Introduction
Prostate cancer (PCa) is a heterogeneous disease. In
2012, there were an estimated 241,740 new cases and 28,170
associated mortalities, making the disease the second most commonly
diagnosed cancer and the second leading cause of cancer-associated
mortality in males (1). With the
recent developments in imaging technology and novel prostate biopsy
protocols, the diagnosis of PCa has improved markedly. At present,
radical prostatectomy is the only effective treatment for such
organ-confined tumors. Despite this, 20–30% of patients experience
recurrence following the complete removal of a tumor, which is
typically detected by a rise in serum prostate-specific antigen
levels (2,3).
Obesity, which is generally measured by the body
mass index (BMI), has become a worldwide public health problem. In
total, >1 billion people are estimated to be overweight (BMI,
≥25), and >300 million of these are considered obese (BMI, ≥30)
(4). Accumulating evidence suggests
that obesity may be associated with an increased risk of cancer at
several sites in the body (5).
However, the association between PCa and obesity remains
controversial. Certain studies have identified positive
correlations between obesity and the incidence and aggressiveness
of PCa (6,7). By contrast, other studies have not
demonstrated such correlation between obesity and an increased
incidence of PCa or associated-mortalities (8,9). The
present study therefore aimed to analyze the correlation between
obesity and the incidence and associated mortalities of PCa in an
updated meta-analysis of cohort studies. This updated analysis of
17 cohort studies will allow for improved risk estimates compared
with the previous analysis (7).
Materials and methods
Search strategy
The present systematic review was conducted
according to the Meta-analysis of Observational Studies in
Epidemiology guidelines (10). On
January 1, 2014, searches were conducted using the MEDLINE and
EMBASE databases for studies that had examined obesity- and
PCa-associated mortalities. The search strategy used medical
subject heading terms and keywords: (Obesity [All Fields] OR BMI
[All Fields] OR body mass index [All Fields] OR overweight [All
Fields] OR weight[All Fields]) and (‘prostate cancer’ [MeSH Terms]
OR ‘PCa’ [MeSH Terms] OR ‘prostatic neoplasm’ [MeSH Terms] OR
‘prostate neoplasm’ [MeSH Terms] OR ‘cancer of the prostate’ [MeSH
Terms] OR ‘prostatic cancer’ [MeSH Terms] and (risk [All Fields])
and (incidence [All Fields]) and (mortality [All Fields]) and
(epidemiological studies [All Fields]). PCa was assessed using
cancer registries, medical records, death certificates, and
ambulatory and inpatient claims. Reference-retrieved reviews,
meta-analyses and other relevant publications were also searched
for study inclusion.
Study selection
Two investigators screened titles and abstracts
according to set inclusion and exclusion criteria. The primary aim
of the present study was to confirm whether a positive association
between obesity and the incidence of PCa existed. The criteria for
study inclusion was as follows: i) A prospective or retrospective
cohort design; ii) an exposure of interest of weight or BMI at
baseline and/or at the end of the follow-up; iii) an outcome of
interest in PCa and/or fatal PCa; and iv) publication in English.
The meta-analysis included studies that reported standardized forms
of relative risk (RR), risk ratio, hazard ratio or odds ratio,
those with estimates on confidence interval (CI), or those that
used RRs to represent various effect estimates. Due to a decreased
risk of selection bias, results from cohort studies, as opposed to
case-control studies, were chosen.
The studies that were excluded from the
meta-analysis were those that consisted of a case-control design.
In addition to these criteria, reviews, meeting abstracts,
editorials and commentaries were also excluded. Other studies that
were not relevant to the meta-analysis, such as non-analytical
epidemiology studies, or those that had not considered obesity and
PCa as primary exposures and outcomes, were also excluded.
Furthermore, studies that had failed to report these estimates, or
those with only univariate estimates, were excluded. In the event
that there were multiple publications from the same study
population, only those that had combined independent studies,
examined weight or BMI as the main interest of exposure, or
presented the largest number of cases or follow-up years were
included.
Data extraction
The data were extracted independently by two
authors, and then cross-checked in order to reach a consensus. The
following variables were recorded: Last name of the first author,
publication year, country in which the study was performed,
participant characteristics, sample size, measure of association,
mean length of follow-up, variables adjusted for in the analysis
and the risk estimates with corresponding 95% CIs. From each study,
the RR estimate that was adjusted for the greatest number of
potential confounders was extracted. The quality of each study was
assessed using the nine-star Newcastle-Ottawa Scale (NOS) (11). The maximum quality score was 10
points, and studies with a quality score >5 points were
considered high quality.
Statistical analysis
The RR value was used to measure the association of
interest. In the event that the RRs were reported separately for
subgroups by different levels of BMI, the results of the subgroups
(BMI, ≥30) were combined, and a common RR was calculated for the
main analysis using a fixed-effects model. The highest levels were
defined as ‘obesity’, and an overall pooled RR was calculated for
the main analysis using a random-effects model. In studies that
included BMI values that were only divided into two open-ended
categories, the RR and CI estimates were assumed for the higher BMI
category.
Heterogeneity among studies was measured by the
Cochrane χ2 test and quantified using the
I2 metric. The random-effects model could have
lead to additional weight and wider CIs being given to smaller
studies than the fixed-effects model, and so was employed when
heterogeneity was present (12).
All analyses were conducted using the STATA statistical software
package version 11 (Stata Corp., College Station, TX, USA) with a
two-sided test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Literature search
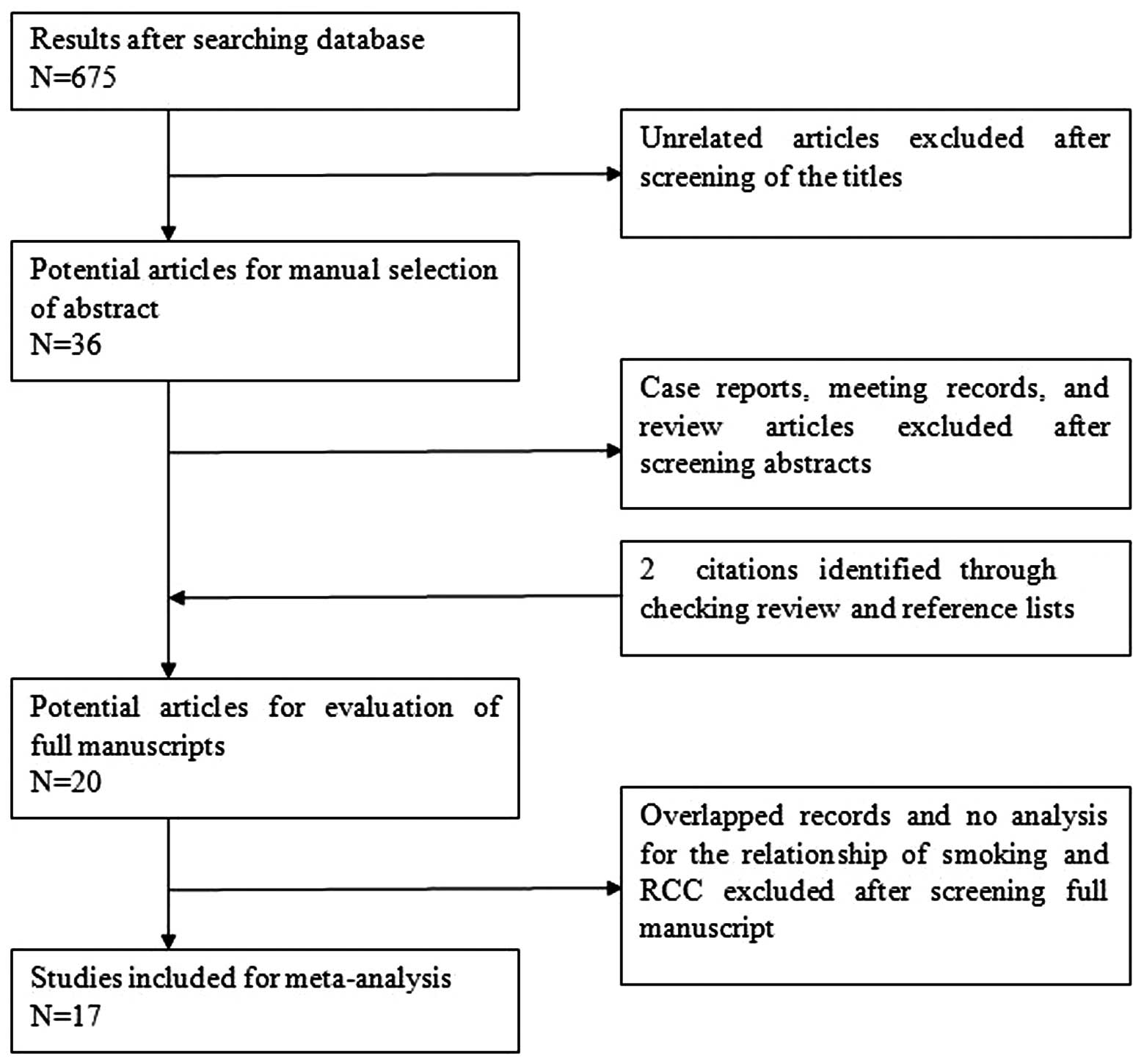
The details of the literature search are presented
in a flow diagram (Fig. 1). In
total, 675 potentially relevant studies (378 from Medline, 265 from
Embase and 32 from the Cochrane Library) were identified that had
examined the association between obesity and the incidence and
mortalities of PCa. Overall, 657 citations were excluded following
abstract- or title-based screening. Subsequently, the 18 remaining
citations, and two retrieved citations, were full-text reviewed.
Following this, three studies were removed; one was a retrospective
cohort study and the other two did not include RRs, a corresponding
95% CI of interest or provide sufficient data in order to calculate
them. Consequently, 17 studies met all eligibility criteria and
were selected for the meta-analysis.
Study characteristics and quality
assessment
The characteristics of the included studies are
shown in Table I. The 17 selected
studies were published between 1997 and 2012 from different
populations; eight from the USA, eight from Europe and one from
Oceania. The sizes of the cohorts ranged between 1,050 and 950,000
individuals. Overall, two studies were adjusted for age, and the
others were controlled for certain conventional risk factors for
PCa, including age and ethnicity. Each study used one of two
end-points, either the incidence of PCa or PCa-associated
mortality. Of the 17 cohort studies, 14 employed incidence rates,
and 10 used mortality rates as the measurement of RR. The median
follow-up time for the study participants ranged between 2 and 49
years. In the present meta-analysis, the median NOS score of the
included studies was seven, with a range between five and nine.
Furthermore, 70.59% of the studies were identified as relatively
high-quality.
 | Table IStudy characteristics. |
Table I
Study characteristics.
| First author
(ref.) | Year | Country | Cohort size, n | Age range, years | Follow-up, years | Obese, % | PCa cases, n | PCa mortalities,
n | NOS score |
|---|
| Haggstrom et
al (13) | 2012 | Norway | 289866 | 44 | 24 | 11 | 6673 | 961 | 7 |
| Bassett et al
(14) | 2012 | Australia | 16510 | 27–76 | 15 | 19 | 1374 | 140 | 6 |
| Dehal et al
(15) | 2011 | USA | 7016 | 25–74 | 17 | 15.4 | 3127 | 44 | 5 |
| Burton et al
(16) | 2010 | UK | 9549 | - | 49 | - | 211 | 111 | 6 |
| Stocks et al
(17) | 2010 | Sweden | 336159 | 30±13a | 22.2 | 5 | 10002 | 2601 | 9 |
| Pischon et
al (18) | 2008 | Europe | 129502 | 25–70 | 8.5 | 60 | 2446 | - | 5 |
| Giovannucci et
al (19) | 2007 | USA | 47750 | 40–75 | 16 | 100 | 3544 | 312 | 7 |
| Littman et
al (20) | 2007 | USA | 34754 | 50–76 | 2 | 24 | 832 | - | 8 |
| Wright et al
(21) | 2007 | USA | 287760 | 50–71 | 6 | 21 | 9986 | 173 | 7 |
| Engeland et
al (22) | 2003 | Norway | 950000 | 20–74 | 21 | - | 33140 | - | 5 |
| Calle et al
(23) | 2003 | USA | 404576 | - | 16 | - | - | 4004 | 8 |
| Discacciati et
al (9) | 2011 | Sweden | 36959 | 45–79 | 10 | 9.69 | 2084 | 225 | 8 |
| Rodriguez et
al (24) | 2001 | USA | 381638/434630 | 52/57b | 13/14 | 5.7/8.4 | - | 1590/3622 | 6/6 |
| Andersson et
al (25) | 1997 | Sweden | 135006 | - | 18 | - | 2368 | 708 | 6 |
| Schuurman et
al (26) | 2000 | Netherlands | 58279 | 55–69 | 6.3 | - | 681 | - | 5 |
| Lee et al
(27) | 2001 | USA | 8922 | Collegec | 5 | 42 | 439 | 34 | 6 |
| Cerhan et al
(28) | 1997 | USA | 1050 | 65–101 | 11 | - | 71 | - | 5 |
Overall analyses
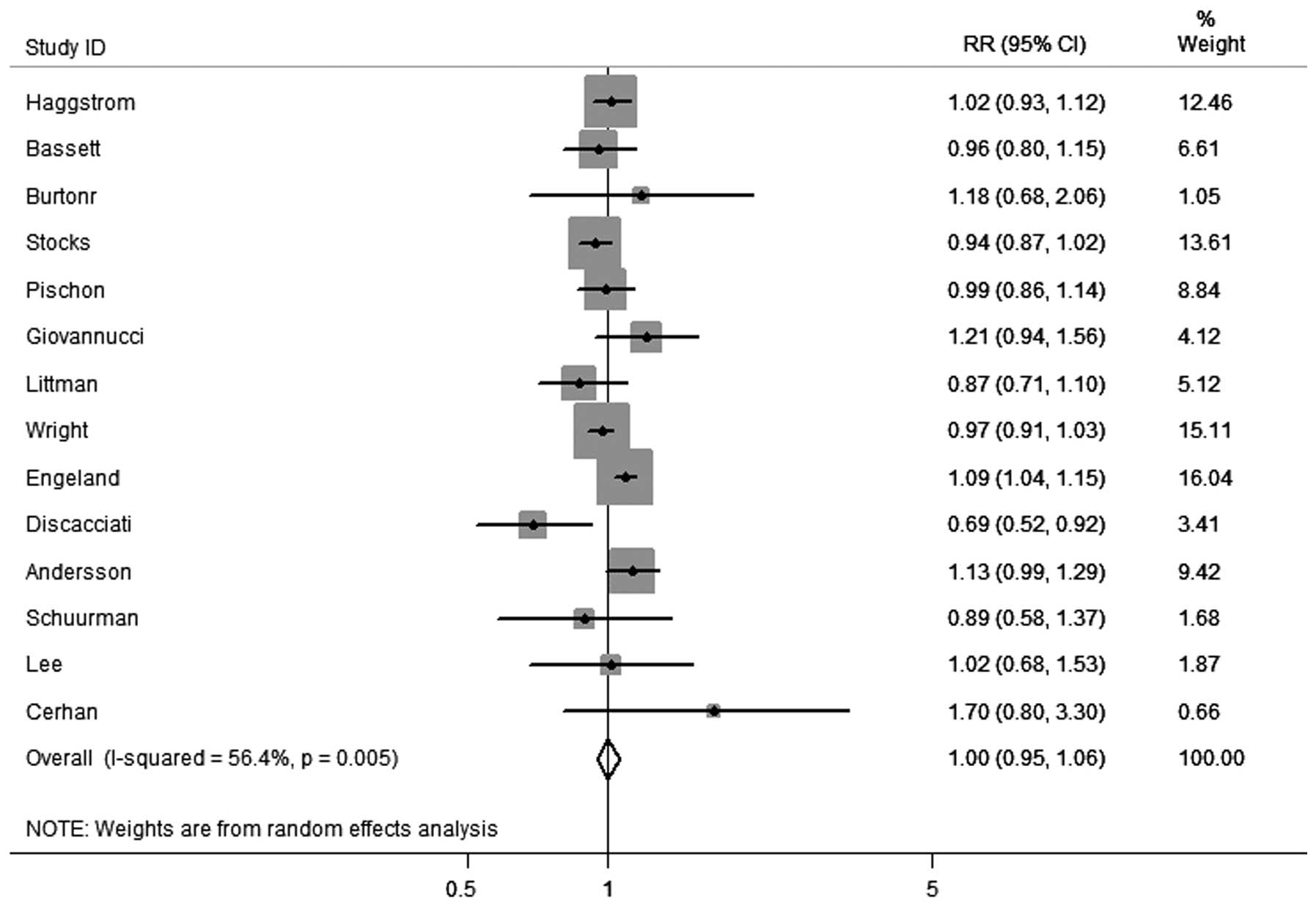
In total, 14 cohort studies were identified that had
recorded results on obesity and the incidence of PCa. As shown in
Fig. 2, obesity was not
significantly correlated with PCa incidence (RR, 1.00; 95% CI,
0.95–1.06) among these studies. However, there was evidence of
heterogeneity between the studies (I2=56.4%; P=0.005).
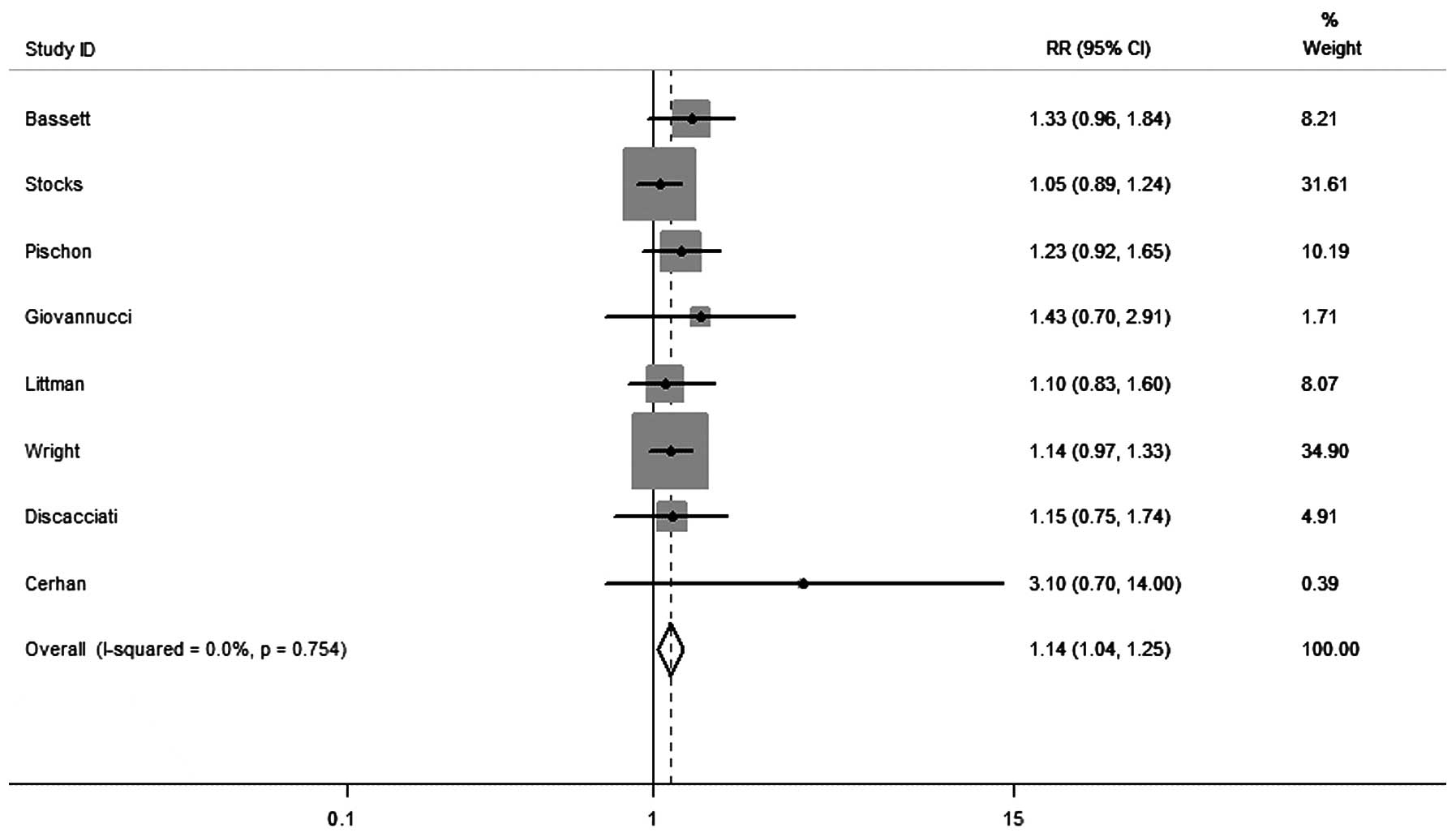
In the subgroup analysis of aggressive disease (Gleason score
between seven and 10), obesity was significantly correlated with an
increased risk of aggressive PCa (RR, 1.14; 95% CI, 1.04–1.25),
without any heterogeneity between studies (I2=0.0%;
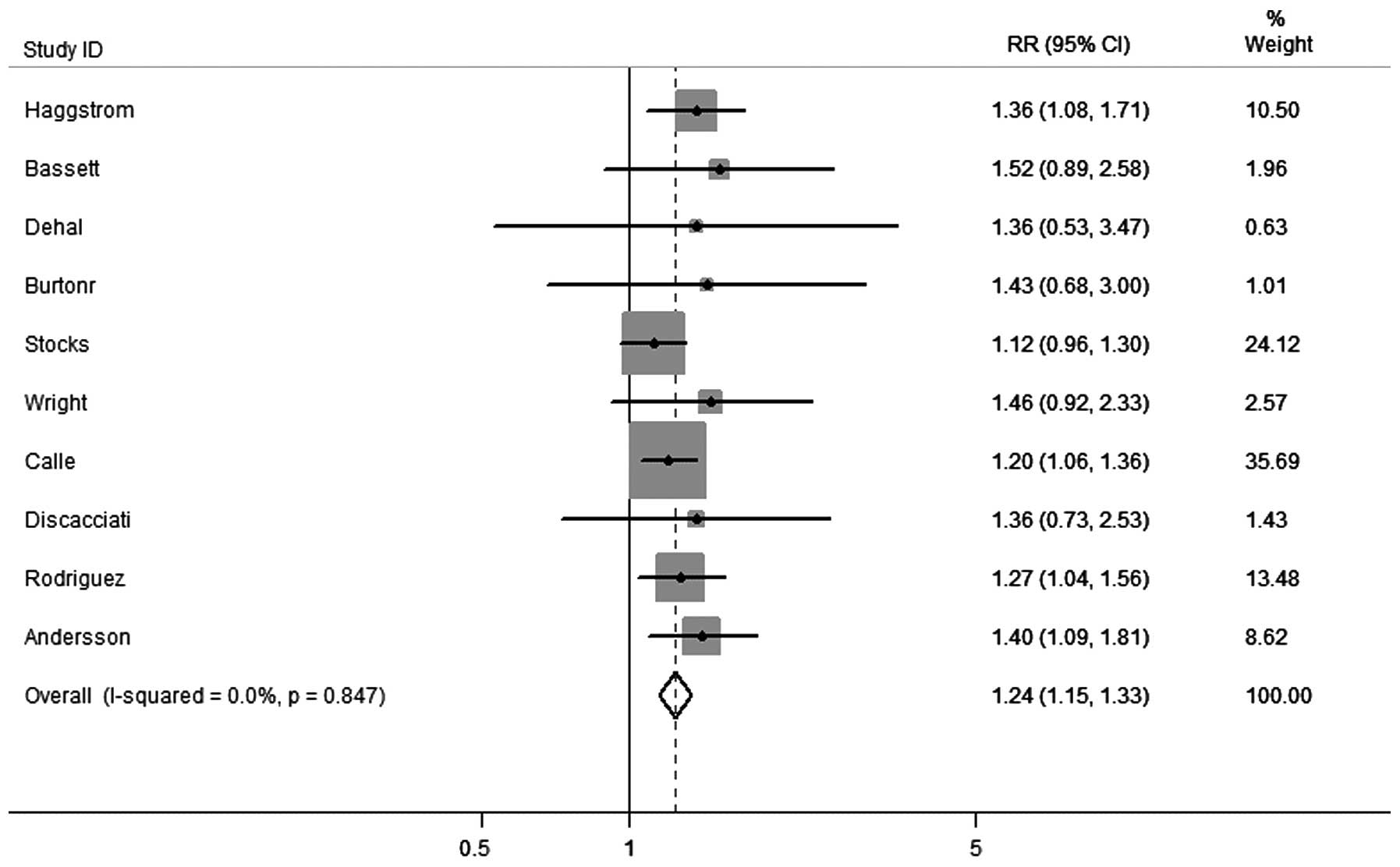
P=0.754) (Fig. 3). Overall, 11
cohort studies were identified that had presented results on
obesity and PCa-associated mortalities. As shown in Fig. 4, the pooled estimates revealed a
significantly higher risk of mortality from PCa when associated
with obesity (RR, 1.24; 95% CI, 1.15–2.33), without any
heterogeneity between studies (I2=0.0%; P=0.847).
Discussion
Obesity is an increasing worldwide health concern,
and an independent risk factor for various types of cancer,
including colon, liver, pancreas, breast and kidney cancer
(23,29). Obesity may contribute to a favorable
carcinogenetic, endocrine and biochemical microenvironment for
tumors (30). Despite a number of
population-based observational epidemiological studies, which have
examined the correlation between obesity and PCa, the association
has remained controversial. A previous meta-analysis of 16 studies
that was conducted in 2006 reported a positive association between
obesity and the incidence of PCa (31). However, the results of the
individual studies that were included in this meta-analysis
differed significantly, with one demonstrating no association
between obesity and PCa (32), two
identifying obesity as a risk factor (22,25)
and others reporting obesity as a protective factor for PCa
(21,33–35).
Since this meta-analysis was published, a number of complementary
studies have yielded inconsistent results on the association. Given
that obesity and PCa affect a large proportion of the male
population, an improved understanding of the association between
the two should have significant implications upon public health and
clinical outcomes. The effect of obesity may not only be linked to
carcinogenesis, but also to disease progression. Therefore, the
present systematic review was conducted.
The present meta-analysis, which included 14 cohort
studies that had examined obesity and/or the incidence of PCa,
concluded that the epidemiological evidence was insufficient to
confirm an association. Although obesity was not significantly
correlated with the incidence of PCa (RR, 1.00; 95% CI, 0.95–1.06)
among these studies, there was evidence of heterogeneity
(I2=56.4%; P=0.005). However, following the analyses of
several parameters of PCa aggressiveness and progression, obesity
was revealed to be significantly associated with high Gleason
scoring or clinically advanced cases of PCa. Obesity was
significantly correlated with an increased risk of aggressive PCa
(RR, 1.14; 95% CI, 1.04–1.25), without any heterogeneity between
studies (I2=0.0%; P=0.754). The association between
obesity and aggressive PCa was supported, as obesity was
biologically linked with advanced disease. At present, the
following three proposed mechanisms exist which aim to explain the
association between obesity and aggressive PCa: The
insulin/insulin-like growth factor-1 axis, the action of sex
hormones and adipokine signaling (36). A previous meta-analysis, which
included multiple prospective studies, demonstrated that although
obesity exhibits a null or marginal protective effect upon
localized disease, it is in fact associated with an increased
incidence of advanced-stage PCa (9). The results of the present study, which
suggested that obesity is associated with an increased risk of
fatal PCa, are in accordance with those of the majority of prior
prospective studies, which also demonstrated a statistically
significant positive association between obesity and the risk of
aggressive PCa.
A previous meta-analysis of prospective cohort
studies conducted by Cao and Ma (7)
evaluated the association between obesity and PCa-specific
mortalities. The study concluded that there was a positive
dose-response association between obesity and fatal PCa. The
presence of a high BMI in a cancer-free population was
significantly correlated with a higher risk of future
PCa-associated mortalities. In addition, evidence from case-control
studies was similar to that from cohort studies, which further
indicated a robust association between obesity and fatal PCa. The
present study reviewed evidence regarding the impact of obesity
upon the rate of PCa-associated mortalities and demonstrated that
obesity is a factor likely to affect disease progression. In total,
11 cohort studies were identified that had presented results on
obesity and PCa-associated mortalities. As shown in Fig. 4, the pooled estimates revealed that
obesity was associated with a significantly higher risk of PCa
mortality (RR, 1.24; 95% CI, 1.15–2.33), without any heterogeneity
between studies (I2=0.0%; P=0.847).
The present study was subject to unavoidable
limitations (37). Firstly, high
heterogeneity was detected among the studies that were reviewed in
the obesity versus PCa incidence correlation analysis.
Heterogeneity could not be eliminated completely, and therefore the
results of the combined analysis may have also been affected.
Secondly, the time expansion of the selected studies was wide, and
the study design, clinical characteristics of the patients and
follow-up durations were varied. Finally, as aforementioned,
publication bias must always be considered in systematic reviews.
The primary aim and outcome of approximately half of the included
studies did not address the effects of obesity upon the incidence
or mortality of PCa. The present study reported the results of only
the most rigorous statistical analyses performed in each study.
Based upon the results of the present meta-analysis,
obesity was identified as an additional risk factor for aggressive
PCa. Furthermore, obesity was associated with a risk of
PCa-specific mortality. These results, which link obesity and PCa,
highlight the importance of considering BMI when screening,
treating and monitoring cases of PCa. Further investigations are
required in order to evaluate the effects of weight loss in
patients with PCa.
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saad F and Pantel K: The current role of
circulating tumor cells in the diagnosis and management of bone
metastases in advanced prostate cancer. Future Oncol. 8:321–331.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pontes-Júnior J, Reis ST, et al:
Association between integrin expression and prognosis in localized
prostate cancer. Prostate. 70:1189–1195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Global Health Risks. Mortality and Burden
of Disease Attributable to Selected Major Risks. Geneva: World
Health Organization; 2009, http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf.
Accessed March 19, 2013
|
|
5
|
Wang YC, McPherson K, Marsh T, Gortmaker
SL and Brown M: Health and economic burden of the projected obesity
trends in the USA and the UK. Lancet. 378:815–825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Freedland SJ, Bañez LL, Sun LL, et al:
Obese men have higher-grade and larger tumors: an analysis of the
duke prostate center database. Prostate Cancer Prostatic Dis.
12:259–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao Y and Ma J: Body mass index, prostate
cancer-specific mortality, and biochemical recurrence: a systematic
review and meta-analysis. Cancer Prev Res (Phila). 4:486–501. 2011.
View Article : Google Scholar
|
|
8
|
Mallah KN, DiBlasio CJ, Rhee AC, et al:
Body mass index is weakly associated with, and not a helpful
predictor of, disease progression in men with clinically localized
prostate carcinoma treated with radical prostatectomy. Cancer.
103:2030–2034. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Discacciati A, Orsini N and Wolk A: Body
mass index and incidence of localized and advanced prostate cancer
- a dose-response meta-analysis of prospective studies. Ann Oncol.
23:1665–1671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stroup DF, Berlin JA, Morton SC, et al:
Meta-analysis of observational studies in epidemiology: a proposal
for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cota GF, de Sousa MR, Fereguetti TO and
Rabello A: Efficacy of anti-leishmania therapy in visceral
leishmaniasis among HIV infected patients: a systematic review with
indirect comparison. PLoS Negl Trop Dis. 7:e21952013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zwahlen M, Renehan A and Egger M:
Meta-analysis in medical research: potentials and limitations. Urol
Oncol. 26:320–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Häggström C1, Stocks T, Ulmert D, et al:
Prospective study on metabolic factors and risk of prostate cancer.
Cancer. 118:6199–6206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bassett JK, Severi G, Baglietto L, et al:
Weight change and prostate cancer incidence and mortality. Int J
Cancer. 131:1711–1719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dehal A, Garrett T, Tedders SH, et al:
Body mass index and death rate of colorectal cancer among a
national cohort of U.S. adults. Nutr Cancer. 63:1218–1225. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burton A, Martin R, Galobardes B, et al:
Young adulthood body mass index and risk of cancer in later
adulthood: historical cohort study. Cancer Causes Control.
21:2069–2077. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stocks T, Hergens MP, Englund A, Ye W and
Stattin P: Blood pressure, body size and prostate cancer risk in
the Swedish Construction Workers cohort. Int J Cancer.
127:1660–1668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pischon T, Boeing H, Weikert S, et al:
Body size and risk of prostate cancer in the European prospective
investigation into cancer and nutrition. Cancer Epidemiol
Biomarkers Prev. 17:3252–3261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giovannucci E1, Liu Y, Platz EA, Stampfer
MJ and Willett WC: Risk factors for prostate cancer incidence and
progression in the health professionals follow-up study. Int J
Cancer. 121:1571–1578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Littman AJ, White E and Kristal AR:
Anthropometrics and prostate cancer risk. Am J Epidemiol.
165:1271–1279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wright ME, Chang SC, Schatzkin A, et al:
Prospective study of adiposity and weight change in relation to
prostate cancer incidence and mortality. Cancer. 109:675–684. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Engeland A, Tretli S and Bjørge T: Height,
body mass index, and prostate cancer: a follow-up of 950000
Norwegian men. Br J Cancer. 89:1237–1242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodriguez C, Patel AV, Calle EE, et al:
Body mass index, height, and prostate cancer mortality in two large
cohorts of adult men in the United States. Cancer Epidemiol
Biomarkers Prev. 10:345–353. 2001.PubMed/NCBI
|
|
25
|
Andersson SO, Wolk A, Bergström R, et al:
Body size and prostate cancer: a 20-year follow-up study among
135006 Swedish construction workers. J Natl Cancer Inst.
89:385–389. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schuurman AG, Goldbohm RA, Dorant E and
van den Brandt PA: Anthropometry in relation to prostate cancer
risk in the Netherlands Cohort Study. Am J Epidemiol. 151:541–549.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee IM, Sesso HD and Paffenbarger RS Jr: A
prospective cohort study of physical activity and body size in
relation to prostate cancer risk (United States). Cancer Causes
Control. 12:187–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cerhan JR, Torner JC, Lynch CF, et al:
Association of smoking, body mass, and physical activity with risk
of prostate cancer in the Iowa 65+ Rural Health Study
(United States). Cancer Causes Control. 8:229–238. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hajian-Tilaki KO, Gholizadehpasha AR,
Bozorgzadeh S and Hajian-Tilaki E: Body mass index and waist
circumference are predictor biomarkers of breast cancer risk in
Iranian women. Med Oncol. 28:1296–1301. 2011. View Article : Google Scholar
|
|
30
|
Puente Vazquez J, Grande Pulido E and
Anton Aparicio LM: Cytokine and endocrine signaling in prostate
cancer. Med Oncol. 29:1956–1963. 2012. View Article : Google Scholar
|
|
31
|
MacInnis RJ and English DR: Body size and
composition and prostate cancer risk: systematic review and
meta-regression analysis. Cancer Causes Control. 17:989–1003. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baillargeon J, Platz EA, Rose DP, et al:
Obesity, adipokines, and prostate cancer in a prospective
population-based study. Cancer Epidemiol Biomarkers Prev.
15:1331–1335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Porter MP and Stanford JL: Obesity and the
risk of prostate cancer. Prostate. 62:316–321. 2005. View Article : Google Scholar
|
|
34
|
Gong Z, Neuhouser ML, Goodman PJ, et al:
Obesity, diabetes, and risk of prostate cancer: results from the
prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev.
15:1977–1983. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rodriguez C, Freedland SJ, Deka A, et al:
Body mass index, weight change, and risk of prostate cancer in the
Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol
Biomarkers Prev. 16:63–69. 2007. View Article : Google Scholar
|
|
36
|
Roberts DL, Dive C and Renehan AG:
Biological mechanisms linking obesity and cancer risk: new
perspectives. Annu Rev Med. 61:301–316. 2010. View Article : Google Scholar
|
|
37
|
Hu Q, Gou Y, Sun C, et al: The prognostic
value of C-reactive protein in renal cell carcinoma: A systematic
review and meta-analysis. Urol Oncol. 32:50.e1–50.e8. 2014.
View Article : Google Scholar
|