Introduction
Cell apoptosis is a form of programmed cell death.
Such cell death is actively regulated by mitochondria-related
intrinsic signaling or death receptor-stimulated extrinsic
signaling, which induces a cascade of caspase-mediated photolytic
degradation of their substrates (1–2).
Consequently, during the process of apoptosis, cells undergo a
series of morphological and biochemical changes (3); these have formed the basis for a
number of methods of apoptotic cell detection (4). For example, the majority of apoptotic
cells exhibit distinct changes in nuclear morphology, including
nuclear condensation and fragmentation, which may be easily
detected by propidium iodide (PI) or 4′,6-diamidine-2′-phenylindole
dihydrochloride (DAPI) staining (5–6). The
egression of phosphatidylserine from within the cell membrane to
the cell surface during apoptosis provides a biological marker for
apoptotic cells; this can be detected by Annexin V staining. In
addition, during apoptosis, the cytoskeleton undergoes a clear
reorganization, which is considered to be an important
characteristic of apoptotic cells (7–9). In
the process of tumorigenesis, apoptosis is inhibited, therefore,
the detection of apoptosis is important for evaluation the efficacy
of chemotherapy drugs. Double staining with V-FITC/PI and DAPI are
classic apoptosis detection methods, however, a more specific
method is required. Few methods using fluorescent-labelled
cytoskeletal components in apoptosis have been reported, therefore,
in the present study, alterations in the cytoskeleton during
apoptosis were examined using fluorescein-conjugated
anti-cytokeratin (CK) 8/18 staining.
Materials and methods
Chemicals
Roswell Park Memorial Institute (RPMI) 1640 medium
was purchased from Invitrogen (Carlsbad, CA, USA). Fetal bovine
serum (FBS) was obtained from Hyclone (Logan, UT, USA). DAPI was
obtained from Sigma-Aldrich (St. Louis, MO, USA). Cisplatin was
purchased from Shandong Qilu Medical Biotechnology (Beijing,
China). The Annexin-fluorescein isothiocyanate (FITC) apoptosis
detection kit was purchased from Beijing Baosai Biotechnology
(Beijing, China). Anti-ck8/18 antibody was purchased from Abcam
(Cambridge, MA, USA, catalog no. ab32118)
Cell culture and treatment
The human gastric cancer cell line SGC-7901
(Shanghai Institute of Biological Science, Chinese Academy of
Sciences; Shanghai, China) was cultured in RPMI 1640 medium
supplemented with 10% FBS, and incubated in an atmosphere of 100%
humidity and 5% CO2, at 37°C. For treatment with
cisplatin, cells were plated in 6-well plates at a density of
1.5×105 cells/well in 2 ml RPMI 1640. Following a 24 h
incubation, the medium was replaced with medium containing
cisplatin at a final concentration of 20 μM to induce apoptosis.
Control cells were treated with cisplatin-free medium. Cells were
incubated at 37°C for the indicated time (12 or 24 h) and
subsequently collected by centrifugation for 5 min at 300 × g at
4°C for further analysis (10).
Detection of apoptosis by flow
cytometry
To evaluate apoptosis in the cells, an Annexin
V-FITC apoptosis detection kit was used according to manufacturer’s
instructions. Cells were collected by centrifugation for 5 min at
300 × g at 4°C and resuspended in 200 μl binding buffer (part of
the Annexin V-FITC apoptosis detection kit; Beijing Baosai
Biotechnology). Annexin V-FITC (10 μl) and PI (5 μl) were added to
the suspension and incubated for 15 min at room temperature. A
further 300 μl binding buffer was subsequently added, and apoptosis
was detected using a flow cytometer (Beckman; Palo Alto, CA, USA)
to identify Annexin V+ and or PI+ cells. Experiments were performed
in triplicate. The apoptosis rate was calculated in accordance with
the formula: (Number of early apoptosis cells + number of late
apoptosis cells)/total cell number.
Immunofluorescence and fluorescence
microscopic imaging
Cells were placed on glass slides by cytospinning at
1,000 rpm for 10 min at 4°C and fixed with 4% paraformaldehyde,
prior to permeabilization with 0.06% of triton X-100 and blocking
in 2% bovine serum albumin. Following overnight incubation with
fluorescein-conjugated anti-CK8/18 antibody at 4°C, the cell nuclei
were counterstained with 10 μl DAPI (stock solution, 1:1
DAPI:glycerol) (11).
Statistical analysis
All statistical comparisons were performed using
SPSS software, version 16.0 (IBM, Armonk, NY, USA). The Student’s
t-test was used to compare differences between the two groups or
association. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cisplatin effectively induces apoptosis
in SGC-7901 cells
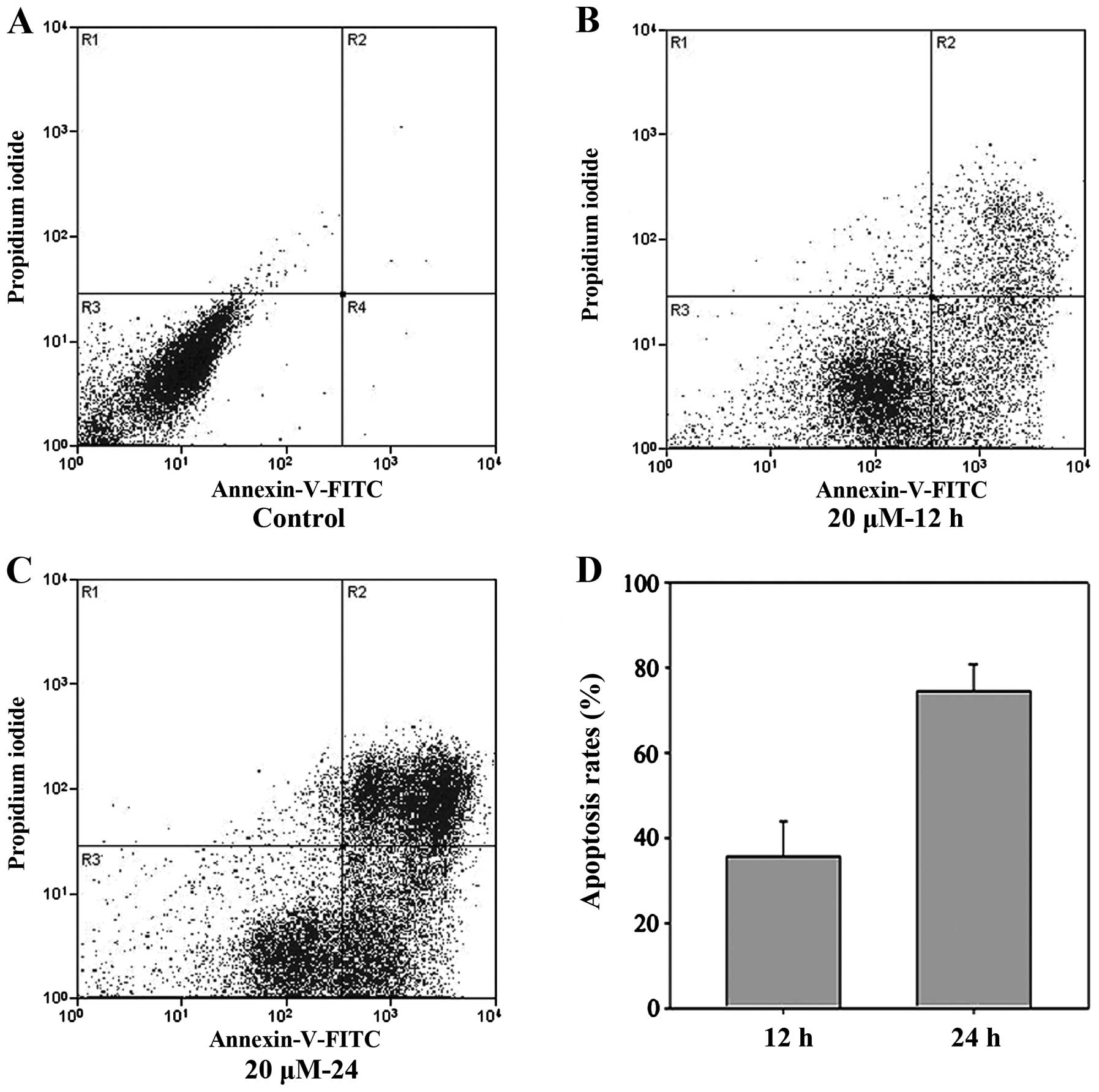
The percentage of apoptotic SGC-7901 cells induced
by cisplatin was evaluated by Annexin V and PI staining followed by
flow cytometric analysis. The results indicated that cisplatin was
able to effectively induce apoptosis in SGC-7901 cells and that the
rate of apoptosis was time dependent. The rate of apoptosis at 12 h
(36.37±3.11%) was lower than that at 24 h (75.87±4.11%) (P<0.01;
Fig. 1); this difference was
significant (P<0.05). Additionally, the difference between the
control and cisplatin-treated cells was also significant
(P<0.05).
Fluorescein-conjugated anti-CK8/18
antibody staining revealed a unique cytoskeletal pattern in
apoptotic cells
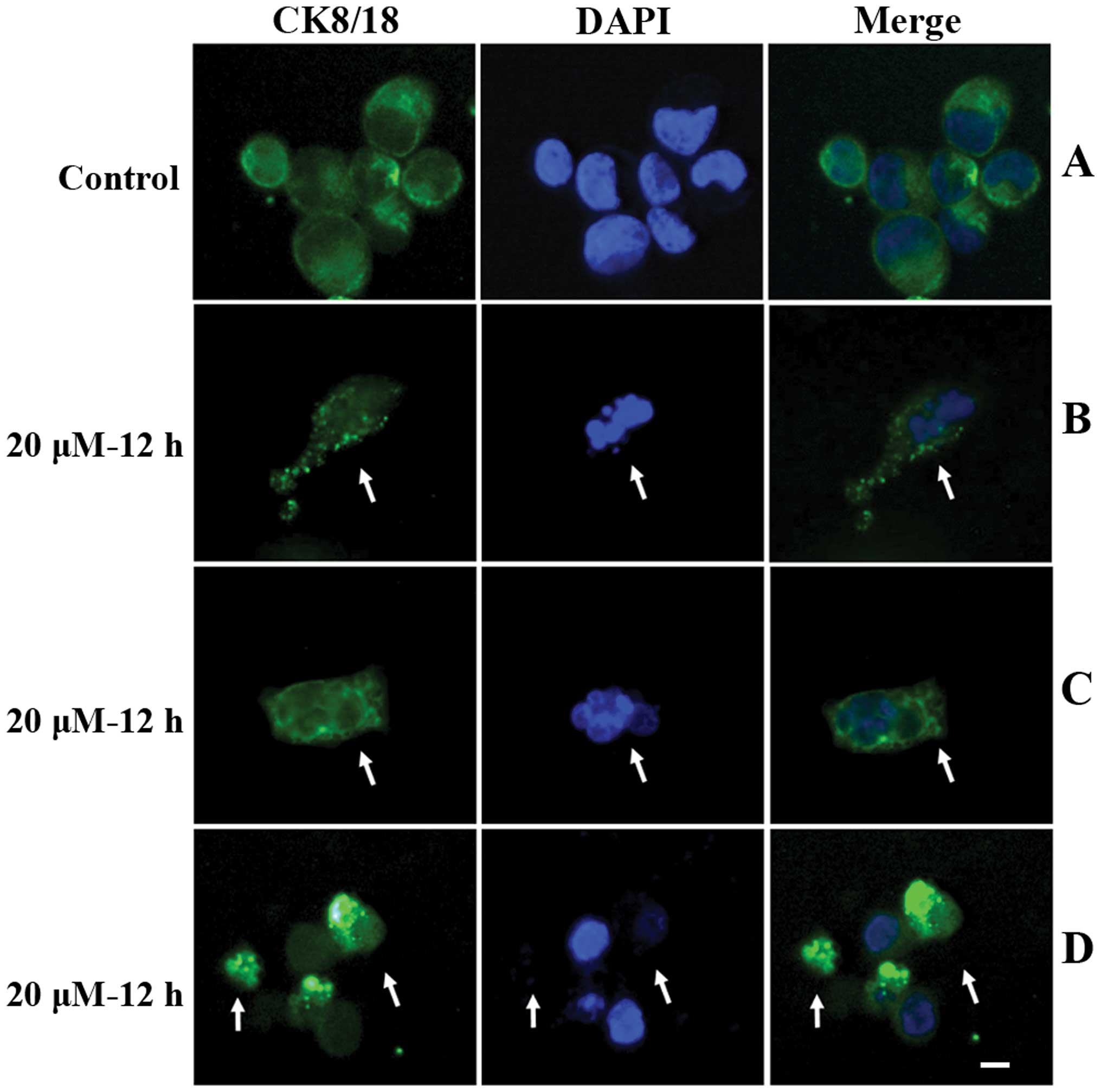
To study the alterations in the cytoskeleton during
apoptosis, SGC-7901 cells were collected following a 12 h
incubation with 20 μM cisplatin. Cells were placed on slides by
cytospinning and stained with fluorescein-conjugated anti-CK8/18
antibody (green) and DAPI (blue). The results are shown in Fig. 2 (arrows). In vehicle-treated control
cells, very few apoptotic cells were detected (rate of apoptosis,
3.64±2.85%) (Fig. 2A), and CK8/18
was uniformly distributed in cytosol of almost all cells. However,
in cisplatin treated cells, DAPI staining revealed a population of
cells with a condensed and/or fragmented nucleus, indicating
apoptosis. In these cells, punctate and/or bubbly CK8/18 staining
was observed in the cytosol (Fig. 2B
and C, arrow). Additionally, in cisplatin treated cells, a
subset of late apoptotic cells with a split nucleus and weak
nuclear DAPI staining also exhibited punctate, bubbly or aggregated
cytosol distribution of fluorescein-conjugated anti-CK8/18 antibody
(Fig. 2D, arrows).
Discussion
CKs are major structural proteins of epithelial
cells, which belong to the intermediate filaments family. They are
highly conserved between species and are important for maintaining
the integrity and continuity of epithelial cells. CKs comprise at
least 20 members that are classified into two categories: The
acidic type I group (CK9-CK20) and the neutral-basic type II group
(CK1-CK8). Type I CKs are co-expressed with type II CKs to form
heterodimers (12–13).
CK18 is a type I keratin, encoded by a gene located
on chromosome 12q13. Its complementary type II keratin is CK8. In
the absence of CK8, CK18 is degraded and keratin intermediate
filaments are not formed (14). CK8
and 18 are co-expressed and serve as structural molecules that
maintain cytoplasmic structure, resist external stresses, and
participate in a number of cellular processes (14). These CKs are primarily expressed in
adult epithelial organs, including the liver, lung,
gastrointestinal tract and kidney, and also in cancer cells arising
from these tissues (15–17). They are involved in the regulation
of tumor metastasis, and have also been associated with progression
and prognosis in various types of epithelial cancers. CK8/18 may
therefore act as a diagnostic marker (18–19).
In esophageal squamous cell carcinoma, CK8/18 expression is
significantly increased in the advanced stages of the disease
(20–21). Similar phenomena are observed in
other malignant diseases, including gastric cancer and lung cancer
(22). The present study used the
gastric cancer cell line SGC-7901 as a model, in which high levels
of cytosolic CK8/18 expression were observed, as indicated by
immunofluorescence staining (Fig.
2A).
Apoptosis is one of the major types of programmed
cell death. During this process, cells experience a series of
morphological and biochemical changes, including membrane blebbing,
cell shrinkage, cytoskeletal rearrangement, nuclear fragmentation,
chromatin condensation, and chromosomal DNA fragmentation (23). The majority of methods used for the
detection of apoptotic cells are based on these changes. Annexin
V-FITC/PI-flow cytometry method is a standard detection method, and
is capable of distinguishing early apoptotic, late apoptotic and
necrotic cells (10,24). Annexin V is a calcium-dependent
phospholipid binding protein. It binds to the membrane component,
phosphatidylserine, which is redistributed to the extracellular
surface of the cell membrane during apoptosis. PI is a nucleic acid
dye. In the present study, Annexin V-FITC/PI-flow cytometry was
used to confirm that 20 μM cisplatin was able to effectively induce
apoptosis in SGC-7901 cells. DAPI staining revealed that nuclear
changes occurred in a population of apoptotic cells.
Cytoskeletal rearrangement during apoptosis was
investigated using a fluorescein-conjugated anti-CK8/18 antibody.
Distinctive cytoskeletal patterning was observed in cells with
specific apoptotic nuclear alterations and also in late apoptotic
cells displaying a split nucleus and weak nuclear DAPI staining
(Fig. 2B–D, arrows), distinct from
that of living cells.
In conclusion, fluorescein-conjugated anti-CK8/18
antibody staining is a novel and effective technique for apoptotic
cell detection, particularly in tissues with high levels of CK8/18
expression. Our findings indicate that immunofluorescence analysis
of cytokeratin 8/18 staining is a sensitive assay for detecting
cell apoptosis. As this study is preliminary, further research is
required to confirm these results. In the future, studies
investigating whether other pro-apoptotic chemicals may induce
these types of changes would be extremely beneficial.
Acknowledgements
This work was supported by the Fundamental Research
Funds for the Central Universities (grant no. lzujbky-2013-150),
the Science and Technology Plan Projects in Gansu Province (grant
no. 1308RJYA071) and the Research Program of the First Hospital of
Lanzhou University (grant no. ldyyynlc201101).
References
|
1
|
Burikhanov R, Shrestha-Bhattarai T, Qiu S,
et al: Novel mechanism of apoptosis resistance in cancer mediated
by extracellular PAR-4. Cancer Res. 73:1011–1019. 2013. View Article : Google Scholar :
|
|
2
|
Li J, Quan H, Liu Q, et al: Alterations of
axis inhibition protein 1 (AXIN1) in hepatitis B virus-related
hepatocellular carcinoma and overexpression of AXIN1 induces
apoptosis in hepatocellular cancer cells. Oncol Res. 20:281–288.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fuchs Y, Brown S, Gorenc T, et al:
Sept4/ARTS regulates stem cell apoptosis and skin regeneration.
Science. 341:286–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cavallo F, Feldman DR and Barchi M:
Revisiting DNA damage repair, p53-mediated apoptosis and cisplatin
sensitivity in germ cell tumors. Int J Dev Biol. 57:273–280. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang AC, Lien JC, Lin MW, et al:
Tetrandrine induces cell death in SAS human oral cancer cells
through caspase activation-dependent apoptosis and LC3-I and LC3-II
activation-dependent autophagy. Int J Oncol. 43:485–494.
2013.PubMed/NCBI
|
|
6
|
Daniel B and DeCoster MA: Quantification
of sPLA2-induced early and late apoptosis changes in neuronal cell
cultures using combined TUNEL and DAPI staining. Brain Res Brain
Res Protoc. 13:144–150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grzanka D, Marszałek A, Izdebska M, et al:
Actin cytoskeleton reorganization correlates with cofilin nuclear
expression and ultrastructural changes in cho aa8 cell line after
apoptosis and mitotic catastrophe induction by doxorubicin.
Ultrastruct Pathol. 35:130–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ndozangue-Touriguine O, Hamelin J and
Bréard J: Cytoskeleton and apoptosis. Biochem Pharmacol. 76:11–18.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schietke R, Bröhl D, Wedig T, et al:
Mutations in vimentin disrupt the cytoskeleton in fibroblasts and
delay execution of apoptosis. Eur J Cell Biol. 85:1–10. 2006.
View Article : Google Scholar
|
|
10
|
Dong Q, Zhang J, Hendricks DT and Zhao X:
GROβ and its downstream effector EGR1 regulate cisplatin-induced
apoptosis in WHCO1 cells. Oncol Rep. 25:1031–1037. 2011.PubMed/NCBI
|
|
11
|
Wang B, Hendricks DT, Wamunyokoli F and
Parker MI: A growth-related oncogene/CXC chemokine receptor 2
autocrine loop contributes to cellular proliferation in esophageal
cancer. Cancer Res. 66:3071–3077. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nguyen TT, Kreisel FH, Frater JL and
Bartlett NL: Anaplastic large-cell lymphoma with aberrant
expression of multiple cytokeratins masquerading as metastatic
carcinoma of unknown primary. J Clin Oncol. 31:e443–e445. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yilmaz Y: Cytokeratins in hepatitis. Clin
Chim Acta. 412:2031–2036. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weng YR, Cui Y and Fang JY: Biological
functions of cytokeratin 18 in cancer. Mol Cancer Res. 10:485–493.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heo CK, Hwang HM, Ruem A, et al:
Identification of a mimotope for circulating anti-cytokeratin 8/18
antibody and its usage for the diagnosis of breast cancer. Int J
Oncol. 42:65–74. 2013.
|
|
16
|
Wang Y, Zhu JF, Liu YY and Han GP: An
analysis of cyclin D1, cytokeratin 5/6 and cytokeratin 8/18
expression in breast papillomas and papillary carcinomas. Diagn
Pathol. 8:82013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song DG, Kim YS, Jung BC, et al: Parkin
induces upregulation of 40S ribosomal protein SA and
posttranslational modification of cytokeratins 8 and 18 in human
cervical cancer cells. Appl Biochem Biotechnol. 171:1630–1638.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ekman S, Eriksson P, Bergström S, et al:
Clinical value of using serological cytokeratins as therapeutic
markers in thoracic malignancies. Anticancer Res. 27:3545–3553.
2007.PubMed/NCBI
|
|
19
|
Szturmowicz M: Cytokeratins - tissue and
biochemical markers of non-small cell lung cancer. Pneumonol
Alergol Pol. 75:315–316. 2007.(In Polish).
|
|
20
|
Makino T, Yamasaki M, Takeno A, et al:
Cytokeratins 18 and 8 are poor prognostic markers in patients with
squamous cell carcinoma of the oesophagus. Br J Cancer.
101:1298–1306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fillies T, Werkmeister R, Packeisen J, et
al: Cytokeratin 8/18 expression indicates a poor prognosis in
squamous cell carcinomas of the oral cavity. BMC Cancer. 6:102006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagashio R, Sato Y, Matsumoto T, et al:
Significant high expression of cytokeratins 7, 8, 18, 19 in
pulmonary large cell neuroendocrine carcinomas, compared to small
cell lung carcinomas. Pathol Int. 60:71–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lukamowicz M, Kirsch-Volders M, Suter W
and Elhajouji A: In vitro primary human lymphocyte flow cytometry
based micronucleus assay: simultaneous assessment of cell
proliferation, apoptosis and MN frequency. Mutagenesis. 26:763–770.
2011. View Article : Google Scholar : PubMed/NCBI
|