Introduction
The rate of morbidity resulting from multiple
primary lung cancer (MPLC) is increasing, and in order to devise an
effective therapeutic strategy, it is important to distinguish
between MPLC and intrapulmonary metastasis (IPM). The incidence of
synchronous MPLCs in a reported clinical series was between 1 and
7% (1). Another study reported that
up to 10% of patients who survive a primary lung carcinoma will go
on to develop a second primary lung tumor (2). A consensus exists regarding the
therapeutic schedule of MPLC, which suggests that surgical
treatment confers improved patient prognoses compared with
chemotherapy. Therefore, a correct differential diagnosis of MPLC,
as opposed to IPM, is conducive to effective individualized
treatment. However, based solely on the Martini and Melamed
criteria (3), which is widely used
in clinical settings, it can be challenging to correctly diagnose
MPLC. Furthermore, several cancers, including lung cancers, arise
as a result of an accumulation of different genetic and epigenetic
alterations (4,5). In a number of organs, carcinogenesis
is considered to be a multistep process due to the accumulation of
several sequential molecular abnormalities. A previous study
identified that the overall frequency of the loss of heterozygosity
(LOH) within cell clones progressively increased as the severity of
histopathological changes progressed from hyperplasia to dysplasia
to carcinoma in situ (6).
Furthermore, the incidence of LOH has been demonstrated to increase
along with the histological progression of lung adenocarcinoma
(7). The definition of field
cancerization has been extended to include cases of multiple
primary tumors of the entire upper aerodigestive tract (8). The pathogenesis of multiple primary
tumors and metastatic tumors is fundamentally different. Tissues in
different fields may develop a unique genetic phenotype under the
action of the same carcinogen (e.g., cigarettes) and proceed to
form multiple primary tumors. By contrast, metastatic tumors are
formed via the hematogenous and/or lymphatic metastasis of primary
tumors. Primary and metastatic tumors exhibit a similar origin of
clonality. The identification of molecular and genetic variations
between tumors will allow for the differential diagnosis of MPLC
and IPM. Recent advances in the study of molecular tumorigenesis
have demonstrated that the genetic alterations acquired during
tumor progression may act as potentially useful markers during
clonality analysis. Certain studies have suggested that the gene
mutational analysis of tumors could be a supplementary method to
distinguish between MPLC and IPM (9–11). We
have formulated two inclusion criteria in order to identify optimal
genetic markers for use during clonality analysis: i) A commonly
occurring and independent mutation that occurs in the early stages
of disease and is maintained throughout tumor progression; and/or
ii) a prognostic marker that is able to evaluate tumor progression.
In total, four genetic markers, p53, p16, p27 and c-erbB2, were
selected in order to examine the differences in clonality between
two separate tumors from the same patient by immunohistochemical
(IHC) staining. In addition, the study aimed to establish a
quantitative differentially-expressed gene mathematical model to
discriminate between cases of MPLC and IPM.
Materials and methods
Patients and clinical features
Of the 111 consecutive patients with primary lung
cancer who had undergone a surgical resection between August 1999
and December 2009 at the Department of Thoracic Surgery, West China
Hospital, Sichuan University (Chengdu, China), 50 patients were
diagnosed with MPLCs according to the Martini and Melamed criteria
(3). Of these patients, 36
exhibited MPLCs of the same histological type, including 34
patients with synchronous MPLCs and two with metachronous MPLCs,
while 14 presented with MPLC of a different histological type.
Finally, the 36 patients with MPLCs of the same histological type,
in which paraffin sections of all tumors were available, were
enrolled in the present study. In addition, 20 patients diagnosed
with IPM during the same period, according to the Martini and
Melamed criteria, were included. In total, 30 patients with lymph
node metastasis, 11 with distant metastasis (eight brain
metastases, two bone metastases and one adrenal metastasis) and 14
MPLC patients with different histological types were selected as
negative or positive controls for the expression analysis of the
four proteins between primary tumors and metastases. The
clinicopathological data were obtained from a retrospective chart
review. The tumor stage was classified according to the 2009
revision of the International System for Staging Lung Cancer
(12). The characteristics of the
patients with MPLCs, IPM or lymph node metastasis are shown in
Table I. The experiments were
approved by the West China Hospital Ethics Committee (no. 201333)
and all participating patients provided informed consent.
 | Table ITumor characteristics. |
Table I
Tumor characteristics.
| A, Intrapulmonary,
distant and lymph node metastases |
|---|
|
|---|
| Characteristics | Intrapulmonary
metastasis | Distant
metastasis | Lymph node
metastasis |
|---|
| No. of patients | 20 | 11 | 30 |
| Age, years
(range) | 62 (46–74) | 55 (42–70) | 60 (38–72) |
| Gender, n (%) |
| Male | 12 (60) | 6 (54.5) | 22 (73.3) |
| Female | 8 (40) | 5 (45.5) | 8 (26.7) |
| Second cancer, n
(%) |
| Metachronous | 3 (15) | | |
| Synchronous | 17 (85) | | |
| No. of tumors |
| 2 | 20 | | |
| 3 | | | |
| Histological type,
n |
|
Adenocarcinoma | 15 | 4 | 17 |
| Squamous cell
carcinoma | 5 | 4 | 10 |
| Other | | 3 | 3 |
| p stage (2009
UICC)b, n |
| IA | | | |
| IB | | | |
| IIA | | | 6 |
| IIB | | | 4 |
| IIIA | 10 | | 18 |
| IIB | 7 | | 1 |
| IV | 3 | 11 | 1 |
|
| B, MPLC |
|
|
Characteristics | MPLC total | Same histological
type | Different
histological type |
| No. of
patients | 50 | 36 | 14 |
| Age, years
(range) | 61 (38–80) | | |
| Gender, n (%) |
| Male | 34 (68) | | |
| Female | 16 (32) | | |
| Second cancer, n
(%) |
| Metachronous | 28 (56) | | |
| Synchronous | 22 (44) | | |
| No. of tumors |
| 2 | 34 | 13 | |
| 3 | 2a | 1 | |
| Histological
type |
|
Adenocarcinoma | | 33 | 11 |
| Squamous cell
carcinoma | | 3 | 11 |
| Other | | | 3 |
| p stage (2009
UICC)b |
| IA | | 15 | 2 |
| IB | | 12 | 2 |
| IIA | | 2 | 3 |
| IIB | | 6 | 1 |
| IIIA | | 1 | 2 |
| IIIB | | | 4 |
| IV | | | |
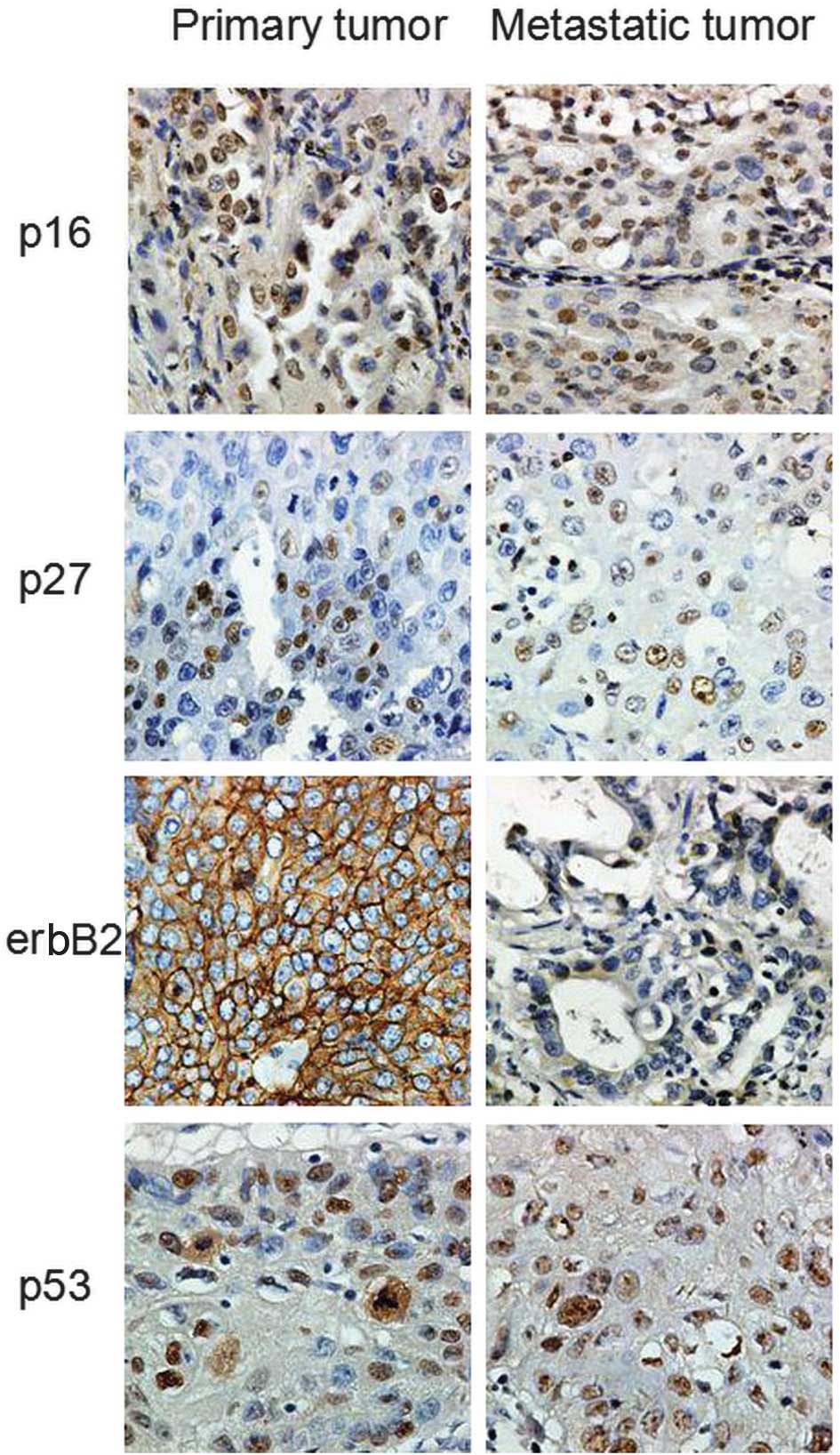
IHC staining
Four proteins, p53, p16, p27 and c-erbB2, which have
been demonstrated to be independent prognostic factors for
non-small cell lung cancer (NSCLC) (13–16),
were selected for the differential diagnostic analysis of MPLC and
IPM. IHC staining was performed using serial sections obtained from
the same paraffin-embedded blocks. The specimens were stained with
hematoxylin and eosin in order to confirm the histological
diagnosis. IHC staining was performed using the
streptavidin-biotin-peroxidase complex method. For the antigen
retrieval, sections were briefly immersed in a citrate buffer (0.01
mol/l citric acid; pH 6.0) and then incubated for 25-min intervals
at 100°C in a microwave oven. Next, the sections were incubated
with a monoclonal mouse anti-p53 antibody (dilution, 1:100;
sc-6243, Santa Cruz Biotechnology, Dallas, TX, USA), a polyclonal
rabbit anti-p16 antibody (dilution, 1:200; ab54210, Abcam,
Cambridge, MA, USA), a monoclonal mouse anti-p27 antibody
(dilution, 1:250; ab32034 Abcam) and a monoclonal mouse
anti-c-erbB2 antibody (dilution, 1:100; ab2428, Abcam) overnight in
a cold room using a labeled streptavidin biotin kit (Dako LSAB kit;
Dako, Carpinteria, CA, USA). The antibodies were diluted in
phosphate-buffered saline containing 2% bovine serum albumin.
Evaluation of the stained specimens
Appropriate positive and negative controls were
selected for use in the present study. The slides were
independently analyzed by two of the authors who were blinded to
the clinicopathological data. A positive result for p53, p16, and
p27 expression was defined as the presence of nuclear staining,
whereas a positive result for c-erbB2 expression was defined as the
appearance of cell membrane staining. Subsequent to the IHC
detection of p53, p16, p27 and c-erbB2 in each of the specimens,
the percentage of immunoreactive tumor cells in five different
randomly-selected fields (magnification, ×400) was recorded. The
final value for the percentage of positive tumor cells was
calculated as the average of the positively-immunostained cells.
The extent of immunostaining was scored according to the percentage
of positive cells in each tumor specimen as follows: No staining,
0; 1–10% staining, 10; 11–20% staining, 20; 21–30% staining, 30;
31–40% staining, 40; 41–50% staining, 50; 51–60% staining, 60;
61–70% staining, 70; 71–80% staining, 80; 81–90% staining, 90; and
91–100% staining, 100.
Results
Establishment of the quantitative
mathematical model based upon the differentially-expressed gene
analysis and its application in the diagnosis of MPLC
First, the differential expression of the four
proteins in the the primary tumors and metastatic lesions of 30
patients with lymph node metastasis and in 11 patients with distant
metastasis were analyzed and subsequently served as a negative
control. The differential expression of p53, p16, p27 and c-erbB2
was compared between the primary lung tumors and the metastatic
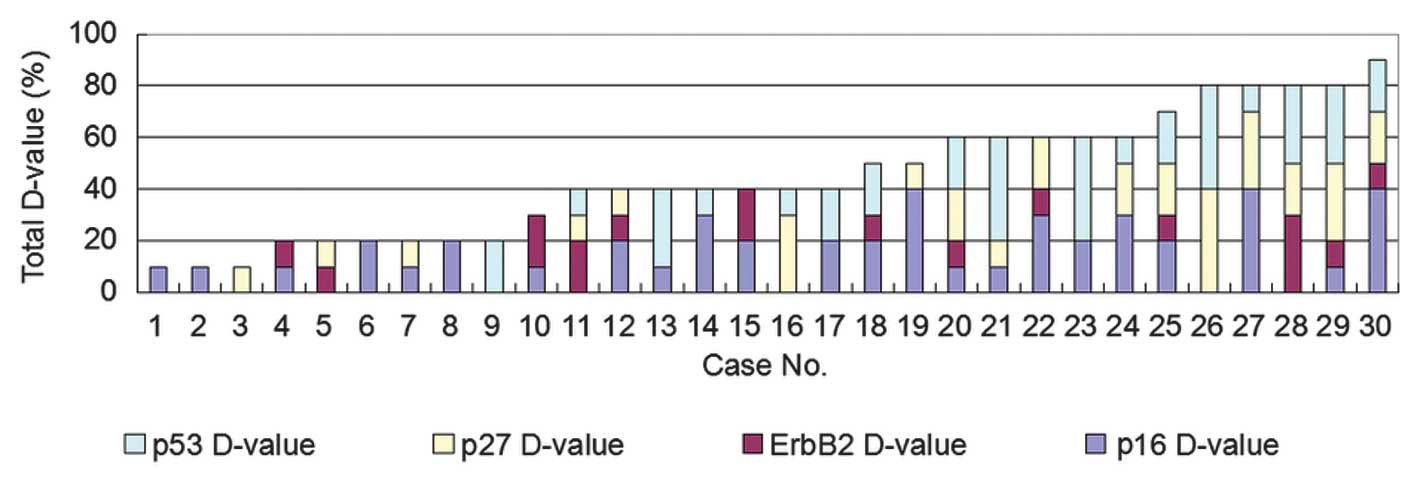
tumors in the lymph nodes of the 30 patients (Table II). The sum value of the
differential expression of the four proteins ranged between 10 and
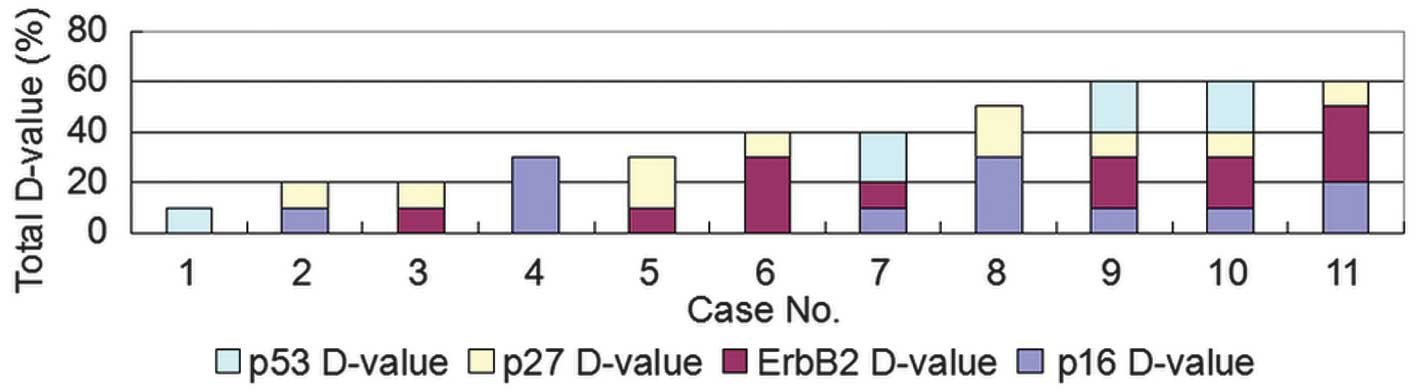
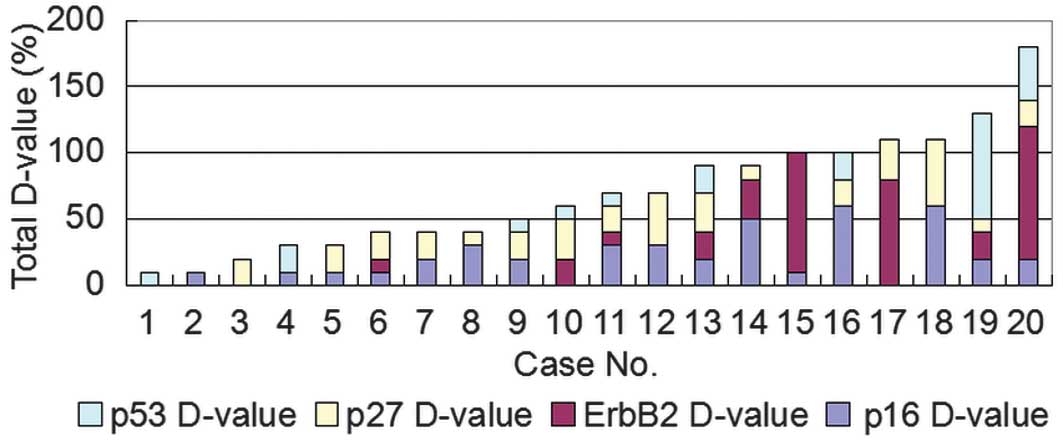
90 (Fig. 1). Next, the differential
expression of the four proteins was compared between the primary
lung tumors and the distant metastases. The sum value of the
differential expression of the four proteins ranged between 10 and
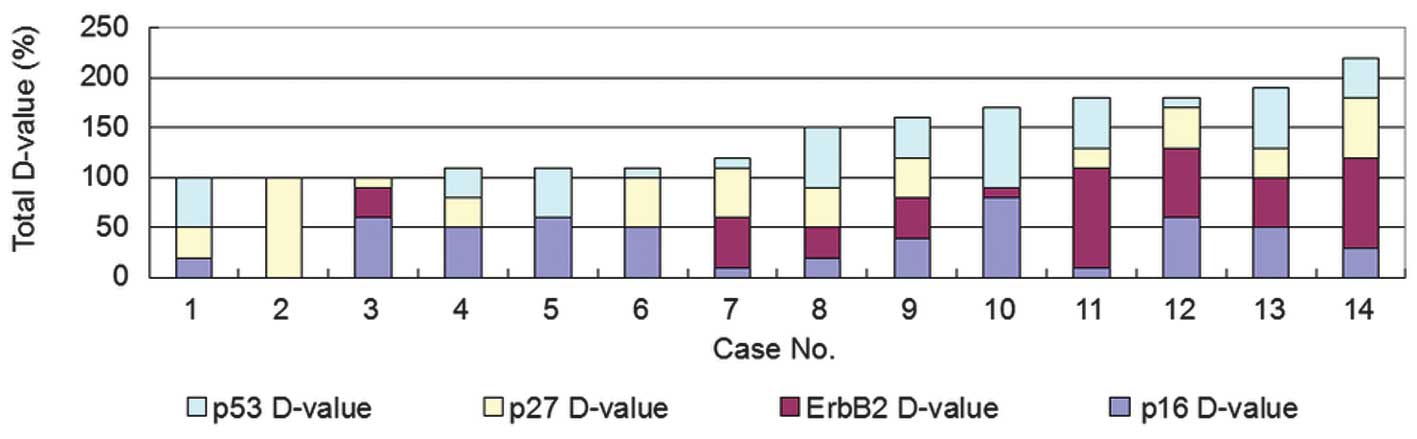
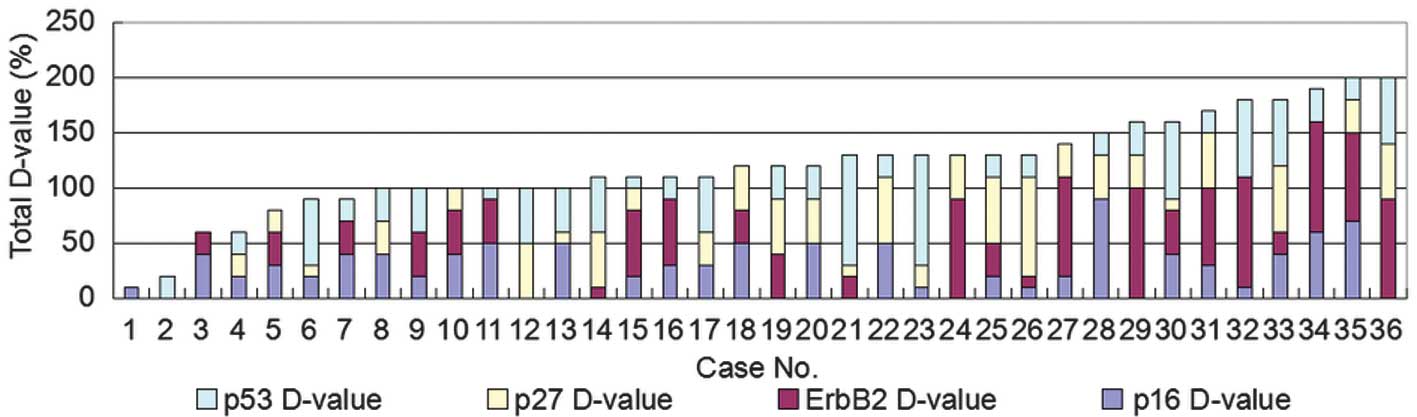
60 (Figs. 2 and 6). The maximum sum value of the
differential expression ratios of the four proteins diagnosed as
the same histological type of MPLC was ≤90. By contrast, the sum
value of the differential expression ratios of the four proteins
ranged between 100 and 220 in the 14 patients diagnosed with MPLCs
of different histological types (Figs.
3 and 6). Therefore, it was
hypothesized that in the case that the difference between two
tumors exceeded a score of 90, the tumors were likely to be
different, i.e., MPLCs.
 | Table IIImmunohistochemical protein
expression of the four genes in primary tumors and metastatic lymph
nodes. |
Table II
Immunohistochemical protein
expression of the four genes in primary tumors and metastatic lymph
nodes.
| Case no. | p16 D-value | ErbB2 D-value | p27 D-value | p53 D-value | Total D-value |
|---|
| 1 | 10 | 10 | 0 | 0 | 20 |
| 2 | 10 | 10 | 20 | 20 | 60 |
| 3 | 0 | 20 | 10 | 10 | 40 |
| 4 | 20 | 10 | 10 | 0 | 40 |
| 5 | 40 | 10 | 20 | 20 | 90 |
| 6 | 10 | 0 | 0 | 30 | 40 |
| 7 | 30 | 0 | 0 | 10 | 40 |
| 8 | 0 | 0 | 40 | 40 | 80 |
| 9 | 40 | 0 | 10 | 0 | 50 |
| 10 | 10 | 0 | 0 | 0 | 10 |
| 11 | 10 | 0 | 10 | 40 | 60 |
| 12 | 0 | 10 | 10 | 0 | 20 |
| 13 | 20 | 10 | 0 | 20 | 30 |
| 14 | 30 | 10 | 20 | 0 | 60 |
| 15 | 40 | 0 | 30 | 10 | 80 |
| 16 | 20 | 0 | 0 | 40 | 60 |
| 17 | 10 | 0 | 0 | 0 | 10 |
| 18 | 20 | 0 | 0 | 0 | 20 |
| 19 | 20 | 10 | 20 | 20 | 70 |
| 20 | 0 | 0 | 10 | 0 | 10 |
| 21 | 20 | 20 | 0 | 0 | 40 |
| 22 | 0 | 0 | 30 | 10 | 40 |
| 23 | 10 | 20 | 0 | 0 | 30 |
| 24 | 10 | 0 | 10 | 0 | 20 |
| 25 | 0 | 30 | 20 | 30 | 80 |
| 26 | 20 | 0 | 0 | 20 | 40 |
| 27 | 30 | 0 | 20 | 10 | 60 |
| 28 | 10 | 10 | 30 | 30 | 80 |
| 29 | 20 | 0 | 0 | 0 | 20 |
| 30 | 0 | 0 | 0 | 20 | 20 |
On the basis of the experimental data, a
quantitative differentially-expressed gene mathematical model was
established as follows: Sum of the differential expression ratios =
p16T1 − T2 + p27T1 − T2 + C-erbB2T1 − T2 + p53T1 − T2, where T1 is
the primary cancer and T2 is the lymph node metastasis, metastatic
cancers or the second separate cancers. According to the
experimental results, tumors can be re-diagnosed as metastatic when
the sum of the differential expression ratios of the four proteins
does not exceed the reference value of 90, and as MPLCs when the
value does exceed 90 (Table
III).
 | Table IIIDifference between the Martini and
Melamed criteria and the mathematical model, which is based upon
differentially-expressed gene analysis, in MPLC. |
Table III
Difference between the Martini and
Melamed criteria and the mathematical model, which is based upon
differentially-expressed gene analysis, in MPLC.
| Clinical diagnosis
(Martini and Melamed criteria) |
|---|
|
|
|---|
|
Differentially-expressed gene
analysis | MPLC | IPM | Total |
|---|
| MPLC | 29 | 6 | 35 |
| IPM | 7 | 14 | 21 |
| Total | 36 | 20 | 56 |
Results of de novo diagnosis based upon a
differentially-expressed gene analysis mathematical model in MPLCs
of the same histological type
Of the 36 patients with the same histological type
of MPLC, who were clinically diagnosed according to the Martini and
Melamed criteria (3), the sum value
of the differential expression ratio was >90 in 29 patients
(80.5%), and <90 in seven patients (19.5%) (Figs. 4 and 6). According to the model, 29 of the 36
patients (82.0%) were diagnosed de novo with
newly-classified MPLCs and seven with newly-classified IPM.
Results of de novo diagnosis based upon a
differentially-expressed gene analysis mathematical model in lung
cancers with IPM
Of the 20 patients with IPM who were clinically
diagnosed according to the Martini and Melamed criteria (3), 14 (70.0%) had a sum value of ≤90 for
the expression ratios of the four proteins. According to the same
criterion, 14 of the 20 patients were diagnosed de novo with
newly-classified IPM, and six with newly-classified MPLCs (Figs. 5 and 6). In total, three of the six patients
(50%) with IPM demonstrated unilateral lung lobe lesions, and the
other three patients presented with bilateral lung lobe lesions.
The pathological stage was diagnosed de novo as being
between T4 and T1 among three of the six patients, and as M1 to T2
in the rest.
Differences in the diagnostic consistency
of the mathematical model, based on differentially-expressed gene
analysis and clinical diagnosis
In total, 29 of the 36 MPLC patients were diagnosed
with newly-classified MPLC and the remaining seven with
newly-classified IPM. Furthermore, 14 of the 20 cases of IPM were
diagnosed with newly-classified IPM and the other six with MPLC.
Overall, 35 patients with multifocal lung cancer were diagnosed
de novo with newly-classified MPLC, and 21 with
newly-classified IPM (Table
III).
Discussion
At present, individuals with lung cancer have an
increased risk of developing a second lung tumor. Cases of MPLC are
distinguished by the presence of a secondary neoplasm. It may be
easy to diagnose cases of MPLC that exhibit different histological
types. Multiple, anatomically distinct, but histologically similar
lung cancers are commonly identified in the same patient. Often, it
can be challenging to distinguish between cases of MPLC and IPM.
The diagnostic criteria for MPLC was proposed by Martini and
Melamed (3) and states that: i)
MPLC tumors must occur in separate lobes or in different regions of
the same lobe, ii) neoplasms may originate from different types of
carcinomas in situ and demonstrate distinct histological
types, and iii) no metastasis should be evident in the lymphatic
system or in any other organs. However, not all patients can be
classified in accordance with these guidelines. Patients with
clinically diagnosed MPLCs occasionally demonstrate extremely poor
five-year survival rates (0–44%), even at stage I of the disease
(3,17–19).
This variation in prognosis is believed to be the result of the
different biological behaviors of the tumors. These results suggest
that a number of patients with clinically diagnosed MPLCs may
possess metastatic lesions. This indicates a potential limitation
in the Martini and Melamed criteria (3), which, at present, is widely used for
the clinical diagnosis of MPLCs.
A universal agreement regarding the particular
approach that should be adhered to for the diagnosis of MPLC is yet
to be established. Therefore, biological analyses are considered to
be a useful approach for distinguishing between cases of MPLC and
IPM, and for determining the correct biological stage of the lung
cancer. In order to overcome this limitation, the use of clonal
analyses for different tumors has been reported to discriminate
between MPLCs and IPM. Previous studies have demonstrated that
multiple gene analyses are able to identify the clonality in a
combination of multiple gene mutations, including a p53 gene
mutation, a K-ras mutation and/or LOH (11,20–28).
In order to differentiate between multifocal tumors and IPM, Chang
et al (29) evaluated p53
somatic aberrations in MPLCs. Of the 58 patients included in the
study, 22 (37.9%) were identified as having the same clonality and
28 (48.3%) as having different clonalities. Furthermore, it was
revealed that the occurrence of lymph node metastasis was more
common in lesions with the same clonality.
In the present study, IHC staining was performed in
order to distinguish between MPLCs and IPM. The IHC expression
levels of p53, p16, p27 and c-erbB2 were revealed to be significant
prognostic factors for cases of lung cancer. The transcription
factor, p53, is activated in response to DNA damage and is involved
in cell cycle regulation, the induction of apoptosis and DNA
repair. However, mutated forms of p53 are unable to effectively
retain these particular functions. A mutated version or an
overexpression of the p53 gene is an unfavorable prognostic factor
that is observed in ~50% of patients with NSCLC (30). In a previous study, the presence of
somatic mutations or an overexpression of p53 were identified in
~23% and ~65% of patients with NSCLC, respectively. Furthermore,
p53 has been extensively investigated as a prognostic marker in
cases of NSCLC, and the majority of results indicate that
alterations in p53 are associated with a poor prognosis (31). The reproduction of human lung
adenocarcinoma phenotypes in the flanks of nude mice has been
successfully completed by introducing a p53 gene alternation
(32). The p16 gene is also a tumor
suppressor gene, which negatively regulates cell cycle progression
by inhibiting cyclin-dependent kinases (CDK) 4/6. Homozygous
deletions (HDs) of p16 have been frequently detected in lung cancer
patients. In a previous study, HDs were detected in eight of 28
(28.6%) primary tumor patients, including two of eight (25.0%)
non-invasive bronchioloalveolar carcinomas, and five of 22 (22.7%)
brain metastases (33). In another
study, abnormal hypermethylation of the p16 promoter was detected
in several tumors types, and was revealed to be inactivated in
40–70% of patients with NSCLC (34). The contribution of p16 deregulation
via alterations in methylation during the carcinogenic process has
been extensively investigated. p16 hypermethylation is considered
to be an independent prognostic factor for poor patient outcomes
(35). p27 is a CDK inhibitor,
which is involved in the regulation of the cell cycle. By
inhibiting retinoblastoma phosphorylation, p27 is able to suppress
the progression of the cell cycle from the G1 phase to
the S phase. A reduced expression of p27 is observed in 70–80% of
patients with NSCLC. In previous studies, this particular
reduced-expression group demonstrated a poorer prognosis compared
with patients from a positive-expression group (15,36,37).
By contrast, high p27 expression is associated with an improved
prognosis (38). The c-erbB-2
(HER-2/neu) proto-oncogene codes for transmembrane receptor
tyrosine kinases, such as epidermal growth factor and human
epidermal growth factor receptor 2, 3 and 4, which are members of
the class 1 receptor tyrosine kinase family. An overexpression of
c-erbB2 is often observed in patients with NSCLC. In a previous
study, c-erbB2 overexpression was identified in 37% of lung
adenocarcinomas cases that were associated with a higher disease
stage and a positive nodal status. Therefore, c-erbB2 has been
suggested to be a potential tumor progression marker in NSCLC
patients, and one that can be observed at the protein level
(39,40).
Two important mechanisms have been proposed, through
which histologically-similar, multifocal tumors are believed to
arise: i) A single clonal event occurs, which results in a tumor
that subsequently spreads within one or two lungs; and ii) multiple
tumors arise independently in a carcinogen-damaged field (41). The difference in protein expression
between the histologically-similar tumors was hypothesized to be
larger in MPLC patients, due to the various clonal origins. The
D-value of protein expression in IPM patients, however, would be
smaller. According to the concept of field cancerization, tissues
from different fields may conduct similar or dissimilar DNA damage
under the control of a carcinogen. The possibility that separate
MPLC tumors may contain a similar genotype could be a default from
the probability theory. When two or more separate tumors share one
genotype, they are likely to be IPMs (41,42).
Based on our preliminary experiments and the results
of a literature review, the reliability of a single gene marker
appeared to be low. Therefore, four markers, p53, p16, p27 and
c-erbB2, were selected in order to distinguish between cases of
MPLC and IPM, according to the early or late emergence of the gene
mutation, the stability of the mutation and the correlation with
prognosis. In the present study, the results indicated that when
the difference between two tumors was >90, the patient could be
newly classified as having MPLC. The 14 patients diagnosed with
MPLCs of different histological types had a sum value of >90 for
the differential expression ratios of the four proteins, which was
concomitant with our hypothesis. By contrast, when the difference
was <90, the patient was newly classified with IPM. Therefore,
36 of the MPLCs cases of the same histological type and 20 of IPM
(based on the Martini and Melamed criteria) were reclassified using
this novel criteria.
The results of the present study demonstrated that
IHC analyses of differential protein expression profiles of
multiple genes can be used to indicate the clonal origins of
multiple separate tumors, and therefore facilitate the
discrimination between a secondary primary cancer and IPM. As a
classical pathological examination method, IHC has a number of
merits, including convenience and sensitivity. For the patients
diagnosed with MPLCs, particularly those with the same histological
type, it was challenging to determine a correct diagnosis of MPLC
or IPM based entirely on the Martini and Melamed criteria (3). Therefore, an IHC test should be
performed in order to confirm the correctly diagnosed ratios. The
quantitative differentially-expressed gene mathematical model is
considered to be a useful approach for distinguishing between MPLCs
of the same histological type and IPM. The precise discrimination
between MPLC and IPM should enable rationalized treatment
strategies, and improve the prognoses of the affected patients.
However, as the number of analyzed cases in the present study was
relatively small, future studies with larger cohorts will be
required in order to confirm these results.
Acknowledgements
This study was supported by grants from the National
Science Foundation (no. 81071929) and the Wu Jieping Medical
Foundation (no. 320.6799.1120).
References
|
1
|
Ferguson MK, DeMeester TR, DesLauriers J,
et al: Diagnosis and management of synchronous lung cancers. J
Thorac Cardiovasc Surg. 89:378–385. 1985.PubMed/NCBI
|
|
2
|
Adebonojo SA, Moritz DM and Danby CA: The
results of modern surgical therapy for multiple primary lung
cancers. Chest. 112:693–701. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martini N and Melamed MR: Multiple primary
lung cancers. J Thorac Cardiovasc Surg. 70:606–612. 1975.PubMed/NCBI
|
|
4
|
Sugio K, Kishimoto Y, Virmani AK, et al:
K-ras mutations are a relatively late event in the pathogenesis of
lung carcinomas. Cancer Res. 54:5811–5815. 1994.PubMed/NCBI
|
|
5
|
Kishimoto Y, Sugio K, Hung JY, et al:
Allele-specific loss in chromosome 9p loci in preneoplastic lesions
accompanying non-small-cell lung cancers. J Natl Cancer Inst.
87:1224–1229. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wistuba II, Behrens C, Milchgrub S, Bryant
D, Hung J, Minna JD and Gazdar AF: Sequential molecular
abnormalities are involved in the multistage development of
squamous cell lung carcinoma. Oncogene. 18:643–650. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aoyagi Y, Yokose T, Minami Y, et al:
Accumulation of losses of heterozygosity and multistep
carcinogenesis in pulmonary adenocarcinoma. Cancer Res.
61:7950–7954. 2001.PubMed/NCBI
|
|
8
|
Strong MS, Incze J and Vaughan CW: Field
cancerization in the aerodigestive tract - its etiology,
manifestation, and significance. J Otolaryngol. 13:1–6.
1984.PubMed/NCBI
|
|
9
|
Matsuzoe D, Hideshima T, Ohshima K, et al:
Discrimination of double primary lung cancer from intrapulmonary
metastasis by p53 gene mutation. Br J Cancer. 79:1549–1552. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hiroshima K, Toyozaki T, Kohno H, Ohwada H
and Fujisawa T: Synchronous and metachronous lung carcinomas:
molecular evidence for multicentricity. Pathol Int. 48:869–876.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitsudomi T, Yatabe Y, Koshikawa T, et al:
Mutations of the P53 tumor suppressor gene as clonal marker for
multiple primary lung cancers. J Thorac Cardiovasc Surg.
114:354–360. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mountain CF: Revisions in the
International System for Staging Lung Cancer. Chest. 111:1710–1717.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsukamoto S, Sugio K, Sakada T, et al:
Reduced expression of cell-cycle regulator p27 (Kip1) correlates
with a shortened survival in non-small cell lung cancer. Lung
Cancer. 34:83–90. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Osaki T, Mitsudomi T, Oyama T, Nakanishi R
and Yasumoto K: Serum level and tissue expression of c-erbB-2
protein in lung adenocarcinoma. Chest. 108:157–162. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dobashi K, Sugio K, Osaki T, Oka T and
Yasumoto K: Micrometastatic P53-positive cells in the lymph nodes
of non-small-cell lung cancer: prognostic significance. J Thorac
Cardiovasc Surg. 114:339–346. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taga S, Osaki T, Ohgami A, et al:
Prognostic value of the immunohistochemical detection of p16INK4
expression in non small cell lung carcinoma. Cancer. 80:389–395.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pommier RF, Vetto JT, Lee JT and Johnston
KM: Synchronous non-small cell lung cancers. Am J Surg.
171:521–524. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mathisen DJ, Jensik RJ, Faber LP and
Kittle CF: Survival following resection for second and third
primary lung cancers. J Thorac Cardiovasc Surg. 88:502–510.
1984.PubMed/NCBI
|
|
19
|
Rosengart TK, Martini N, Ghosn P and Burt
M: Multiple primary lung carcinomas: prognosis and treatment. Ann
Thorac Surg. 52:773–779. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lau DH, Yang B, Hu R and Benfield JR:
Clonal origin of multiple lung cancers: K-ras and p53 mutations
determined by nonradioisotopic single-strand conformation
polymorphism analysis. Diagn Mol Pathol. 6:179–184. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Christiani DC, Mark EJ, et al:
Carcinogen exposure, p53 alteration, and K-ras mutation in
synchronous multipleprimary lung carcinoma. Cancer. 85:1734–1739.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ribeiro U, Safatle-Ribeiro AV, Posner MC,
et al: Comparative p53 mutational analysis of multiple primary
cancers of the upper aerodigestive tract. Surgery. 120:45–53. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leong PP, Rezai B, Koch WM, et al:
Distinguishing second primary tumors from lung metastases in
patients with head and neck squamous cell carcinoma. J Natl Cancer
Inst. 90:972–977. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Califano J, Leong PL, Koch WM, et al:
Second esophageal tumors in patients with head and neck squamous
cell carcinoma: an assessment of clonal relationships. Clin Cancer
Res. 5:1862–1867. 1999.PubMed/NCBI
|
|
25
|
van Oijen MG, Leppers Vd Straat FG,
Tilanus MG and Slootweg PJ: The origins of multiple squamous cell
carcinoma in the aerodigestive tract. Cancer. 88:884–893. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimizu S, Yatabe Y, Koshikawa T, et al:
High frequency of clonally related tumors in cases of multiple
synchronous lung cancers as revealed by molecular diagnosis. Clin
Cancer Res. 6:3994–3999. 2000.PubMed/NCBI
|
|
27
|
Sozzi G, Miozzo M, Pastorino U, et al:
Genetic evidence for an independent origin of multiple
preneoplastic and neoplastic lung lesions. Cancer Res. 55:135–140.
1995.PubMed/NCBI
|
|
28
|
van der Sijp JR, van Meerbeeck JP, Maat
AP, et al: Determination of the molecular relationship between
multiple tumors within one patient is of clinical importance. J
Clin Oncol. 20:1105–1114. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang YL, Wu CT, Lin SC, Hsiao CF, Jou YS
and Lee YC: Clonality and prognostic implications of p53 and
epidermal growth factor receptor somatic aberrations in multiple
primary lung cancers. Clin Cancer Res. 13:52–58. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mitsudomi T, Hamajima N, Ogawa M and
Takahashi T: Prognostic significance of p53 alterations in patients
with non-small cell lung cancer: a meta-analysis. Clin Cancer Res.
6:4055–4063. 2000.PubMed/NCBI
|
|
31
|
Mogi A and Kuwano H: TP53 mutations in
nonsmall cell lung cancer. J Biomed Biotechnol. 2011:5839292011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sasai K, Sukezane T, Yanagita E, et al:
Oncogene-mediated human lung epithelial cell transformation
produces adenocarcinoma phenotypes in vivo. Cancer Res.
71:2541–2549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iwakawa R, Kohno T, Anami Y, et al:
Association of p16 homozygous deletions with clinicopathologic
characteristics and EGFR/KRAS/p53 mutations in lung adenocarcinoma.
Clin Cancer Res. 14:3746–3753. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Otterson GA, Kratzke RA, Coxon A, Kim YW
and Kaye FJ: Absence of p16INK4 protein is restricted to the subset
of lung cancer lines that retains wildtype RB. Oncogene.
9:3375–3378. 1994.PubMed/NCBI
|
|
35
|
Lou-Qian Z, Rong Y, Ming L, et al: The
prognostic value of epigenetic silencing of p16 gene in NSCLC
patients: a systematic review and meta-analysis. PLoS One.
8:e549702013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Esposito V, Baldi A, De Luca A, et al:
Prognostic role of the cyclin-dependent kinase inhibitor p27 in
non-small cell lung cancer. Cancer Res. 57:3381–3385.
1997.PubMed/NCBI
|
|
37
|
Catzavelos C, Tsao MS, DeBoer G, et al:
Reduced expression of the cell cycle inhibitor p27Kip1 in non-small
cell lung carcinoma: a prognostic factor independent of Ras. Cancer
Res. 59:684–688. 1999.PubMed/NCBI
|
|
38
|
Zhuang Y, Yin HT, Yin XL, Wang J and Zhang
DP: High p27 expression is associated with a better prognosis in
East Asian non-small cell lung cancer patients. Clin Chim Acta.
412:2228–2231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kristiansen G, Yu Y, Petersen S, et al:
Overexpression of c-erbB2 protein correlates with disease-stage and
chromosomal gain at the c-erbB2 locus in non-small cell lung
cancer. Eur J Cancer. 37:1089–1095. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Turken O, Kunter E, Cermik H, Isitmangil
T, Kandemir G, Yaylaci M and Ozturk A: Prevalence and prognostic
value of c-erbB2 expression in non-small cell lung cancer (NSCLC).
Neoplasma. 50:257–261. 2003.PubMed/NCBI
|
|
41
|
Gazdar AF and Minna JD: Multifocal lung
cancers - clonality vs field cancerization and does it matter? J
Natl Cancer Inst. 101:541–543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dali C, Huan X and Guowei C: Differential
diagnosis of multiple tumor: multiple primary cancer or metastatic
cancer of lung. Chin J Cancer Prev Treat. 9:721–724. 2014.
|