Introduction
Ultrasound (US) is a diagnostic tool used
clinically, however, this technique is also used therapeutically.
US therapy has been clinically investigated as a tumor treatment
with a particular focus on the thermal effects generated by
high-intensity focused US (1).
However, few clinical studies have investigated the non-thermal
mechanisms induced by low-frequency US, such as acoustic cavitation
(2–4). Ultrasonic transducers with a frequency
within the kHz range produce the most evident cavitation effects
(5). In vitro cavitation
studies have demonstrated the ability of US to induce morphological
changes, plasma membrane wounding (6) and cell lysis (7). A previous study showed that cavitation
induced by 24 kHz US resulted in a loss of viability of cellular
membranes, apoptotic death and immediate necrosis (8). Furthermore, in vivo study on
organs that contain air, such as the lungs, has demonstrated that
US may cause tissue hemorrhage (9).
In vivo cavitation may alter the permeability of individual
cells, leading to improved delivery of genes and drugs (10). Inertial cavitation has also been
found to lead to marked destructive effects in vivo, as
shown by reduced rates of tumor growth (11,12)
and increased survival time of mice (13). Our previous findings indicated that
low-intensity US with microbubbles (MBs) may exhibit
anti-angiogenic effects on subcutaneous tumors in nude mice
(14,15). In vitro and in vivo
bioeffects of kHz-range US have been reported previously. However,
the application of US in combination with microbubbles in a
clinical setting remains unclear. The aim of the present study was
to investigate the clinically therapeutic effect of 20 kHz US
combined with MBs for the treatment of hepatic carcinoma.
Case report
Clinical data
A 71-year-old male presented to Nantong University
Affiliated Nantong Tumor Hospital (Nantong, China) with abdominal
pain and weight loss. Laboratory tests showed elevated carbohydrate
antigen 19-9 (CA19-9) levels (2,007 U/ml; normal range, 0–39 U/ml)
and negativity for hepatitis B virus-related antigens and
antibodies. However, α-fetoprotein (1.5 ng/ml; normal range, 0–20
ng/ml), carcinoembryonic antigen (1.77 ng/ml; normal range, 0–5
ng/ml), alanine aminotransferase (25 U/ml; normal range, 4–40
U/ml), alkaline phosphatase (84 U/ml; normal range, 66–220 U/ml)
and γ-glutamyl transferase (49 U/ml; normal range, 8–87 U/ml) were
within the normal ranges. Imaging examinations including
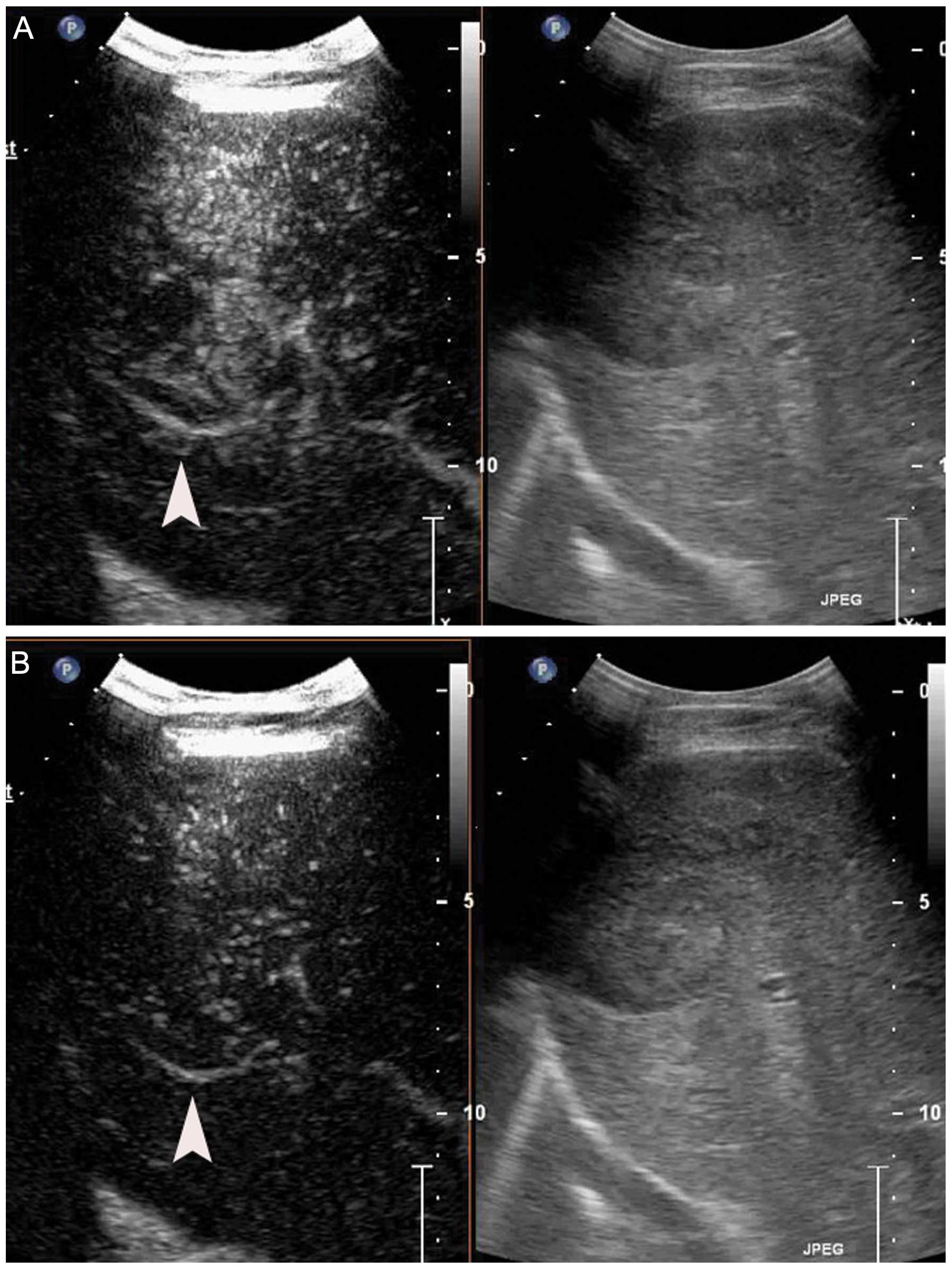
contrast-enhanced US (CEUS) (Fig.
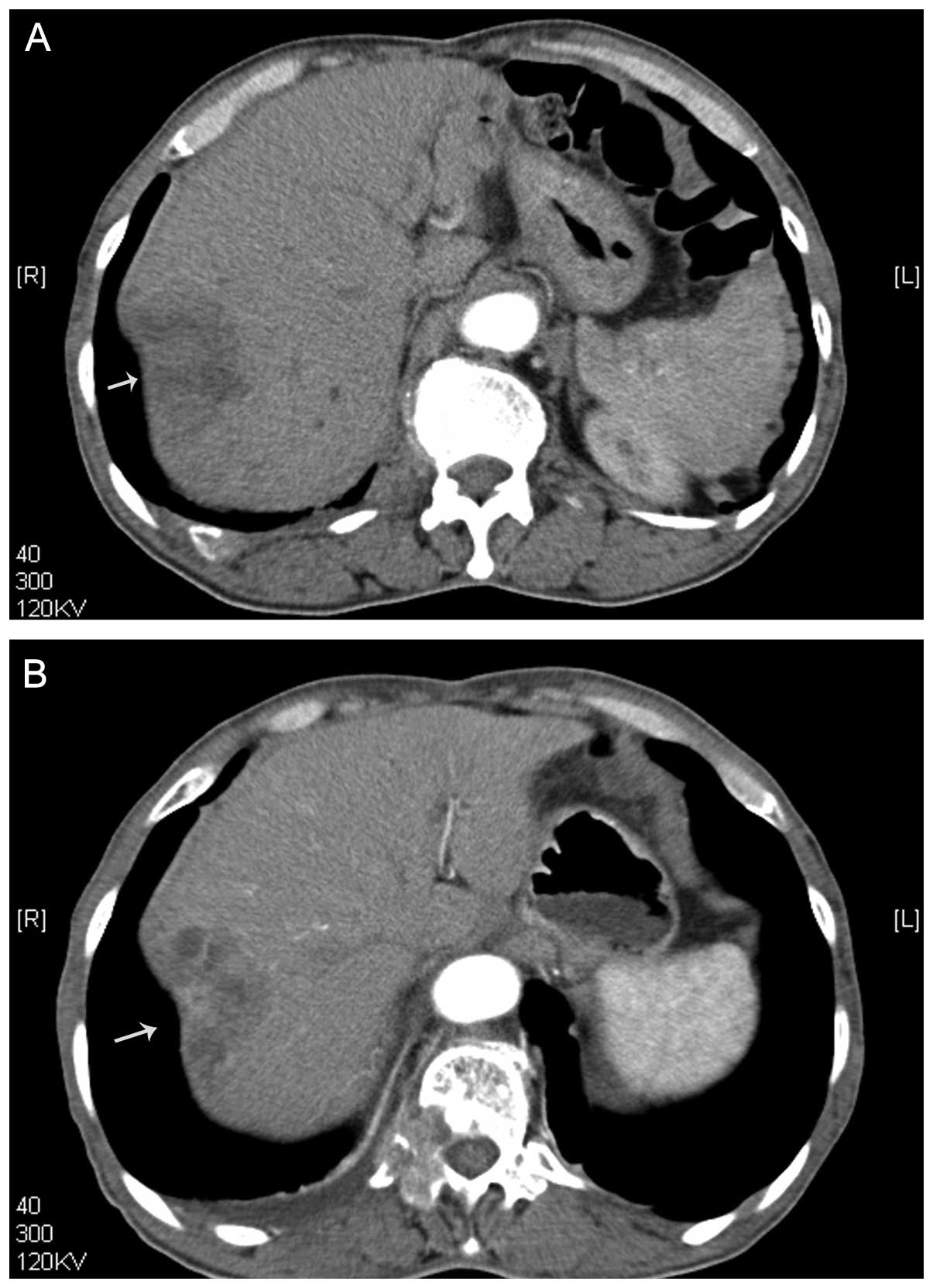
1) and computed tomography (CT) (Fig. 2) identified a heterogeneously
enhancing liver tumor in the hepatic arterial phase. A malignant
tumor was suspected and an intrahepatic cholangiocarcinoma (ICC)
was clinically diagnosed. The patient was not suitable for surgery,
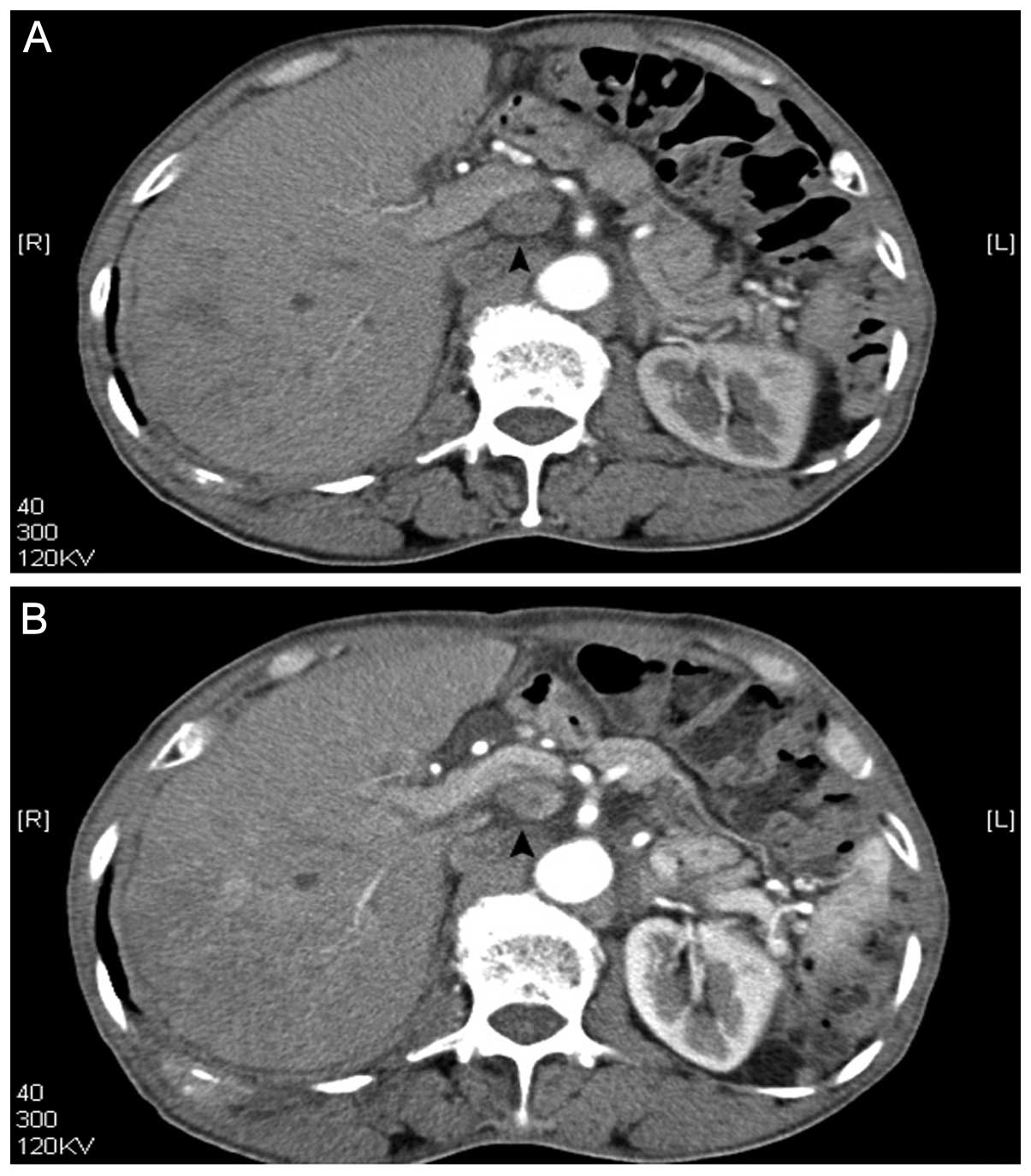
due to abdominal lymph nodes (Fig.
3) and spinal metastasis. Treatment with radiofrequency
ablation was excluded, as the tumor was located in the posterior
portion of the right lobe near the diaphragm. Doctors decided
against transarterial chemoembolization, as ICC is not suitable for
an arterial embolization (16).
Therefore, the patient was offered other palliative therapy, such
as low-frequency US. Prior to and following US treatment, CEUS and
CT imaging, as well as the examination of CA19-9 levels, were used
to evaluate the therapeutic effect. This study was approved by the
human ethics committee of Nantong University Affiliated Nantong
Tumor Hospital. Written informed consent was obtained from the
patient’s family. Clinical data, laboratory tests, imaging data, US
treatment and results were obtained from the patient’s medical
records.
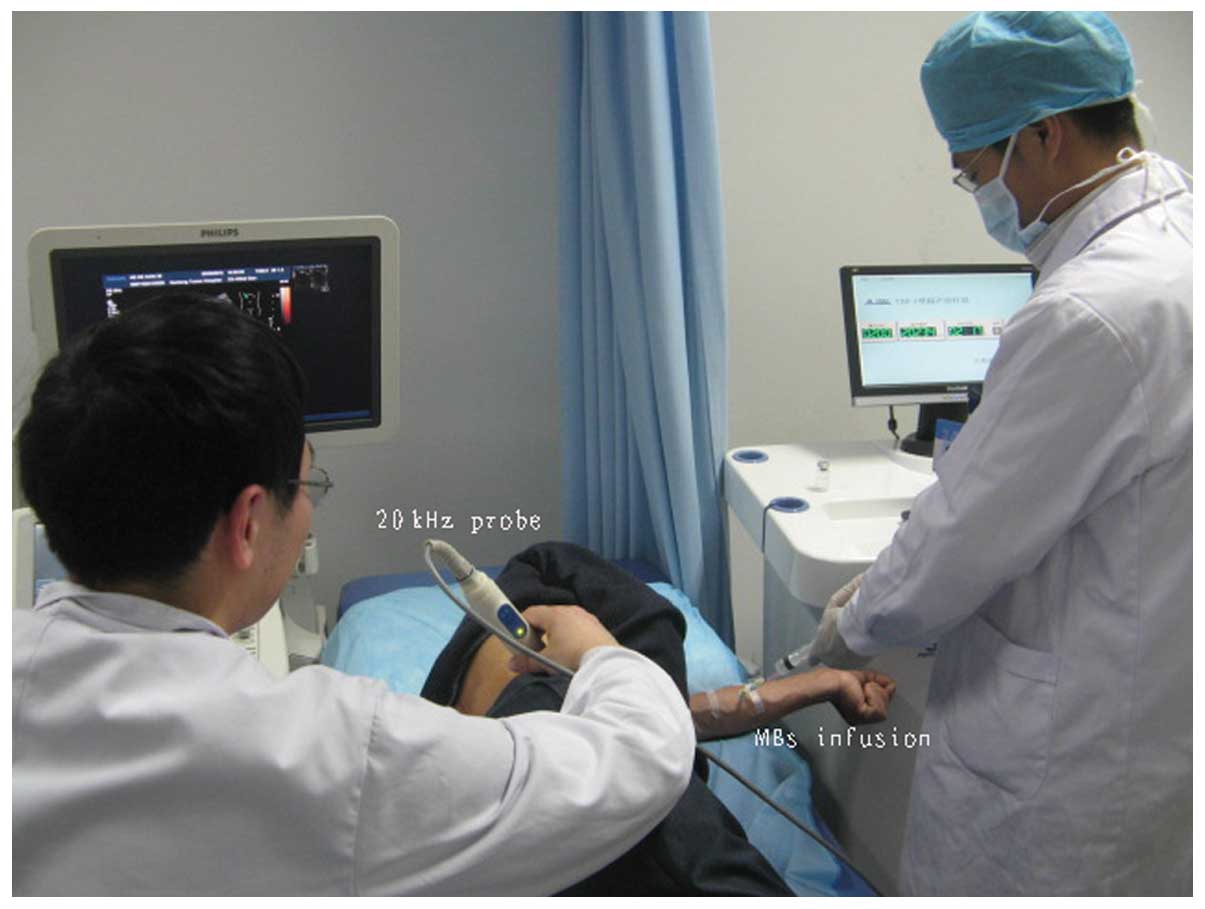
Therapeutic set-up
The tumor was exposed to low-frequency US, which was
accompanied simultaneously with MBs injected through the ulnar
vein. The low-frequency US therapeutic unit (Fig. 4) was manufactured by the Jiangsu
Hanmei Biology Technology Ltd., Co., (Taizhou, China). Prior to
treatment, the ultrasonic instrument (iU22 US system; Philips
Medical Systems, Bothell, WA, USA) was used to identify the hepatic
tumor. The tumor was subsequently sonicated using a low-frequency
US transducer. The diameter of the therapeutic US transducer was
~24 mm (Fig. 4). The probe was
placed on the patient’s skin using US transmission gel (Aquasonic
100; Parker Laboratories, Inc., Fairfield, NJ, USA), which was
interposed to ensure US propagation. The low-frequency US
parameters were set at 20 kHz, 2 W/cm2, duty cycle 40%
(on 2 sec, off 3 sec) for a duration of 5 min each day for a total
of five days. MBs of the contrast agent were administered
simultaneously with the ultrasonic irradiation by continuous
infusion through the ulnar vein (Fig.
5). Each MB was composed of a phospholipid shell containing
sulfur hexafluoride. A 5-ml dose of contrast agent was administered
for each treatment at a concentration of 1.8×109
microbubbles/ml.
CEUS
CEUS is a useful method for assessing tumor
neovascularity and monitoring anti-angiogenic therapies. In this
study, the hepatic tumor was examined by CEUS after initiation and
completion of treatment. CEUS images of the tumor were captured by
an experienced examiner using the iU22 US system (Philips Medical
Systems). The frequency of the probe was 3.5 MHz. Sulfur
hexafluoride MBs (SonoVue; Bracco, Milan, Italy) were used. The
agent (25 mg) was agitated for ~1 min with 0.9% saline solution (5
ml) and 2.4 ml suspension was injected manually as a bolus through
a 5-ml syringe placed in the vein. Following injection of the
bolus, the real-time enhancement pattern of the contrast agent was
observed inside the tumor for 3–5 min and an imaging video was
captured.
CT scanning
Multiphasic CT was performed using a 64-section
multidetector CT scanner (Somatom Sensation 64; Siemens Medical
Solutions, Erlangen, Germany) with a gantry rotation speed of 500
msec, generator of 60 KW, pitch 1–1.5, 120 kVp and 320 mAs. The
patient received a nonionic contrast medium [iomeprol, 400 mg of
iodine per milliliter (Iomeron 400; Bracco Imaging, Milan, Italy)]
at a dose of 1.3 ml (520 mg of iodine) per kilogram of body weight.
The contrast medium was administered using a dual-chamber
mechanical power injector (Stellant D CT; Medrad, Inc., Indianola,
PA, USA) at a rate of 3 ml/sec through an 18-gauge intravenous
catheter, which was inserted into an antecubital vein. This was
followed by a 40-ml saline flush at the same injection rate.
Results
The patient was treated in one session. Prior to
treatment, the tumor size on CT was 5.4 cm. After treatment, the
tumor size on the CT increased to 5.6 cm. After treatment with US
and MBs, the intensity and enhanced-areas on the CEUS and the CT
images were reduced (Figs. 1 and
2). Thus, insonation of tumors by
US and MBs may be effective in reducing blood supply to tumors.
After US treatment, abdominal lymph nodes decreased in size from
2.2 cm to 1.9 cm (Fig. 3), as shown
by CT. The CA19-9 level decreased from 2,007 U/ml prior to therapy
to 734 U/ml after therapy.
Discussion
CEUS is useful for the evaluation of anti-angiogenic
treatments. CT is also generally used for assessment of the
response to anticancer therapy, as tumor size, necrotic lesions,
vascularity and perfusion are evaluated simultaneously (17).
Although the tumor size, as shown on CT scans,
increases marginally after treatment, the intensity-enhanced area
of the tumor is decreased when compared with that prior to
treatment. Another possible reason for the increased
intensity-enhanced area observed in the tumor following therapy is
that edema may have occurred (18,19).
After treatment, the tumor exhibits a poor
parenchymal vascular network. Atrophy of the arterioles in the
parenchyma may occur, and decreased blood perfusion may decrease
the onset of tumor enhancement (14). These results lead to a reduction of
the enhanced areas in the CEUS and CT scans in the arterial phase.
Low-frequency US with a lower energy, such as a frequency of ~20
kHz, an intensity of 2 W/cm2, and a duration of ~5 min,
may improve the efficiency of anti-angiogenesis therapy.
In the present study, the MBs were injected through
the vein simultaneously with the US treatment. The MBs can function
as cavitation nuclei, which reduce the threshold of US intensity
required to induce bio-effects (20). Constrained within the blood vessels,
the MBs excited by the US not only affect the vascular endothelium
(21), but may also rupture the
vessel (20,22). In a previous study, it was found
that the confinement caused by the vessels and the surrounding
tissue did not prevent the bubbles from undergoing large volumetric
oscillations, which included inertial collapse. The blood vessels
were deformed on the same microsecond time-scale as the bubble
oscillations (21). Blood vessel
deformations on μsec timescales caused by ultrasonic cavitation
(23,24), including vessel distention,
invagination and liquid jets, may contribute to vessel rupture.
These deformations may also lead to a transient increase in
temperature that alters the fluidity of the cell membrane (25). Local deposition of such high energy
may result in cell damage that enhances the permeability of the
endothelial cell layers (26).
Another possibility is asymmetric bubble collapse and the formation
of liquid jets (21). All three
mechanisms, vessel distention, invagination and liquid jets, may
contribute to vessel rupture (24).
In our previous in vivo study, it was also found that 20 kHz
US combined with MBs lead to vascular endothelial cell wall
rupture, widened endothelial cell gaps and interstitial erythrocyte
leakage in rabbit hepatic tumors (27). Following treatment, vessel rupture
damaged the vascular network, decreased angiogenic activity, and
decreased blood perfusion. These phenomena may explain why the
onset of tumor enhancement (on CEUS and CT scans) was reduced.
In the present study, it was found that the
antitumor treatment lead to a decrease in abdominal lymph node size
on CT scans (Fig. 3).
High-frequency US (10–12 MHz) has a superior resolution compared
with that of low-frequency US on superficial organs, such as breast
and thyroid. However, the penetration of this technique is inferior
to that of low-frequency US. A total of 20 kHz US penetrates deep
into tissues, while maintaining selectivity by being focused. The
penetration of this method is better than that of higher frequency
US. This presents a unique advantage of using 20 kHz US as a
non-invasive treatment for deep tumors. The reason for the observed
decrease in lymph node size may be that low-frequency US penetrates
deeply into the tissues and focuses on the area of the abdominal
lymph node. Furthermore, therapy appears to stimulate an immune
response (28), which could thus
inhibit lymph node growth.
In this study, the post-treatment level of CA19-9
was 734 U/ml, while the pre-treatment level was 2,007 U/ml. CA19-9
is a diagnostic and prognostic factor for ICC (29,30).
The reduced levels of the serum marker indicated that the therapy
used to treat the liver tumor was effective.
Approximately 70% of patients who present with
hepatic malignant tumors are not eligible for curative treatment as
they exhibit middle or advanced stage disease, and the prognosis is
poor (31). In patients with tumors
that exhibit a rich blood supply, anti-angiogenic treatment is
required and will become widespread. Low-frequency US appears to
present a potential minimally invasive and convenient method of
tumor treatment. This modality may gain attention in the future as
a minimally invasive method for the treatment of hepatic malignant
tumors with a copious blood supply and with surgical
contraindications. As low-frequency US remains a novel technology,
to the best of our knowledge, this report is the first to
investigate the effects of low-frequency US combined with MBs for
the treatment of a patient with a hepatic malignant tumor. Future
studies are required before low-frequency US can become a
well-established method for tumor therapy.
Acknowledgements
The authors would like to thank Mrs. Xin Yue Gan and
Mr. Xun Shen for their provision of the US therapy apparatus, Mr.
Jun Yan Yang and Mr. Jia Jin Liu for providing the US contrast
agent and Mrs. Yu Qing Zhou, Mrs. Xiao Li Gu, Mrs. Jun Lian Yuan,
Mrs. Lu Hua Yang and Mrs. Yu Feng Chen for their assistance with
imaging examinations. This study was partially supported by Nantong
Municipal Science and Technology Project (grant no. HS149072).
References
|
1
|
Jung SE, Cho SH, Jang JH and Han JY:
High-intensity focused ultrasound ablation in hepatic and
pancreatic cancer: complications. Abdom Imaging. 36:185–195. 2011.
View Article : Google Scholar
|
|
2
|
Tonucci LB, Mourão DM, Ribeiro AQ and
Bressan J: Noninvasive body contouring: biological and aesthetic
effects of low-frequency, low-intensity ultrasound device.
Aesthetic Plast Surg. 38:959–967. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baron C, Aubry JF, Tanter M, Meairs S and
Fink M: Simulation of intracranial acoustic fields in clinical
trials of sonothrombolysis. Ultrasound Med Biol. 35:1148–1158.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubiera M and Alexandrov AV:
Sonothrombolysis in the management of acute ischemic stroke. Am J
Cardiovasc Drugs. 10:5–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Son Y, Lim M, Khim J and Ashokkumar M:
Acoustic emission spectra and sonochemical activity in a 36 kHz
sonoreactor. Ultrason Sonochem. 19:16–21. 2012. View Article : Google Scholar
|
|
6
|
Schlicher RK, Hutcheson JD, Radhakrishna
H, Apkarian RP and Prausnitz MR: Changes in cell morphology due to
plasma membrane wounding by acoustic cavitation. Ultrasound Med
Biol. 36:677–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miller DL and Dou C: The influence of
octyl β-D-glucopyranoside on cell lysis induced by ultrasonic
cavitation. J Acoust Soc Am. 130:3482–3848. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hutcheson JD, Schlicher RK, Hicks HK and
Prausnitz MR: Saving cells from ultrasound-induced apoptosis:
quantification of cell death and uptake following sonication and
effects of targeted calcium chelation. Ultrasound Med Biol.
36:1008–1021. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Skyba DM, Price RJ, Linka AZ, Skalak TC
and Kaul S: Direct in vivo visualization of intravascular
destruction of microbubbles by ultrasound and its local effects on
tissue. Circulation. 98:290–293. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frenkel V: Ultrasound mediated delivery of
drugs and genes to solid tumors. Adv Drug Deliv Rev. 60:1193–1208.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wood AK, Ansaloni S, Ziemer LS, et al: The
antivascular action of physiotherapy ultrasound on murine tumors.
Ultrasound Med Biol. 31:1403–1410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bunte RM, Ansaloni S, Sehgal CM, Lee WM-F
and Wood AK: Histopathological observations of the antivascular
effects of physiotherapy ultrasound on a murine neoplasm.
Ultrasound Med Biol. 32:453–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wood AK, Schultz SM, Lee WM, Bunte RM and
Sehgal CM: Antivascular ultrasound therapy extends survival of mice
with implanted melanomas. Ultrasound Med Biol. 36:853–857. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen ZY, Shen E, Zhang JZ, et al: Effects
of low-frequency ultrasound and microbubbles on
angiogenesis-associated proteins in subcutaneous tumors of nude
mice. Oncol Rep. 30:842–850. 2013.PubMed/NCBI
|
|
15
|
Shen ZY, Shen E, Diao XH, et al:
Inhibitory effects of subcutaneous tumors in nude mice mediated by
low-frequency ultrasound and microbubbles. Oncol Lett. 7:1385–1390.
2014.PubMed/NCBI
|
|
16
|
Herber S, Otto G, Schneider J, et al:
Transarterial chemoembolization (TACE) for inoperable intrahepatic
cholangiocarcinoma. Cardiovasc Intervent Radiol. 30:1156–1165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marcus C, Ladam-Marcus V, Cucu C, et al:
Imaging techniques to evaluate the response to treatment in
oncology: current standards and perspectives. Crit Rev Oncol
Hematol. 72:217–238. 2009. View Article : Google Scholar
|
|
18
|
Spina JC, Ulla M, Yeyati EL, et al: MDCT
findings after hepatic chemoembolization with DC-beads: what the
radiologist needs to know. Abdom Imaging. 38:778–784. 2013.
View Article : Google Scholar
|
|
19
|
Dierckx R, Maes A, Peeters M and Van De
Wiele C: FDG PET for monitoring response to local and locoregional
therapy in HCC and liver metastases. Q J Nucl Med Mol Imaging.
53:336–342. 2009.PubMed/NCBI
|
|
20
|
Qin S, Caskey CF and Ferrara KW:
Ultrasound contrast microbubbles in imaging and therapy: physical
principles and engineering. Phys Med Biol. 54:R27–R57. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen H, Kreider W, Brayman AA, Bailey MR
and Matula TJ: Blood vessel deformations on microsecond time scales
by ultrasonic cavitation. Phys Rev Lett. 106:0343012011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miller DL, Averkiou MA, Brayman AA, et al:
Bioeffects considerations for diagnostic ultrasound contrast
agents. J Ultrasound Med. 27:611–632. 2008.PubMed/NCBI
|
|
23
|
Chen H, Kreider W, Brayman AA, Bailey MR
and Matula TJ: Blood vessel deformations on microsecond time scales
by ultrasonic cavitation. Phys Rev Lett. 106:0343012011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen H, Brayman AA, Bailey MR and Matula
TJ: Blood vessel rupture by cavitation. Urol Res. 38:321–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Holland CK and Apfel RE: An improved
theory for the prediction of microcavitation thresholds. IEEE Trans
Ultrason Ferroelectr Freq Control. 36:204–208. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Basta G, Venneri L, Lazzerini G, et al: In
vitro modulation of intracellular oxidative stress of endothelial
cells by diagnostic cardiac ultrasound. Cardiovasc Res. 58:156–161.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen ZY, Xia GL, Wu MF, et al: The effects
of low-frequency ultrasound and microbubbles on rabbit hepatic
tumors. Exp Biol Med (Maywood). 239:747–757. 2014. View Article : Google Scholar
|
|
28
|
Zitvogel L and Kroemer G: Anticancer
effects of imatinib via immunostimulation. Nat Med. 17:1050–1051.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu ZH, Chen Z, Ma LL, Li XH and Wang LX:
Factors influencing the prognosis of patients with intrahepatic
cholangiocarcinoma. Acta Gastroenterol Belg. 75:215–218.
2012.PubMed/NCBI
|
|
30
|
Tangkijvanich P, Thong-ngam D,
Theamboonlers A, et al: Diagnostic role of serum interleukin 6 and
CA 19-9 in patients with cholangiocarcinoma.
Hepatogastroenterology. 51:15–19. 2004.PubMed/NCBI
|
|
31
|
Yoshida K, Hirokawa T, Moriyasu F, et al:
Arterial-phase contrast-enhanced ultrasonography for evaluating
anti-angiogenesis treatment: a pilot study. World J Gastroenterol.
17:1045–1050. 2011. View Article : Google Scholar : PubMed/NCBI
|