of the
PVT1 locus can be induced by p53 and consequently upregulates
miR-1204, which inhibits HCT116 cell proliferation (53). Therefore, the precise interplay
between miRs and other ncRNAs of the PVT1 locus within the context
of the p53 pathway requires further exploration.
Loss of imprinting (LOI) of H19 genes in CRC is
associated with CRC progression (10,54),
and another lncRNA, long QT intronic transcript 1 (LIT1) (55), also called Kcnq1ot1 (56), also frequently exhibits LOI in CRC.
Additionally, Nakano et al (57) further found that the LOI of LIT1 via
epigenetic disruption plays an important role in CRC.
Recently, Zhai et al (58) identified that long intergenic ncRNA
(lincRNA)-p21 expression is significantly higher in patients with
stage III tumors compared with those with stage I tumors, and that
elevated lincRNA-p21 levels are significantly associated with a
higher degree of vascular invasion. However, another study
(59) showed decreases in
lincRNA-p21 in CRC tissues. Furthermore, the enforced expression of
lincRNA-p21 enhances sensitivity to radiotherapy in CRC by
promoting apoptosis, the reason for which may be suppression of the
β-catenin signaling pathway. The Warburg effect is known to play an
important role in CRC progression, yet it remains unclear whether
lncRNAs are involved in this phenomenon. Yang et al
(60) first showed that lincRNA-p21
is a hypoxia-responsive lncRNA that is essential for
hypoxia-enhanced glycolysis, which suggested the involvement of
lincRNA-p21 in the regulation of the Warburg effect.
ncRNA expressed in aggressive neuroblastoma
(ncRAN)-lncRNA was first recognized in a chromosomal gain, behaving
as an oncogene in aggressive neuroblastomas (61). Thereafter, Qi et al (62) proved that the in vitro
migration and invasion of CRC cells is mediated by ncRAN,
suggesting that this lncRNA may be a novel prognostic indicator and
biomarker for the early diagnosis of CRC. Recently, a novel lncRNA,
prostate cancer associated-transcript 1 (PCAT1), was identified to
be highly overexpressed in aggressive prostate cancer (63). In addition, PCAT1 was also revealed
to be overexpressed in CRC tissues compared with matched normal
tissues, and there was a significant association between higher
PCAT1 expression and distant metastasis and poor overall survival
(64). Notably, a search of the
University of California Santa Cruz Human Genome Browser database
(Feb 2009 assembly) (65) revealed
that one adjoining neighbor of PCAT1 on chromosome 8q24, named
prostate cancer-associated non-coding RNA 1 (PRNCR1; also known as
PCAT8) (66) is also correlated
with CRC. Li et al (67)
conducted a case-control study and genotyped five tag SNPs in
PRNCR1 in 313 patients with CRC and 595 control subjects using a
PCR-restriction fragment length polymorphism assay. The results
showed that rs13252298 and rs1456315 were associated with
significantly decreased risks of CRC, indicating that SNPs in
PRNCR1-lncRNA may contribute to the susceptibility to CRC. Low
expression in tumor (LET)-lncRNA was reported to be downregulated
in CRC as a regulator of hypoxia signaling, offering novel avenues
for therapeutic intervention against the progression of cancer
(68).
5. Challenges and future perspectives
From a clinical perspective, a large number of
dysregulated ncRNAs represent a number of useful biomarkers for the
diagnosis and prognosis of patients with CRC (Table I). However, a series of challenges
remain to be addressed. Firstly, there is a considerable lack of
understanding with regard to the regulation of lncRNA expression
and the detailed mechanisms (Fig.
1) involved in lncRNA-mediated effects on tumor progression.
Therefore, it may be an over-simplification to classify all
tumor-associated lncRNAs into CRC activator or suppressor genes.
Secondly, the problems associated with the variability in lncRNA
detection in CRC samples should be overcome using standardized
techniques for tissue isolation and preparation, platforms and
software analysis to avoid selection bias. Thirdly, a number of
prognostic or predictive biomarkers for CRC that are based on in
vitro observation fail when they are translated into clinical
management. To succeed in the future, further validation of these
potential lncRNA biomarkers in additional cohorts (particularly in
different ethnic groups) or prospective randomized trials is
required to aid in the measurement of the true effect of these
lncRNAs and their possible roles in CRC treatment. Recent studies
have also suggested the possibility of a widespread interaction
network involving competing endogenous RNAs, whereby lncRNAs
modulate regulatory RNAs by binding to and titrating them away from
their mRNA-binding sites (69,70).
In addition to providing the possibility of an additional level of
post-transcriptional regulation, such a network also necessitates
the reassessment of the existing regulatory pathways involved in
CRC progression and metastasis.
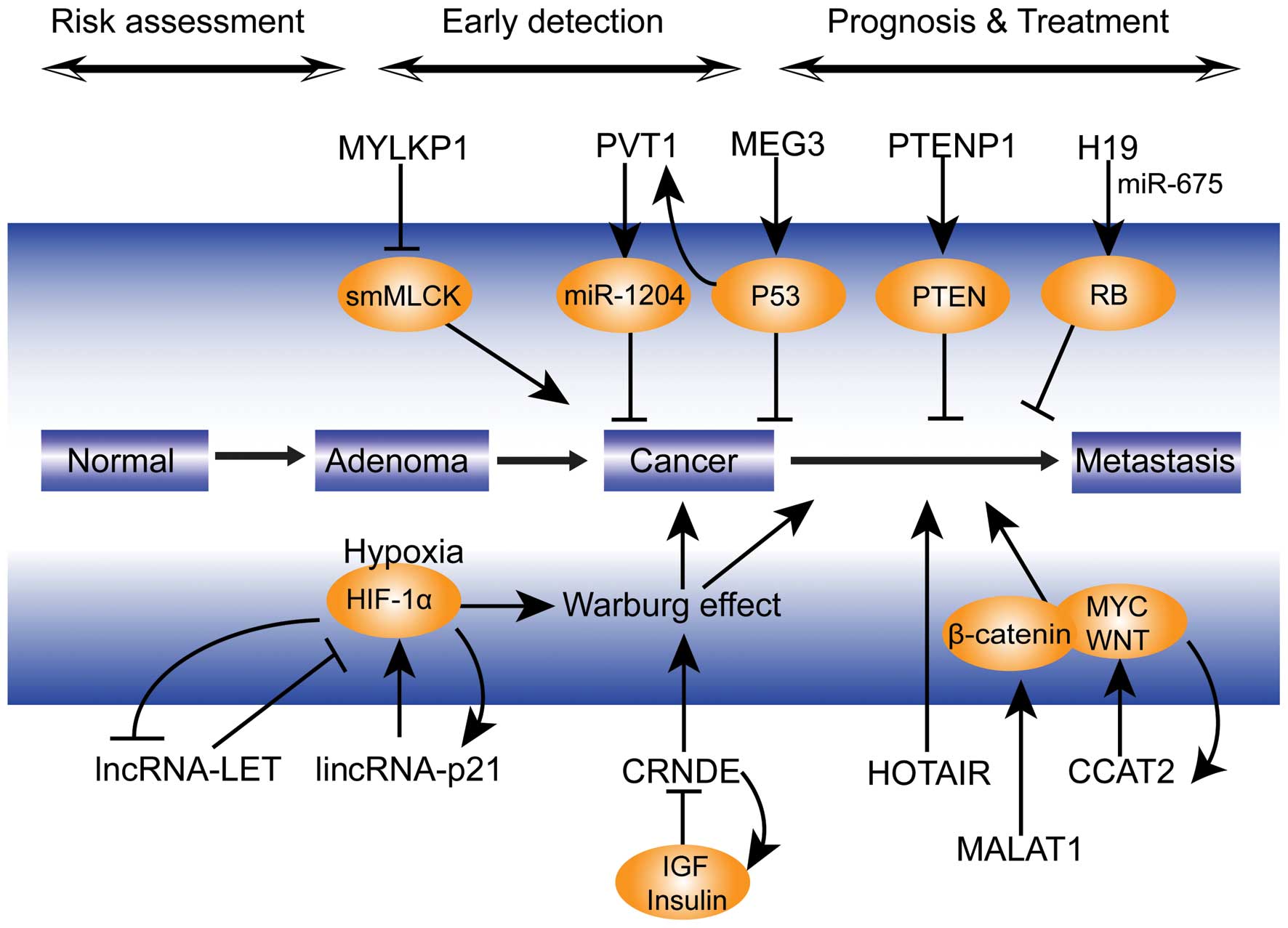 | Figure 1Potential mechanism of lncRNAs
involved in colorectal cancer progression. lncRNA, long non-coding
RNA; lincRNA, long intergenic non-coding RNA; miR, microRNA; HIF,
hypoxia inducible factor; IGF, insulin-like growth factor; PTEN,
phosphatase and tensin homolog; PTENP1, PTEN pseudogene 1; RB,
retinoblastoma protein; smMLCK, smooth muscle myosin light chain
kinase; HOTAIR, HOX transcript antisense RNA; MALAT1,
metastasis-associated lung adenocarcinoma transcript 1; CRNDE,
colorectal neoplasia differentially expressed; MYLKP1, myosin light
chain kinase pseudogene 1; CCAT2, colorectal cancer-associated
transcript 2; PVT1, plasmacytoma variant translocation 1; MEG3,
maternally-expressed gene 3; LET, low expression in tumor. |
 | Table ICRC-associated lncRNAs. |
Table I
CRC-associated lncRNAs.
| lncRNA | Potential
mechanism | Expression | Function | Locus | Size (kb) | Reference |
|---|
| H19 | Control of
imprinting | Upregulated | ⇅↓
proliferation? | Chr11p15.5 | 2.3 | (7,11,12) |
| HOTAIR | Gene silencing by
binding to PRC2 and LSD1 | Upregulated |
↑metastasis
↑poor prognosis | Chr12q13.13 | 2.2 | (15,16) |
| MALAT1 | RNA splicing; small
RNA production; protein interaction | Upregulated | ↑invasion
↑metastasis | Chr11q13.1 | ~7 | (19) |
| HULC | RNA-DNA (CREB) | Upregulated | N.D. | Chr6p24.3 | 0.5 | (22) |
| MEG3 | Increases p53
levels by suppressing MDM2 levels | Downregulated |
↓proliferation
↑apoptosis | Chr14q32 | 1.6–1.8 | (25) |
| CCAT1 | Unknown | Upregulated | ↑risk of CRC | Chr8q24.21 | 2.6 | (27,28) |
| CCAT2 | Regulates Myc and
Wnt | Upregulated |
↑proliferation
↑metastasis | Chr8q24 | 0.4 | (29) |
| CRNDE | Provides scaffolds
for regulatory complexes | Upregulated | ↑Warburg
effect
↑risk of CRC |
Chr16:hCG_1815491 | ~10 | (30) |
| LOC285194 | Unknown | Downregulated | ↑metastasis | Chr3q13.31 | 2.1 | (33) |
| OCC-1 | Unknown | Upregulated | N.D. | Chr12q24.1 | 1.2–1.3 | (34) |
| lincRNA-p21 | Binds to hnRNP;
guides it to p53-targeted gene promoters | Up- or
downregulated | ↑invasion
↑radiation sensitivity
↑Warburg effect | Upstream of
p21/Cdkn1a | ~3.1 | (60) |
| UC.388 | Unknown | Downregulated | ↓metastasis | Near
BX641000/TCF12/FLJ14957 genes | 0.2–0.8 | (37,38) |
| UC.73A | Unknown | Upregulated |
↑proliferation
↓apoptosis | Near AK126774,
BC017741, ZFHX1B | 0.2 | (37,38) |
| LIT1
(Kcnq1ot1) | LOI | LOI occurs
frequently | N.D. | Chr11p15.5 | 91 | (57) |
| PTENP1 | Pseudogene of
PTEN | Downregulated | ↓proliferation | Chr9q13.3 | ~3.9 | (47) |
| MYLKP1 | Pseudogene of
MYLK | Upregulated | ↑proliferation | Chr3p12.3 | 106 | (49) |
| pou5f1p1
(OCT4) | Pseudogene of
pou5f1 | Upregulated | ↑risk of CRC | Chr8q24 | 0.4 | (44) |
| UCA1 | Unknown | Upregulated | N.D. | Chr19p13.12 | 1.4, 2.2, 2.7 | (71) |
| PCAT1 | Inhibits BRCA2 | Upregulated |
↑proliferation
↑poor prognosis | Chr8q24 | 1.9 | (64) |
| PRNCR1 | Unknown | Upregulated | ↑proliferation | Chr8q24 | 13 | (66,67) |
| LET | Regulator of
hypoxia signaling | Downregulated | ↓metastasis | Chr15q24.1 | 2.3 | (68) |
| ncRAN | | Upregulated |
↑migration
↑invasion | Chr17q25.1 | 2.3 | (62) |
| PVT1 | A p53-inducible
target miR-1204 | Upregulated |
↓apoptosis
↑invasion
↑poor prognosis | Chr8q24.21 | >300 | (52) |
In conclusion, evidence is accumulating that lncRNAs
have a significant role in the CRC process and may serve as
potential CRC biomarkers for diagnosis and prognosis. However,
further lncRNAs involved in the metastasis of CRC remain to be
identified. Therefore, continued investigation is necessary to
yield additional information on CRC-associated lncRNAs for future
use in clinical practice.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81372315) and the Shanghai
Scientific Research Plan Project (no. 13JC1401601).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kapranov P, Cheng J, Dike S, et al: RNA
maps reveal new RNA classes and a possible function for pervasive
transcription. Science. 316:1484–1488. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gutschner T and Diederichs S: The
hallmarks of cancer: a long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsang WP, Ng EK, Ng SS, et al: Oncofetal
H19-derived miR-675 regulates tumor suppressor RB in human
colorectal cancer. Carcinogenesis. 31:350–358. 2010. View Article : Google Scholar
|
|
8
|
Yoshimizu T, Miroglio A, Ripoche MA, et
al: The H19 locus acts in vivo as a tumor suppressor. Proc Natl
Acad Sci USA. 105:12417–12422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moulton T, Crenshaw T, Hao Y, et al:
Epigenetic lesions at the H19 locus in Wilms’ tumour patients. Nat
Genet. 7:440–447. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui H, Onyango P, Brandenburg S, Wu Y,
Hsieh CL and Feinberg AP: Loss of imprinting in colorectal cancer
linked to hypomethylation of H19 and IGF2. Cancer Res.
62:6442–6446. 2002.PubMed/NCBI
|
|
11
|
Fellig Y, Ariel I, Ohana P, et al: H19
expression in hepatic metastases from a range of human carcinomas.
J Clin Pathol. 58:1064–1068. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohana P, Schachter P, Ayesh B, et al:
Regulatory sequences of H19 and IGF2 genes in DNA-based therapy of
colorectal rat liver metastases. J Gene Med. 7:366–374. 2005.
View Article : Google Scholar
|
|
13
|
Sorin V, Ohana P, Mizrahi A, et al:
Regional therapy with DTA-H19 vector suppresses growth of colon
adenocarcinoma metastases in the rat liver. Int J Oncol.
39:1407–1412. 2011.PubMed/NCBI
|
|
14
|
Gupta RA, Shah N, Wang KC, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pádua Alves C, Fonseca AS, Muys BR, et al:
Brief report: The lincRNA Hotair is required for
epithelial-to-mesenchymal transition and stemness maintenance of
cancer cell lines. Stem cells. 31:2827–2832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kogo R, Shimamura T, Mimori K, et al: Long
noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gutschner T, Hämmerle M, Eissmann M, et
al: The noncoding RNA MALAT1 is a critical regulator of the
metastasis phenotype of lung cancer cells. Cancer Res.
73:1180–1189. 2013. View Article : Google Scholar :
|
|
18
|
Chang JL, Li ZG, Wang XY and Yang MH:
Detection of p53, MALAT1, ki-67 and β-catenin mRNA expression and
its significance in molecular diagnosis of colorectal carcinoma.
World Chinese J Digestol. 16:3849–3854. 2008.
|
|
19
|
Xu C, Yang M, Tian J, Wang X and Li Z:
MALAT-1: a long non-coding RNA and its important 3′ end functional
motif in colorectal cancer metastasis. Int J Oncol. 39:169–175.
2011.PubMed/NCBI
|
|
20
|
Ji Q, Liu X, Fu X, et al: Resveratrol
inhibits invasion and metastasis of colorectal cancer cells via
MALAT1 mediated Wnt/β-catenin signal pathway. PLoS One.
8:e787002013. View Article : Google Scholar
|
|
21
|
Panzitt K, Tschernatsch MM, Guelly C, et
al: Characterization of HULC, a novel gene with striking
up-regulation in hepatocellular carcinoma, as noncoding RNA.
Gastroenterology. 132:330–342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matouk IJ, Abbasi I, Hochberg A, Galun E,
Dweik H and Akkawi M: Highly upregulated in liver cancer noncoding
RNA is overexpressed in hepatic colorectal metastasis. Eur J
Gastroenterol Hepatol. 21:688–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie H, Ma H and Zhou D: Plasma HULC as a
promising novel biomarker for the detection of hepatocellular
carcinoma. Biomed Res Int. 2013:1361062013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyoshi N, Wagatsuma H, Wakana S, et al:
Identification of an imprinted gene, Meg3/Gtl2 and its human
homologue MEG3, first mapped on mouse distal chromosome 12 and
human chromosome 14q. Genes Cells. 5:211–220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Zhou Y, Mehta KR, et al: A
pituitary-derived MEG3 isoform functions as a growth suppressor in
tumor cells. J Clin Endocrinol Metab. 88:5119–5126. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Zhang X and Klibanski A: MEG3
noncoding RNA: a tumor suppressor. J Mol Endocrinol. 48:R45–R53.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nissan A, Stojadinovic A,
Mitrani-Rosenbaum S, et al: Colon cancer associated transcript-1: a
novel RNA expressed in malignant and pre-malignant human tissues.
Int J Cancer. 130:1598–1606. 2012. View Article : Google Scholar
|
|
28
|
Kam Y, Rubinstein A, Naik S, et al:
Detection of a long non-coding RNA (CCAT1) in living cells and
human adenocarcinoma of colon tissues using FIT-PNA molecular
beacons. Cancer Lett. 352:90–96. 2014. View Article : Google Scholar
|
|
29
|
Ling H, Spizzo R, Atlasi Y, et al: CCAT2,
a novel noncoding RNA mapping to 8q24, underlies metastatic
progression and chromosomal instability in colon cancer. Genome
Res. 23:1446–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Graham LD, Pedersen SK, Brown GS, et al:
Colorectal neoplasia differentially expressed (CRNDE), a novel gene
with elevated expression in colorectal adenomas and
adenocarcinomas. Genes Cancer. 2:829–840. 2011. View Article : Google Scholar
|
|
31
|
Ellis BC, Molloy PL and Graham LD: CRNDE:
A long non-coding RNA Involved in CanceR, Neurobiology, and
DEvelopment. Front Genet. 3:2702012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ellis BC, Graham LD and Molloy PL: CRNDE,
a long non-coding RNA responsive to insulin/IGF signaling,
regulates genes involved in central metabolism. Biochim Biophys
Acta. 1843:372–386. 2014. View Article : Google Scholar
|
|
33
|
Liu Q, Huang J, Zhou N, et al: LncRNA
loc285194 is a p53-regulated tumor suppressor. Nucleic Acids Res.
41:4976–4987. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pibouin L, Villaudy J, Ferbus D, et al:
Cloning of the mRNA of overexpression in colon carcinoma-1: a
sequence overexpressed in a subset of colon carcinomas. Cancer
Genet Cytogenet. 133:55–60. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng JC, Shen J and Ran ZH: Transcribed
ultraconserved region in human cancers. RNA Biol. 10:1771–1777.
2013. View Article : Google Scholar
|
|
36
|
Scaruffi P: The transcribed-ultraconserved
regions: a novel class of long noncoding RNAs involved in cancer
susceptibility. ScientificWorldJournal. 11:340–352. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Calin GA, Liu CG, Ferracin M, et al:
Ultraconserved regions encoding ncRNAs are altered in human
leukemias and carcinomas. Cancer Cell. 12:215–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sana J, Hankeova S, Svoboda M, Kiss I,
Vyzula R and Slaby O: Expression levels of transcribed
ultraconserved regions uc.73 and uc388 are altered in colorectal
cancer. Oncology. 82:114–118. 2012. View Article : Google Scholar
|
|
39
|
Wojcik SE, Rossi S, Shimizu M, et al:
Non-coding RNA sequence variations in human chronic lymphocytic
leukemia and colorectal cancer. Carcinogenesis. 31:208–215. 2010.
View Article : Google Scholar :
|
|
40
|
Ng SY, Gunning P, Eddy R, et al: Evolution
of the functional human beta-actin gene and its multi-pseudogene
family: conservation of noncoding regions and chromosomal
dispersion of pseudogenes. Mol Cell Biol. 5:2720–2732.
1985.PubMed/NCBI
|
|
41
|
Poliseno L: Pseudogenes: newly discovered
players in human cancer. Sci Signal. 5:re52012.PubMed/NCBI
|
|
42
|
Wezel F, Pearson J, Kirkwood LA and
Southgate J: Differential expression of Oct4 variants and
pseudogenes in normal urothelium and urothelial cancer. Am J
Pathol. 183:1128–1136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kastler S, Honold L, Luedeke M, et al:
POU5F1P1, a putative cancer susceptibility gene, is overexpressed
in prostatic carcinoma. Prostate. 70:666–674. 2010.
|
|
44
|
Panagopoulos I, Möller E, Collin A and
Mertens F: The POU5F1P1 pseudogene encodes a putative protein
similar to POU5F1 isoform 1. Oncol Rep. 20:1029–1033.
2008.PubMed/NCBI
|
|
45
|
Ali A, Saluja SS, Hajela K, Mishra PK and
Rizvi MA: Mutational and expressional analyses of PTEN gene in
colorectal cancer from Northern India. Mol Carcinog. 53(Suppl 1):
E45–E52. 2014. View Article : Google Scholar
|
|
46
|
Johnsson P, Ackley A, Vidarsdottir L, et
al: A pseudogene long-noncoding-RNA network regulates PTEN
transcription and translation in human cells. Nat Struct Mol Biol.
20:440–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Poliseno L, Salmena L, Zhang J, Carver B,
Haveman WJ and Pandolfi PP: A coding-independent function of gene
and pseudogene mRNAs regulates tumour biology. Nature.
465:1033–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lazar V and Garcia JG: A single human
myosin light chain kinase gene (MLCK; MYLK). Genomics. 57:256–267.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Han YJ, Ma SF, Yourek G, Park YD and
Garcia JG: A transcribed pseudogene of MYLK promotes cell
proliferation. FASEB J. 25:2305–2312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rack KA, Delabesse E, Radford-Weiss I, et
al: Simultaneous detection of MYC, BVR1, and PVT1 translocations in
lymphoid malignancies by fluorescence in situ hybridization. Genes
Chromosomes Cancer. 23:220–226. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guan Y, Kuo WL, Stilwell JL, et al:
Amplification of PVT1 contributes to the pathophysiology of ovarian
and breast cancer. Clin Cancer Res. 13:5745–5755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Takahashi Y, Sawada G, Kurashige J, et al:
Amplification of PVT-1 is involved in poor prognosis via apoptosis
inhibition in colorectal cancers. Br J Cancer. 110:164–171. 2014.
View Article : Google Scholar :
|
|
53
|
Barsotti AM, Beckerman R, Laptenko O,
Huppi K, Caplen NJ and Prives C: p53-Dependent induction of PVT1
and miR-1204. J Biol Chem. 287:2509–2519. 2012. View Article : Google Scholar :
|
|
54
|
Nakagawa H, Chadwick RB, Peltomaki P,
Plass C, Nakamura Y and de La Chapelle A: Loss of imprinting of the
insulin-like growth factor II gene occurs by biallelic methylation
in a core region of H19-associated CTCF-binding sites in colorectal
cancer. Proc Natl Acad Sci USA. 98:591–596. 2001. View Article : Google Scholar :
|
|
55
|
Murakami K, Oshimura M and Kugoh H:
Suggestive evidence for chromosomal localization of non-coding RNA
from imprinted LIT1. J Hum Genet. 52:926–933. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mitsuya K, Meguro M, Lee MP, et al: LIT1,
an imprinted antisense RNA in the human KvLQT1 locus identified by
screening for differentially expressed transcripts using
monochromosomal hybrids. Hum Mol Genet. 8:1209–1217. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nakano S, Murakami K, Meguro M, et al:
Expression profile of LIT1/KCNQ1OT1 and epigenetic status at the
KvDMR1 in colorectal cancers. Cancer Sci. 97:1147–1154. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhai H, Fesler A, Schee K, Fodstad O,
Flatmark K and Ju J: Clinical significance of long intergenic
noncoding RNA-p21 in colorectal cancer. Clin Colorectal Cancer.
12:261–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang G, Li Z, Zhao Q, et al: LincRNA-p21
enhances the sensitivity of radiotherapy for human colorectal
cancer by targeting the Wnt/β-catenin signaling pathway. Oncol Rep.
31:1839–1845. 2014.PubMed/NCBI
|
|
60
|
Yang F, Zhang H, Mei Y and Wu M:
Reciprocal regulation of HIF-1α and lincRNA-p21 modulates the
Warburg effect. Mol Cell. 53:88–100. 2014. View Article : Google Scholar
|
|
61
|
Yu M, Ohira M, Li Y, et al: High
expression of ncRAN, a novel non-coding RNA mapped to chromosome
17q25.1, is associated with poor prognosis in neuroblastoma. Int J
Oncol. 34:931–938. 2009.PubMed/NCBI
|
|
62
|
Qi P, Xu MD, Ni SJ, et al: Down-regulation
of ncRAN, a long non-coding RNA, contributes to colorectal cancer
cell migration and invasion and predicts poor overall survival for
colorectal cancer patients. Mol Carcinog. 2014. View Article : Google Scholar
|
|
63
|
Yang L, Lin C, Jin C, et al:
lncRNA-dependent mechanisms of androgen-receptor-regulated gene
activation programs. Nature. 500:598–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ge X, Chen Y, Liao X, et al:
Overexpression of long noncoding RNA PCAT-1 is a novel biomarker of
poor prognosis in patients with colorectal cancer. Med Oncol.
30:5882013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Meyer LR, Zweig AS, Hinrichs AS, et al:
The UCSC Genome Browser database: extensions and updates 2013.
Nucleic Acids Res. 41:D64–D69. 2013. View Article : Google Scholar :
|
|
66
|
Chung S, Nakagawa H, Uemura M, et al:
Association of a novel long non-coding RNA in 8q24 with prostate
cancer susceptibility. Cancer Sci. 102:245–252. 2011. View Article : Google Scholar
|
|
67
|
Li L, Sun R, Liang Y, et al: Association
between polymorphisms in long non-coding RNA PRNCR1 in 8q24 and
risk of colorectal cancer. J Exp Clin Cancer Res. 32:1042013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yang F, Huo XS, Yuan SX, et al: Repression
of the long noncoding RNA-LET by histone deacetylase 3 contributes
to hypoxia-mediated metastasis. Mol Cell. 49:1083–1096. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: the Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang F, Li X, Xie X, Zhao L and Chen W:
UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma
and embryo, influencing cell growth and promoting invasion. FEBS
Lett. 582:1919–1927. 2008. View Article : Google Scholar : PubMed/NCBI
|















